Abstract
Background
Some abnormalities of mouse corneal epithelial maintenance can be identified by the atypical mosaic patterns they produce in X-chromosome inactivation mosaics and chimeras. Human FLNA/+ females, heterozygous for X-linked, filamin A gene (FLNA) mutations, display a range of disorders and X-inactivation mosaicism is sometimes quantitatively unbalanced. FlnaDilp2/+mice, heterozygous for an X-linked filamin A (Flna) nonsense mutation have variable eye, skeletal and other abnormalities, but X-inactivation mosaicism has not been investigated. The aim of this study was to determine whether X-inactivation mosaicism in the corneal epithelia of FlnaDilp2/+mice was affected in any way that might predict abnormal corneal epithelial maintenance.
Results
X-chromosome inactivation mosaicism was studied in the corneal epithelium and a control tissue (liver) of FlnaDilp2/+and wild-type (WT) female X-inactivation mosaics, hemizygous for the X-linked, LacZ reporter H253 transgene, using β-galactosidase histochemical staining. The corneal epithelia of FlnaDilp2/+and WT X-inactivation mosaics showed similar radial, striped patterns, implying epithelial cell movement was not disrupted in FlnaDilp2/+corneas. Corrected stripe numbers declined with age overall (but not significantly for either genotype individually), consistent with previous reports suggesting an age-related reduction in stem cell function. Corrected stripe numbers were not reduced in FlnaDilp2/+compared with WT X-inactivation mosaics and mosaicism was not significantly more unbalanced in the corneal epithelia or livers of FlnaDilp2/+than wild-type Flna+/+ X-inactivation mosaics.
Conclusions
Mosaic analysis identified no major effect of the mouse FlnaDilp2 mutation on corneal epithelial maintenance or the balance of X-inactivation mosaicism in the corneal epithelium or liver.
Similar content being viewed by others
Background
Filamin A is a cytoskeleton protein with multiple roles that binds to actin and other proteins in many cell types. It is encoded by an X-linked gene, designated FLNA in humans and Flna in mice; mutations in human FLNA cause several types of congenital birth defects [1, 2]. Females heterozygous for a loss of function mutation are viable but have periventricular nodular heterotopia (PVNH), a developmental defect in neuronal migration, whereas hemizygous males do not survive. FLNA missense mutations cause four genetic syndromes belonging to the otopalatodigital (OPD) spectrum disorders. The phenotypes of heterozygous females vary according to the syndrome but include skeletal dysplasia, urogenital defects and deafness. Hemizygous males with the more severe syndromes die in utero or shortly after birth but those with the mildest syndrome survive.
A mutant Flna mouse with misshapen pupils was recovered from a mutagenesis screen [3]. This new mutant was initially called Dilp2 (dilated pupils 2) but renamed FlnaDilp2 once it was mapped to the Flna locus and the underlying defect identified [4]. The FlnaDilp2 mutation results in nonsense-mediated decay of the Flna mRNA and, therefore, absence of any Flna protein. Heterozygous FlnaDilp2/+female mice are mostly viable and fertile but have mild defects of the eyes, sternum and palate whereas FlnaDilp2 /Y males die in utero with heart defects [4]. After X-chromosome inactivation, heterozygous FlnaDilp2/+and FLNA/+ females are X-inactivation mosaics with two genetically distinct cell populations. Although the FlnaDilp2/+phenotype has been characterised [4], X-inactivation mosaicism was not investigated. This has been reported to be very unbalanced in some human FLNA/+ females [1, 5]. Unbalanced X-inactivation mosaicism may be caused by primary non-random X-chromosome inactivation (e.g. heterozygosity for different Xce alleles in mice [6–11]) or by various secondary selection processes.
