Abstract
Background
Imidazo[1,2-a]pyridines and pyrimidines are important organic fluorophores which have been investigated as biomarkers and photochemical sensors. The effect on the luminescent property by substituents in the heterocycle and phenyl rings, have been studied as well. In this investigation, series of 3-hydroxymethyl imidazo[1,2-a]pyridines and pyrimidines were synthesized and evaluated in relation to fluorescence emission, based upon the hypothesis that the hydroxymethyl group may act as an enhancer of fluorescence intensity.
Results
Compounds of both series emitted light in organic solvents dilutions as well as in acidic and alkaline media. Quantitative fluorescence spectroscopy determined that both fused heterocycles fluoresced more intensely than the parent unsubstituted imidazo[1,2-a]azine fluorophore. In particular, 3-hydroxymethyl imidazo[1,2-a]pyridines fluoresced more intensely than 3-hydroxymethyl imidazo[1,2-a]pyrimidines, the latter emitting blue light at longer wavelengths, whereas the former emitted purple light.
Conclusion
It was concluded that in most cases the hydroxymethyl moiety did act as an enhancer of the fluorescence intensity, however, a comparison made with the fluorescence emitted by 2-aryl imidazo[1,2-a]azines revealed that in some cases the hydroxymethyl substituent decreased the fluorescence intensity.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
In the past few years a growing interest in the chemistry of imidazo[1,2-a]pyrimidines and pyridines has been developed due to the extent of their applications in pharmacological science. Indeed they are known for their anxiolytic [1], cardiovascular [2], analgesic [3, 4], antihypertensive [4] and neuroleptic [5, 6] among other activities [7–9]. However, imidazo[1,2-a]pyridines and pyrimidines are also attractive due to their physicochemical properties exhibited, namely the fluorescent activity. Several studies concerning the effects of substituents on the fluorescent properties of imidazo[1,2-apyridines have been carried out, for instance, 2-phenyl (or 2-(2-naphthyl)) and/or 7-methyl substitution caused no deterioration of the fluorescent property. The amino or dimethylamino substitution at the 4'-position of 2-phenyl imidazo[1,2-apyridine shifted the fluorescence to the visible region in polar solvents [10]. From a comparative spectroscopy study performed on several imidazo[1,2-a]pyridines and pyrimidines, it was observed that substitution of a proton for methyl, carboxyl, or amino group increased the fluorescence intensity. Fluorescence was destroyed when a ring position carried a nitro group or when the pyridine or pyrimidine ring is catalytically reduced to the 5,6,7,8-tetrahydro derivative [11]. In the latter study, it was also observed that imidazo[1,2-a]pyrimidines fluoresced more intensely and ca. 60 nm higher than the analogous pyridines. Taking advantage of the fluorescence property, a imidazo1,2-a]pyridine derivative was used as a biomarker of hypoxic tumor cells [12]. A (4-piperidinylfluorophenyl) imidazo1,2-a]pyridine was applied to a multiple fluorescent chemosensor [13]. Recently, an imidazopyrimidine based compound was used in an electron transport layer of an organic light emission device [14].
However, a great disadvantage of many current fluorophores is their very short time of life and susceptibility to physicochemical environments [14], therefore interest in the development of more efficient fluorophores is growing. In this study, the hydroxymethyl group was investigated as an enhancer of the fluorescence property of derivatives of imidazo[1,2-a]pyridines and imidazo[1,2-a]pyrimidines with the aim of obtaining fluorophores with potential use as biomarkers.
Results and discussion
The hydroxymethyl group has proved to be a promoter of fluorescence in naphthyl thioureas [15]. In another study, it was reported that 2-(3,4,5,6-tetrafluoro-2-hydroxyphenyl)imidazo[1,2-a]pyridine emitted long wavelength light around 540 nm both in polar and in nonpolar solvents [16]. Based upon this information, it was hypothesized that introduction of a hydroxymethyl group at position 3 of the imidazo[1,2-a]azines should enhance fluorescence (probably through a spatial non-covalent interaction of the hydroxyl non-bonding electrons with the aryl rings) as compared to the parent fluorophore (the unsubstituted imidazo[1,2-a]azine).
The two series of compounds were prepared straightforward (Scheme 1). Condensation of 2-amino pyridine or 2-amino pyrimidine with the appropriately substituted 2-bromo acetophenone afforded the imidazo[1,2-a]azine nucleus. A Vilsmeier Haack treatment on the fused heterocycles 3 and 4 led to the corresponding 3-formyl substituted derivatives 5. Reduction of the formyl moiety with NaBH4 in alkaline ethyl alcohol delivered the 3-hydroxymethyl derivatives 6 and 7.
Some of these products are already known but have not been studied in relation to a fluorescence property. Products were fully identified by spectroscopic methods and were obtained in moderate to good yields as shown in Tables 1 and 2. New compounds were submitted to combustion analysis for complete characterization.
A series of qualitative assays aimed to evaluate the stability of the products and permanence of the fluorescent property followed: Compound 6 g did not fluoresce. Dilutions of the compounds (0.05 g) in common organic solvents (10 mL) such as ethyl alcohol, acetone, ethyl acetate, acetonitrile and dichloromethane showed fluorescence, this being considerably intense in ethyl acetate and dichloromethane. Solutions of 3-hydroxymethyl imidazo[1,2-a]pyridine 6 and pyrimidine 7 derivatives at various concentrations of aqueous HCl (0.1, 1 and 8 N) preserved the fluorescence, however, this was suppressed with 10 N HCl for the case of imidazo[1,2-a]pyrimidines 7 and deteriorated for the case of 6 at both λ 250 and 365 nm. Addition of weakly acidic materials also preserved fluorescence. Thus, heterogeneous 5% aqueous NH4Cl mixtures of 3-hydroxymethyl 6d showed a decreased fluorescence but 7d preserved it. A powdered mixture of silica gel (0.5 g), for column chromatography thoroughly mixed with the alcohol derivative 7d (10 mg) fluoresced only at the long wavelength λ 365 nm whereas 6d (10 mg) fluoresced at both wavelengths. These results indicate that the protonated species 8 holds the fluorescence activity to a certain extent. Fluorescence of both 6d and 7d in solutions 0.1, 1 and 10 N NaOH gradually diminished but prevailed at both wavelenghts. Biological materials such as egg yolk, pig blood, albumina and Giardia lamblia cultures were fluorescent upon addition of the imidazo[1,2-a]azines. The fluorescence of these products remained after several days.
The quantitative fluorescence analysis performed on one hundred-fold dilutions of the compounds showed that imidazo[1,2-a]pyridines absorbed and emitted energy at long wavelengths (Table 3) while imidazo[1,2-a]pyrimidines absorbed at lower wavelengths but emitted light at longer wavelengths (Table 4).
In the case of the imidazo[1,2-a]pyridines 6, all compounds fluoresced more intensely than the parent unsubstituted imidazo[1,2-a]pyridine 1, with the exception of the nitro substituted 6 g, which showed no fluorescence as was expected. It is also worth noting that the 2-(4-chlorophenyl) substituted imidazopyridine derivative 6b showed a rather low intensity.
As for the case of the imidazo[1,2-a]pyrimidine series, again the 2-(4’-chlorophenyl) substituted imidazopyrimidine 7b showed a very low intensity. Surprisingly, the electron donor substituted 7d and 7e and the nitro substituted derivative 7 g registered a similar intensity close to that given by the parent fluorophore 2.
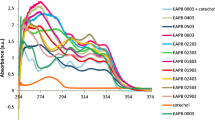
A comparative UV absorption graph for both series of compounds is shown in Figure 1.
Figure 2 shows a comparison of the emission intensities. From these, it is clearly appreciated that imidazo[1,2-a]pyrimidines emitted light at longer wavelengths than analogous imidazo[1,2-a]pyridines but the latter fluoresced more intensely, in contrast to previous analyses on imidazo[1,2-a[azines [12]. The effects of the phenyl substituents on the outcome of the emission intensities did not follow a consistent pattern, some interesting results are worth pointing out. The strong electron-donating methoxy group caused a marked increased intensity on the imidazopyridine (6e) but not on the imidazopyrimidine (7e). The fluorescence intensity of the imidazo[1,2-a]azines with the phenyl carrying the electron-withdrawing chlorine was low in 6b and dramatically decreased in 7b, whereas the intensity of the derivative with the phenyl bearing the fluorine substituent, 7c was enhanced to make it the most fluorescent of the imidazopyrimidine series. Interestingly in the case of the 4’-nitrophenyl substituted imidazo[1,2-a]pyridine, fluorescence was completely absent, while in the 4’-nitrophenyl substituted imidazo[1,2-a]pyrimidine fluorescence did not vanish.