Some abnormalities of mouse corneal epithelial maintenance can be identified by the abnormal mosaic patterns they produce in X-chromosome inactivation mosaics and chimeras. According to the widely accepted, limbal epithelial stem cell (LESC) hypothesis, the corneal epithelium is maintained by stem cells located in the limbus between the cornea and conjunctiva [12, 13]. These produce daughter transient (or transit) amplifying cells (TACs), which move centripetally in the basal epithelial layer and divide several times before they leave the basal layer and move apically towards the surface from where they are shed. Mosaic patterns in the adult corneal epithelia of wild-type (WT), adult mouse chimeras, X-inactivation mosaics and other types of mosaics change from a randomly orientated patchwork to radial stripes between 5 and 8 weeks after birth [14–17]. This change in pattern is thought to reflect the activation of LESCs at the corneal periphery and the radial stripes are presumed to be formed by lineages of TACs, which move centripetally from the limbus towards the centre of the cornea. This is consistent with more direct evidence for centripetal movement of corneal epithelial cells [18, 19]. The radial striped pattern is disrupted in some mutant mice, including Pax6+/Sey-Neu and Pax6+/Leca4 X-inactivation mosaics [20, 21] and transgenic mosaics that are also Dstncorn1/corn1homozygotes [22], implying that cell movement is affected. Numerical analysis of these striping patterns provides an indirect estimate of the number of coherent clones of LESCs maintaining the corneal epithelium [14, 16] and has been used to predict that stem cell function declines with age and may already be reduced in young (15-week) Pax6+/Sey-Neu mice [20, 21].
Although the radial striped pattern is disturbed in Pax6+/Sey-Neu X-inactivation mosaics, where all the cells are Pax6+/Sey-Neu , it is normal when Pax6+/Sey-Neu and WT cells are combined in Pax6+/Sey-Neu ↔ WT chimeras. This suggests that the presence of WT cells can rescue the defect in cell movement [20]. However, the Pax6+/Sey-Neu cells were under-represented in corneal epithelia, relative to other tissues in these chimeras, implying that active Pax6+/Sey-Neu LESCs were depleted.
X-chromosome linkage of the FlnaDilp2 mutation provides an opportunity to use X-chromosome inactivation mosaics to investigate the effect of the mutant FlnaDilp2 allele on stem cell function in ways that would normally require production of experimental chimeras. FlnaDilp2 XLacZ- /Flna+ XLacZTg X-inactivation mosaics are expected to be equivalent to chimeras with separate populations of β-galactosidase (β-gal)-positive cells expressing the WT, Flna+ allele and β-gal-negative cells expressing the mutant FlnaDilp2 allele. If expression of the FlnaDilp2 mutation affected stem cell function, β-gal-negative cells (expressing FlnaDilp2 ) would be expected to be under-represented in corneal epithelia, relative to other tissues in FlnaDilp2 XLacZ- /Flna+ XLacZTg X-inactivation mosaics in the same way that Pax6+/Sey-Neu cells were under-represented in corneal epithelia, relative to other tissues in Pax6+/Sey-Neu ↔ WT chimeras [20].
The aim of the present study was to identify whether X-inactivation mosaicism in the corneal epithelia of FlnaDilp2/+heterozygotes was abnormal in any way that predicted that Flna has a role in LESC function or corneal maintenance.
Methods
Mice and genotyping
Animal work was performed in accordance with institutional guidelines and UK Home Office regulations. FlnaDilp2/+mice were maintained by crossing FlnaDilp2/+females with WT C57BL/6 or (C57BL/6 × CBA/Ca)F1 males. Females for analysis were produced by crossing FlnaDilp2/+females with XLacZTg /Y, H253 strain males [23, 24], which ubiquitously express an X-linked nLacZ transgene (abbreviated to XLacZTg to produce FlnaDilp2 XLacZ- /Flna+ XLacZTg and wild-type Flna+ XLacZ- /Flna+ XLacZTg females. Both groups of females were X-inactivation mosaics and showed mosaic transgene expression of the XLacZTg transgene, after X-gal staining for β-galactosidase (β-gal) activity.
FlnaDilp2/+ and Flna+/+ females were distinguished by PCR of Flna exon 44 DNA from ear clip biopsies. DNA was prepared using direct PCR lysis reagent (Viagen 401-E). 1 μl DNA was used in 20 μl PCR reaction mixtures containing forward primer TGA AGG GGA TGT TAA CCA ATT C, reverse primer TCT ATC TCA CTG GCT TCC TTG C and DNA polymerase (BIO-X-ACT, Bioline). DNA was amplified in a thermocycler by incubating at 94°C for 3 min., followed by 30 cycles of 30 s at 94°C, 45 s at 55°C, 1 min. extension at 68°C and a final incubation of 2 min at 68°C. The Dilp2 mutation creates an AluI restriction site. Therefore, the PCR products were digested with AluI restriction enzyme at 37°C for 2 h., separated by electrophoresis on 2% agarose gels, containing ethidium bromide, and visualised on a UV transilluminator.