According to the previous results, the role of the hydroxymethyl moiety was not quite clear, therefore we decided to revise the influence of the aryl group on the luminescent response of the 2-aryl imidazo[1,2-a]azines. Results for the 2-aryl imidazo[1,2-a]pyridines 3 (Table 5) showed that the phenyl, as well as the 4’-chloro, 4’-fluoro and 4’-methoxy substituted phenyl increased the fluorescence intensity as compared to 1 and interestingly the 3,4-dimethoxyphenyl caused a drop-off of the intensity. In most cases, the hydroxymethyl group increased fluorescence intensity even more than the aryl substituent, therefore it acted as enhancer. However, the intensity was decreased in the 4’-fluoro substituted phenyl and even more in the case of the 4’-chloro substituted phenyl.
As for the case of imdazopyrimidines (Table 6), substitution by phenyl did increase the intensity as compared to the parent fluorophore 2, the 4’-nitro substituted phenyl did not show fluorescence and it was interesting to observe that the 4’-methoxy phenyl (4f) showed a very low intensity. With this information, it was concluded that the hydroxymethyl moiety was a promoter of fluorescence in compound (4 g) and acted as an enhancer of the intensity in all other derivatives except in 2-(4’-chlorophenyl) imidazo[1,2-a]pyrimidine where a decrement was found. Figure 3 shows a comparative graph of the emission wavelengths and fluorescence obtained for compounds 3 and 4.
Thermal properties
The thermal stability of the most fluorescent compounds, 6a, 6e, 6f, 7a, 7c and 7f was estimated through a thermo gravimetric analysis (TGA) within an interval 0 – 600°C under an inert atmosphere. From the TG curves shown in Figure 4 it was determined that these compounds were thermally stable up to 280°C. The thermodynamic most stable was 7f, which completely decomposed above 600°C (47.75% mass loss at 600°C). The chemically most stable was 6f (55.25% weight loss at 600°C) and compound 7a showed a 66.47% weight loss at 600°C, the least stable was 6a (95.25% weight loss at 600°C).
Experimental
Melting points were measured on an Electrothermal melting point apparatus and are uncorrected. The FT-IR spectra were recorded on Perkins-Elmer 257 spectrometer using KBr discs. 1 H and 13C nmr spectral data were recorded with a Varian Mercury 300 MHz or a Varian 500 spectrometer. The chemical shifts (δ) are referenced to internal (CH3)4Si (δ 1 H = 0, δ 13C = 0). Fluorescence measurements were performed with a Shimadzu spectrofluorophotometer (RF-5000), equipped with a 150-W Xenon lamp, 12” color video display, 1 x 1 cm quartz cells. Both, operational performance and instrument sensitivity were revised by running a Raman spectrum of methanol. Cells were mounted in the holding device. The cell was filled out with a dilution 1:100 of the compounds in question for various lengths and then washed four times with methanol. Thermo gravimetric analysis was performed on a TGA 2950 Thermogravimetric Analyzer TA Instruments in a range 0-600°C, at a rate of 10°C/min on a platinum tray, under a nitrogen atmosphere and a flux rate 37.5 and 25.
General procedure for the synthesis of 3-hydroxymethyl imidazo[1,2-a]pyridines and pyrimidines
In a round bottom flask equipped with a magnetic stirrer, 1 g of the formyl derivative was suspended in ethanol (30 mL). The mixture was heated to 30°C and NaBH4 dissolved in 2 mL of 0.1 N NaOH added (2 molar equivalents for imidazo[1,2-a]pyridine and 1 molar equivalent for imidazo[1,2-a]pyrimidine). The reaction mixture was left stirring and reaction progress monitored by thin layer chromatography. After completion, ethanol was removed under vacuum. The solid formed was re-suspended in water and concentrated HCl added dropwise to neutral pH. The solid was collected by filtration, washed with water and dried off with hexane.
2-Phenyl-3-hydroxymethy imidazo[1,2-a]pyridine 6a
IR υ max cm-1: 3290 (OH); 2966.57, 2916.51, 2848.34 (C-H); 1734.11 (C=C).
1H NMR (300MHz, DMSO-d6) δ: 8.46 (1H, dd, J5,6=6.9, J5,7=1.2, H5); 7.84 (2H, d, J2’,3’=6.9, H2’,6’); 7.61 (1H, dd, J8,7=8.1, J8,6=1.2, H8); 7.48 (2H, td, J3’,2’=6.9, J3’,4’=7.2, J3’,5’=1.6 H3’,H5’); 7.38 (1H, td, J4’,3’=7.2, J4’,2’=1.6 H4’); 7.3 (1H, td, J7,8=8.1, J7,6=6.9, J7,5=1.2, H7); 6.98 (1H, td, J6,5=6.9, J6,7=6.9, J6,8=1.2, H6); 5.44 (1H, s, OH); 4.92 (2H, s, CH2).
13C NMR (75.5MHz, DMSO-d6) δ: 143.9 (C5), 142.8 (C8a); 134.3 (C1’), 128.4 (C2’); 128.1 (C3’); 128.2 (C4’); 124.8 (C7); 120.4 (C3); 116.6 (C8); 111.9 (C6); 52.8 (CH2).
2-(4’-Chlorophenyl)-3-hydroxymethyl imidazo[1,2-a]pyridine 6b
Isolated as a white solid in 61.3% yield, mp 229 - 230ºC (Lit 15, mp >240°C).
IR υ max cm-1: 3398 (OH); 2930 (C-H); 1635 (C=C).
1H NMR (300MHz, DMSO-d6) δ: 8.46 (1H, d, J5,6=6.9, H5); 7.87 (2H, d, J2’,3’=8.8, H2’); 7.6 (1H, d, J8,7=9.0, H8); 7.52 (2H, d, J3’,2’=8.8, H3’,5’); 7.31 (1H, td, J7,8=9.0, J7,6=6.8, J7,5=1.2, H7); 6.98 (1H, td, J6,5=6.9, J6,7=6.8, J6,8=1.2, H6); 5.47 (1H, t, J=4.8, OH); 4.92 (2H, d, J=4.8, CH2).
13C NMR (125MHz, DMSO-d6) δ: 143.9 (C5); 142.8 (C8a); 134.3 (C1’); 128.4 (C2’); 128.1 (C3’); 128.2 (C4’); 125.0 (C2); 124.8 (C7); 120.4 (C3); 116.6 (C8); 111.9 (C6); 52.8 (CH2).
2-(4’-Fluorophenyl)-3-hydroxymethyl imidazo[1,2-a]pyridine 6c
Isolated as a white solid in 70.3% yield, mp 154 - 155ºC (Lit 15, mp 153 - 155°C).
IR υ max cm-1: 3427 (OH); 3137 and 3048 (C-H); 1600 (C=C).
1H NMR (300MHz, DMSO-d6) δ: 8.52 (1H, d, J5,6=6.9, H5); 8.0 (2H, dd, J2’,3’=9.0, Jm=5.7, H2’ and H6’); 7.57 (1H, d, J8,7=9.3, H8); 7.37 – 7.29 (3H, m, H7,3’,5’); 6.87 (1H, d, J6,5=6.9, H6); 5.54 (1H, s, OH); 4.91 (2H, s, CH2).
13C NMR (125MHz, DMSO-d6) δ: 163.4 and 161.0 (C4); 143.91 (C8a); 130.9 and 130.8 (C2); 125.1 (C1); 120.4 (C3); 116.6 (C8); 115.6 and 115.3 (C3); 112.1 (C6); 52.0 (CH2).
2-(4’-Methylphenyl)-3-hydroxymethyl imidazo[1,2-a]pyridine 6d
Isolated as a white solid in 76.7% yield, mp 274 - 275ºC (Lit 15, mp >290°C).
IR υ max cm-1: 3279 (OH); 3040 y aprox 2950 (C-H); 1654 (C=C).
1H NMR (500MHz, DMSO-d6) δ: 8.45 (1H, d, J5,6=6.9, H5); 7.74 (2H, d, J2’,3’=9.0, H2’,6’); 7.61 (1H, d, J8,7=9.3, H8); 7.33 – 7.28 (3H, m, H7,3’.5’); 6.98 (1H, dd, J6,5=6.9, J6,7=6.9, H6); 5.45 (1H, t, J=5.1, OH); 4.91 (2H, d, J=5.1, CH2); 2.36 (3H, s, CH3).
13C NMR (125MHz, DMSO-d6) δ: 143.9 (C5); 142.9 (C8a); 136.9 (C1’); 131.6 (C4’); 129.1 (C3’); 128.1 (C2’); 125.1 (C2); 124.9 (C7); 120.2 (C3); 116.5 (C8); 112.0 (C6); 52.2 (CH2); 20.8 (CH3).
2-(4’-Methoxyphenyl)-3-hydroxymethyl imidazo[1,2-a]pyridine 6e
Isolated as a white solid in 66.7% yield, mp 166 - 167ºC (Lit 15, mp 165 - 167°C).
IR υ max cm-1: 3427 (OH); ca. 2950 (C-H); 1610 (C=C).