β-galactosidase staining and analysis of X-inactivation mosaicism
For analysis of corneas, mice were culled and the eyes were fixed in 0.2% glutaraldehyde solution, stained overnight in X-gal staining solution as described previously [14], post-fixed in 4% paraformaldehyde and stored in 70% ethanol. Mosaic patterns in the adult corneal epithelium occurred as radial stripes and were analysed as described previously to estimate the percentage of β-gal-positive cells and the 'corrected stripe number' [14, 16, 20, 21] except that a semi-automated method was used for analysis [25]. This involved using the 'Circular Clonal Analysis' tool provided by the ClonalTools ImageJ plugin (http://imagejdocu.tudor.lu/doku.php?id=plugin:analysis:clonaltools:start) to define a circular line selection that was concentric with the corneal epithelium. To avoid errors arising from sampling too close to the limbal epithelium, the radius of the circle was only 80% of the corneal radius. Lengths of β-gal-positive and β-gal-negative tissue were measured along the circular line selection (across the widths of the stripes) to estimate the percentage of β-gal-positive cells and the observed mean stripe width [21, 25].
The corrected stripe number adjusts for the likely mean number of adjacent radial, β-gal-positive corneal epithelial clones within a single β-gal-positive radial stripe. The corrected mean stripe width is derived by dividing the observed mean stripe width by the function 1/(1-p), where p is the proportion of β-gal-positive cells around the circumference, as described previously [14, 16]. If the corrected mean stripe width is expressed as a percentage of the circumference of the corneal epithelium, it can be readily converted to the corrected stripe number per circumference. This provides an estimate of the total number of active corneal epithelial coherent clones (both β-gal positive and β-gal negative) per circumference. The corrected stripe number is useful for comparing numbers of active clones of stem cells between different groups because it is believed that each coherent clone in the corneal epithelium is maintained by a coherent clone of stem cells in the limbus at the periphery of the cornea. However, because the number of stem cells per coherent clone may vary it is not a direct estimate of the number of active stem cells [14, 16].
Livers were analysed as frozen sections. Mice were culled and their livers were removed, washed with cold PBS, cut into small pieces and fixed in 4% paraformaldehyde (2 h. at 4°C). Fixed samples were washed in PBS (3 × 10 min), transferred to 30% glycerol in PBS (4°C, overnight), 50% glycerol (4°C, overnight) and stored in 80% glycerol at 4°C. Before sectioning, tissues were re-hydrated (50% and 30% glycerol for 1 h. each and then to PBS for 30 min.). Samples were immersed in OCT embedding medium in plastic moulds and snap-frozen on dry ice. Frozen sections were cut at 10 μm thickness, transferred to Polysine slides and stained for β-gal activity using a modification of an established protocol [14]. Slides were soaked in wash buffer (0.1 M Na phosphate, 2 mM MgCl2, 0.01% sodium deoxycholic acid and 2% Igepal) 3 × 10 min., then incubated in X-gal staining solution at 37°C in the dark overnight. After staining, slides were washed in PBS (3 × 10 min) and counterstained with 1% neutral red in 1.48 mM acetate buffer, pH 4.8 for 5 min. and rinsed in water. Sections were dehydrated rapidly through a graded ethanol series, cleared in xylene and mounted with DPX mounting medium (BDH, Poole, UK) and cover slips. β-gal positive and β-gal negative cells were counted in five square scoring regions in one section from each sample (Zeiss Axioplan-2 microscope; ×40 objective). Random number tables were used to position each scoring region randomly on the section. Each scoring region, containing ~200 cells, was divided into 100 smaller squares, with a 10 × 10 eyepiece graticule, for cell counting and the person scoring was blinded to the genotypes.
Statistical analysis
Genotype frequencies were compared to Mendelian expectations by goodness of fit χ2 tests. Percentages of β-gal positive cells and corrected stripe numbers in different groups were compared by 2-way analysis of variance (ANOVA) using GraphPad Prism software. For β-gal positive cells and corrected stripe numbers in the corneal epithelium, individual values analysed were means of left and right eyes of each mouse.