1H NMR (300MHz, DMSO-d6) δ: 8.43 (1H, dd, J5,6=6.9, J5,7=1.5 H5); 7.77 (2H, d, J2’,3’=9.0, H2’,6’); 7.57 (1H, d, J8,7=9.0, H8); 7.28 (1H, td, J7,8=9.0, J7,6=6.6, J7,5=1.5, H7); 7.04 (2H, d, J3’,2’=9.0, H3’,5’); 6.95 (1H, d J6,7=6.6, H6); 5.37 (1H, d, J=5.1, OH); 4.9 (2H, d, J=5.1, CH2); 3.82 (3H, s, OCH 3 ).
13C NMR (75.5MHz, DMSO-d6) δ: 157 (C4’); 141.9 (C5); 140.9 (C8a); 127.4 (C2’); 124.9 (C4); 123 (C1’); 122.7 (C2’); 117.8 (C3); 114.5 (C8); 112 (C3’); 109.8 (C6); 53.2 (CH2).
2-(3’,4’-Dimethoxyphenyl)-3-hydroxymethyl imidazo[1,2-a]pyridine 6f
Isolated as a brown solid in 71% yield, mp 171 - 172ºC (Lit 15, mp 170 - 172°C).
IR υ max cm-1: 3004.19 (OH); 2933.8 and 2834.93 (C-H); 1633.76 (C=C).
1H NMR (500MHz, DMSO-d6) δ: 8.46 (1H, d, J5,6=7.2 H5); 8.44 (1H, dd, J8,7=7.0, J8,6=1.6, H7); 7.61 (1H, d, J2’,6’=1.2, H2’); 7.56 (1H, dd, J6’,5’=6.4, J6’,2’=1.2 H6’); 7.50 (1H, dd, J7,8=7.0, J7,5=1.6 H7); 6.9 (1H, d, J5’,6’=8.0, H5’); 6.8(1H, td, J6,5=7.2, J6,7=7.2 J6,8=1.2 H6) ; 5.4 (1H, s, OH); 4.9 (2H, s, CH2); 3.83 (3H, s, OCH3), 3.81, (3H, s, OCH3).
13C NMR (125MHz, DMSO-d6) δ: 148.7 (C7); 148.5 (C4’); 143.7 (C3’); 142.9 (C8a); 127.03 (C2); 124.6 (C5); 120.4 (C1’); 119.8 (C6’); 116.4 (C3); 111.8 (C2’ and C5’); 111.7 (C6), 55.46 and 55.38 (OCH3); 52.1 (CH2).
2-(4’-Nitrophenyl)-3-hydroxymethyl imidazo[1,2-a]pyridine 6g
Isolated as a yellow solid in 70% yield, mp 225 - 226ºC (Lit 15, mp 224 - 226°C).
IR υ max cm-1: 3417 (OH); 3130, 2943 (C-H); 1600 (C = C).
1 H NMR (300 MHz, DMSO-d6) δ: 8.5 (1 H, d, J5,6 = 6.9); 8.49 (2 H, d, J2’,3’ = 8.9); 8.14 (2 H, d, J2’,3’ = 8.9); 7.62 (1 H, d, J7,8 = 9.0); 7.34 (1 H, ddd, J5,7 = 1.2, J6,7 = 6.9, J7,8 = 9.0); 7.0 (1 H, ddd, J6,8 = 1.2, J5,6 = 6.9, J6,7 = 6.9); 5.55 (1 H, t, J = 5.1, OH); 4.98 (2 H, d, J = 5.1, CH2).
13C NMR (125MHz, DMSO-d6) δ: 146.3 (C5); 144.2 (C8a); 140.8 (C1); 140.3 (C3); 128.6 (C3’); 125.4 (C4’); 125.0 (C2); 123.4 (C2’); 122.2 (C7); 116.8 (C8); 112.3 (C6); 52.0 (CH2).
2-Phenyl-3-hydroxymethyl imidazo[1,2-a]pyrimidine 7a
Isolated as a yellow solid in 60.6% yield, mp 241 - 242ºC.
IR υ max cm-1: 3216 (OH); 3082, 2948 y 2884 (C-H); 1614 (C=C).
1H NMR (300MHz, DMSO-d6) δ: 8.92 (1H, dd, J5,6=6.9, J5,7=1.2, H5); 7.84 (2H, d, J2’,3’=6.9, H2’,6’); 7.61 (1H, dd, J7,6=8.1, J7,5=1.2, H7); 7.53 (2H, dd, J3’,2’=6.9, J3’,4’=7.2, H3’,5’); 7.4 (1H, dd, J4’,3’=7.2, H4’); 7.1 (1H, td, J6,5=6.9, J6,7=6.9, J6,8=1.2, H6); 5.45 (1H, s, OH); 4.96 (2H, s, CH2).