Results
Survival of FlnaDilp2/+heterozygotes
Crosses between FlnaDilp2/+females with XLacZTg /Y males produced 50 FlnaDilp2/+ females, 88 wild-type Flna+/+ females and 81 males (not genotyped) at weaning. The frequencies of the two female genotypes differed significantly from a 1:1 ratio (χ2 = 10.46; P = 0.0012) and implied a 43% deficiency of FlnaDilp2/+females, which is consistent with previously reported findings [4]. It was shown previously that FlnaDilp2 /Y males die before birth [4]. The frequencies of surviving males and Flna+/+ females differed significantly from the 2:1 ratio expected if all males survived (χ2 = 26.70; P < 0.0001) but did not differ significantly from a 1:1 ratio (χ2 = 0.290; P = 0.590), which is consistent with the previously reported lethality of FlnaDilp2 /Y males [4].
X-inactivation mosaicism in the corneal epithelium
We compared X-chromosome mosaicism in corneal epithelia and a control tissue (liver) from FlnaDilp2 XLacZ- /Flna+ XLacZTg and Flna+ XLacZ- /Flna+ XLacZTg X-inactivation mosaic females, as described in the Methods. Cells expressing the X-chromosome carrying the XLacZTg transgene were identified as β-gal-positive by histochemistry [23].
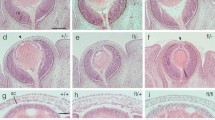
Non-mosaic XLacZTg /Y males and XLacZTg/Tgfemales showed uniform blue staining in the cornea (Figure 1A). When the intact eye is stained all 4-6 layers of the corneal epithelium become stained but the underlying stroma remains unstained unless the epithelium is damaged [14, 16]. Mosaic patterns in the corneal epithelium were examined in 15- and 30-week old adults, by which time radial stripe patterns are established in WT corneas [14, 16, 20, 21]. Both heterozygous FlnaDilp2/+and WT X-inactivation mosaics showed normal radial striped patterns (Figure 1B, C).
β-gal staining of tissues from FlnaDilp2/+and wild-type Flna+/+ X-inactivation mosaics. (A-C) Corneas from (A) an XLacZTg/Tg positive control (100% β-gal-positive), (B) a 15-week, WT (Flna+/+) XLacZTg/- X-inactivation mosaic (68.7% β-gal-positive) and (C) a 15-week, FlnaDilp2/+ X-inactivation mosaic (84.5% β-gal-positive). (B) and (C) show similar radial stripes of β-gal-positive (blue) and β-gal-negative (unstained) cells. (D-F) Sections of livers from (D) XLacZTg/Tg positive control (100% β-gal-positive), (E) a WT X-inactivation mosaic and (F) a FlnaDilp2/+ X-inactivation mosaic. (E) and (F) show similar randomly orientated patches of β-gal-positive (blue) and β-gal-negative (pink) cells.
Quantitative analysis was based on means of left and right eyes for each mouse with 8-22 mice per group as shown in Figure 2. A 2-way ANOVA showed there were no overall significant differences, in percentage of β-gal-positive cells (expressing the WT, Flna+ allele) in the corneal epithelium (Figure 2A), for age (P = 0.347) or genotype (P = 0.342) and the interaction was also non-significant (P = 0.081). Thus, the percentage of β-gal-positive cells was not significantly greater in FlnaDilp2/+ than WT X-inactivation mosaics at either 15 or 30 weeks. Overall, a 2-way ANOVA showed that the 'corrected stripe number' (Figure 2B) declined significantly with age (P = 0.040), as previously reported for WT X-inactivation mosaics [16], and was significantly higher in FlnaDilp2/+ than WT X-inactivation mosaics (P = 0.024) but the interaction was non-significant (P = 0.434). However, age differences were not significant for either genotype separately and genotype differences were not significant when 15- and 30-week old mice were analysed separately by Bonferroni post-hoc tests.
Quantitative comparison of heterozygous FlnaDilp2/+and wild-type Flna+/+X-inactivation mosaics. (A) Percentage of β-gal-positive cells in corneal epithelia from FlnaDilp2 and WT (Flna+/+) X-inactivation mosaics at 15 and 30 weeks. (Individual values were means of left and right eyes of each mouse.) There were no significant differences between genotypes or ages by 2-way ANOVA. (B) Corrected stripe numbers in corneal epithelia from FlnaDilp2/+and WT X-inactivation mosaics at 15 and 30 weeks. (Individual values were means of left and right eyes of each mouse.) Statistical analysis showed significant differences between both ages (P = 0.040) and genotypes (P = 0.024) by 2-way ANOVA. However, neither age nor genotype differences were significant for individual pairwise comparisons when analysed separately by Bonferroni post-hoc tests. (C) Percentage of β-gal-positive cells in livers from FlnaDilp2/+and WT X-inactivation mosaics at 3, 6 and 15 weeks. There were no significant differences between genotypes or ages by 2-way ANOVA. In each case the results are presented as mean ± SEM and the number of mice is shown above each bar.
X-inactivation mosaicism in the liver
More than 98% of cells in frozen sections of livers from non-mosaic XLacZTg /Y males and XLacZTg/Tg females were β-gal-positive so we used the liver as a control tissue and quantitatively compared the mosaicism in livers of FlnaDilp2/+and Flna+/+female, X-inactivation mosaics (Figure 1D). Staining patterns in mosaic livers were examined at three, six and 15 weeks (Figure 1E, F) in an attempt to identify whether any unbalanced mosaicism resulted from cell selection over this period. A 2-way ANOVA on the results of a quantitative analysis (Figure 2C) revealed no significant differences in the percentage of β-gal-positive cells in livers of FlnaDilp2/+and Flna+/+X-inactivation mosaics at any of the ages examined. For age comparisons P = 0.161, for genotypes P = 0.652 and for the interaction, P = 0.307.
Discussion
Our study revealed no significant adverse effect of the FlnaDilp2/+genotype on X-inactivation mosaicism in either the corneal epithelium or liver. FlnaDilp2/+heterozygotes had qualitatively normal radial striped patterns, implying that corneal epithelial cell movement was normal. Although, overall the corrected stripe numbers were higher in FlnaDilp2/+corneas than WT, they were within the WT range observed previously [14, 16, 20, 21]. As FlnaDilp2/+corrected stripe numbers were not reduced, there was no evidence from the mosaic analysis for any overall reduction in stem cell function in FlnaDilp2/+corneas. Furthermore, as β-gal-negative cells, expressing the FlnaDilp2 mutation were not significantly under-represented in the corneal epithelium, there was no evidence that stem cells expressing the FlnaDilp2 mutation were depleted or less active than those expressing the WT allele. We conclude that mosaic analysis identified no major effect of the mouse FlnaDilp2 mutation on corneal epithelial cell movement or maintenance of the corneal epithelium by clones of limbal epithelial stem cells.
A previous investigation estimated lethality among heterozygous FlnaDilp2/+females as approximately 30% [4] and in the present study we estimated this loss as about 43%. Hart et al. suggested that survival might be lower for FlnaDilp2/+females with a higher proportion of cells with the mutant X-chromosome active [4]. This predicts that the average percentage of β-gal-positive cells (not expressing the FlnaDilp2 mutation) would be higher among surviving heterozygous FlnaDilp2/+mosaic females than among the WT Flna+/+mosaic females, either generally or in one or more critical tissue(s). This was not the case for either the corneal epithelium or liver, so there is no evidence that the composition of these tissues correlates with anything that affects the survival of FlnaDilp2/+mosaic females.
The failure to detect an imbalance in X-inactivation mosaicism in the FlnaDilp2/+mouse liver or corneal epithelium, as reported for leukocytes from human FLNA/+ females [1, 5], could reflect the type of mutation, species differences and/or the tissues studied. Although heterozygotes for FLNA mutations causing OPD spectrum disorders showed a pronounced imbalance in X-inactivation mosaicism in leukocytes [1, 5] this does not appear to be true of heterozygotes for mutations causing PVNH [26]. FlnaDilp is a loss of function mutation and, therefore, is more similar to FLNA mutations causing PVNH than those causing OPD disorders but the mouse FlnaDilp/+phenotype differs from the human PVNH phenotype [4]. It may be informative to compare the phenotypes of other mouse Flna alleles and their effects on X-inactivation mosaicism and to extend the range of tissues examined.
Conclusions
Analysis of patterns of X-inactivation mosaicism revealed no evidence that mouse FlnaDilp2 mutation has a major detrimental effect on maintenance of the corneal epithelium by clones of limbal epithelial stem cells. In contrast to some reports for human FLNA/+ heterozygotes, there was no evidence for unbalanced X-inactivation mosaicism in the tissues we examined from FlnaDilp2/+ mice.
Abbreviations
- ANOVA:
-
Analysis of variance
- ß-gal:
-
ß-galactosidase
- LESC:
-
Limbal epithelial stem cell
- OPD:
-
Otopalatodigital
- PBS:
-
Phosphate buffered saline
- PVNH:
-
Periventricular nodular heterotopia
- SEM:
-
Standard error of the mean
- TAC:
-
Transient (or transit) amplifying cell
- UV:
-
Ultraviolet
- WT:
-
Wild-type
- X-gal:
-
Bromo-chloro-indolyl-galactopyranoside
- XLacZ Tg/- :
-
Female X-inactivation mosaic mice: hemizygous for the H253 X-linked nLacZ transgene.
References
Robertson SP, Twigg SRF, Sutherland-Smith AJ, Biancalana V, Gorlin RJ, Horn D, Kenwrick SJ, Kim CA, Morava E, Newbury-Ecob R, et al: Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat Genet. 2003, 33: 487-491. 10.1038/ng1119.
Robertson SP: Filamin A: phenotypic diversity. Curr Opin Genet Dev. 2005, 15: 301-307. 10.1016/j.gde.2005.04.001.
Thaung C, West K, Clark BJ, McKie L, Morgan JE, Arnold K, Nolan PM, Peters J, Hunter AJ, Brown SDM, et al: Novel ENU-induced eye mutations in the mouse: models for human eye disease. Hum Mol Genet. 2002, 11: 755-767. 10.1093/hmg/11.7.755.
Hart AW, Morgan JE, Schneider J, West K, McKie L, Bhattacharya S, Jackson IJ, Cross SH: Cardiac malformations and midline skeletal defects in mice lacking filamin A. Hum Mol Genet. 2006, 15: 2457-2467. 10.1093/hmg/ddl168.
Robertson SP, Jenkins ZA, Morgan T, Ades L, Aftimos S, Boute O, Fiskerstrand T, Garcia-Minaur S, Grix A, Green A, et al: Frontometaphyseal dysplasia: Mutations in FLNA and phenotypic diversity. Am J Med Genet A. 2006, 140A: 1726-1736. 10.1002/ajmg.a.31322.
Cattanach BM, Williams CE: Evidence of non-random X chromosome activity in the mouse. Genet Res. 1972, 19: 229-240. 10.1017/S001667230001449X.
West JD, Chapman VM: Variation for X-chromosome expression in mice detected by electrophoresis of phosphoglycerate kinase. Genet Res. 1978, 32: 91-102. 10.1017/S0016672300018565.
Johnston PG, Cattanach BM: Controlling elements in the mouse. 4. Evidence of non-random X-inactivation. Genet Res. 1981, 37: 151-160. 10.1017/S0016672300020127.
Courtier B, Heard E, Avner P: Xce haplotypes show modified methylation in a region of the active X-chromosome lying 3' to Xist. Proc Natl Acad Sci USA. 1995, 92: 3531-3535. 10.1073/pnas.92.8.3531.
Plenge RM, Percec I, Nadeau JH, Willard HF: Expression-based assay of an X-linked gene to examine effects of the X-controlling element (Xce) locus. Mamm Genome. 2000, 11: 405-408. 10.1007/s003350010077.
Chadwick LH, Pertz LM, Broman KW, Bartolomei MS, Willard HF: Genetic control of X chromosome inactivation in mice: Definition of the Xce candidate interval. Genetics. 2006, 173: 2103-2110. 10.1534/genetics.105.054882.
Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM: Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989, 57: 201-209. 10.1016/0092-8674(89)90958-6.
Lehrer MS, Sun TT, Lavker RM: Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci. 1998, 111: 2867-2875.
Collinson JM, Morris L, Reid AI, Ramaesh T, Keighren MA, Flockhart JH, Hill RE, Tan SS, Ramaesh K, Dhillon B, West JD: Clonal analysis of patterns of growth, stem cell activity, and cell movement during the development and maintenance of the murine corneal epithelium. Dev Dyn. 2002, 224: 432-440. 10.1002/dvdy.10124.
Collinson JM, Hill RE, West JD: Analysis of mouse eye development with chimeras and mosaics. Int J Dev Biol. 2004, 48: 793-804. 10.1387/ijdb.041885jc.
Mort RL, Ramaesh T, Kleinjan DA, Morley SD, West JD: Mosaic analysis of stem cell function and wound healing in the mouse corneal epithelium. BMC Dev Biol. 2009, 9: 4-10.1186/1471-213X-9-4.
Endo M, Zoltick PW, Chung DC, Bennett J, Radu A, Muvarak N, Flake AW: Gene transfer to ocular stem cells by early gestational intraamniotic injection of lentiviral vector. Mol Therap. 2007, 15: 579-587. 10.1038/sj.mt.6300092.
Buck RC: Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Invest Ophthalmol Vis Sci. 1985, 26: 1296-1299.
Nagasaki T, Zhao J: Centripetal movement of corneal epithelial cells in the normal adult mouse. Invest Ophthalmol Vis Sci. 2003, 44: 558-566. 10.1167/iovs.02-0705.
Collinson JM, Chanas SA, Hill RE, West JD: Corneal development, limbal stem cell function, and corneal epithelial cell migration in the Pax6+/- mouse. Invest Ophthalmol Vis Sci. 2004, 45: 1101-1108. 10.1167/iovs.03-1118.
Mort RL, Bentley AJ, Martin FL, Collinson JM, Douvaras P, Hill RE, Morley SD, Fullwood NJ, West JD: Effects of aberrant Pax6 gene dosage on mouse corneal pathophysiology and corneal epithelial homeostasis. PLoS ONE. 2011, 6: e28895-10.1371/journal.pone.0028895.
Zhang W, Zhao J, Chen L, Urbanowicz MM, Nagasaki T: Abnormal epithelial homeostasis in the cornea of mice with a destrin deletion. Mol Vis. 2008, 14: 1929-1939.
Tan S-S, Williams EA, Tam PPL: X-chromosome inactivation occurs at different times in different tissues of the post-implantation mouse embryo. Nat Genet. 1993, 3: 170-174. 10.1038/ng0293-170.
Tan SS, Breen S: Radial mosaicism and tangential cell dispersion both contribute to mouse neocortical development. Nature. 1993, 362: 638-640. 10.1038/362638a0.
Mort RL: Quantitative analysis of patch patterns in mosaic tissues with ClonalTools software. J Anat. 2009, 215: 698-704. 10.1111/j.1469-7580.2009.01150.x.
Fox JW, Lamperti ED, Eksioglu YZ, Hong SE, Feng YY, Graham DA, Scheffer IE, Dobyns WB, Hirsch BA, Radtke RA, et al: Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998, 21: 1315-1325. 10.1016/S0896-6273(00)80651-0.
Acknowledgements
We thank staff at BRR, University of Edinburgh, for expert animal husbandry and specialised technical services and Prof. Seong-Seng Tan for permission to use H253 mice. The FlnaDilp2 mutation (GENA379) was derived in a mutagenesis screen that was funded, in part, by GlaxoSmithKline and we thank them for permitting us access to these mice via a materials transfer agreement. This work was supported, in part, by the Barbour Watson Fund (Edinburgh) and a PhD studentship from Fight for Sight (UK) and the RS MacDonald Charitable Trust, awarded to JDW for PD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PD carried out the staining and photography of the mosaic corneas, established the protocol for staining frozen sections, stained some of the tissue sections and contributed to the data analysis and the draft manuscript, WL carried out staining and analysis of the livers, RM carried out the quantitative analysis of the corneal epithelia, LM and KMW genotyped the mice, SHC managed the primary FlnaDilp2 mouse colony, SDM participated in the study design and helped to draft the manuscript. JDW conceived and coordinated the study and wrote most of the first draft of the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Douvaras, P., Liu, W., Mort, R.L. et al. Normal X-inactivation mosaicism in corneas of heterozygous FlnaDilp2/+ female mice-a model of human Filamin A (FLNA) diseases. BMC Res Notes 5, 122 (2012). https://doi.org/10.1186/1756-0500-5-122
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-0500-5-122