13C NMR (75.5MHz, DMSO-d6) δ: 150.5 (C5), 147.2 (C8a); 143.6 (C1’), 133.8 (C2’); 128.6 (C3’); 128.3 (C4’); 128 (C7); 119.2 (C3); 108.6 (C8); 111.9 (C6); 51.8 (CH2).
Anal. calcd for C13H11N3O: C, 69.33 H, 4.88, N, 18.76; found: C, 69.41, H, 5.2, N, 18.46.
2-(4’-Chlorophenyl)-3-hydroxymethyl imidazo[1,2-a]pyrimidine 7b
Isolated as a yellow solid in 63.8% yield, mp 164 - 165ºC.
IR υ max cm-1: 3400 - 1900 (OH and C-H); 1662 and 1618 (C=C).
1H NMR (500MHz, DMSO-d6) δ: 8.97 (1H, d, J5,6=7.0, H5); 8.61 (1H, dd, J7,6=4.0, J7,5=2.0, H7); 7.91 (2H, d, J2’,3’=8.5, H2’,6’); 7.58 (2H, dd, J3’,2’=8.5, H3’,5’); 7.15 (1H, dd, J6,5=7.0, J6,7= 4.0, H6); 5.65 (1H, s, OH); 4.92 (2H, s, CH2).
13C- NMR (125MHz, DMSO-d6) δ: 150.6 (C7); 147.2 (C8a); 142.2 (C2); 133.6 (C5); 132.8 (C1’); 132.6 (C4’); 129.9 (C2’); 128.7 (C3’); 119.4 (C6); 108.7 (C3); 56.0 (CH2).
Anal. calcd for C13H10N3OCl: 60.11 H, 3.85, N, 16.19; found: C, 60.23, H, 4.01, N, 15.92.
2-(4’-Fluorophenyl)-3-hydroxymethyl imidazo[1,2-a]pyrimidine 7c
Isolated as a yellow solid in 30.3% yield, mp 232 - 233ºC.
IR υ max cm-1: 3300 (OH); 3076 (C-H); 1659 and 1615 (C=C).
1H NMR (500MHz, DMSO-d6) δ: 9.01 (1H, dd, J5,6=6.5, J5,7=2.0, H5); 8.59 (1H, dd, J7,6=4.0, J7,5=2.0, H7); 7.92 (2H, dd, J2’,3’=12.0, J2’F=5.0, H2’); 7.34 (2H, dd, J2’,3’=12.0, J3’F=9.0, H3’); 7.14 (1H, dd, J6,7=4.0, J6,5=6.5 H6); 5.72 (1H, s, OH); 4.9 (2H, s, CH2).
13C NMR (125MHz, DMSO-d6) δ: 163.4 and 161.0 (C4); 150.4 (C7); 147.0 (C8a); 142.4 (C2); 133.6 (C5); 130.2 (C2’); 130.1 (C1’); 119.2 (C6); 115.6 and 115.4 (C3’); 108.6 (C3); 51.5 (CH2).
Anal. calcd for C13H10N3OF: 64.19 H, 4.11, N, 17.28; found: C, 65.26, H, 4.23, N, 16.98.
2-(4’-Methylphenyl)-3-hydroxymethyl imidazo[1,2-a]pyrimidine 7d
Isolated as a yellow solid in 32% yield, mp 199 - 200ºC.
IR υ max cm-1: 3369 (OH); 2926 (C-H); 1617 (C=C).
1H NMR (500MHz, DMSO-d6) δ: 8.99 (1H, dd, J5,6=6.5, J5,7=1.5, H5); 8.59 (1H, dd, J7,6=7.0, J7,5=1.5, H7); 7.9 (2H, d, J2’,3’=8.0, H2’,6’) 7.57 (2H, d, J3’,2’=8.0, H3’,5’); 7.13 (1H, dd, J6,7=7.0, J6,5=6.5, H6); 5.7 (1H, s, OH); 4.91 (2H, s, CH2); 3.38 (3H, s, CH3).
13C NMR (125MHz, DMSO-d6) δ: 150.7 (C7); 147.1 (C8a); 142.2 (C2); 133.6 (C5); 132.7 (C1’); 132.7 (C4’); 129.8 (C3’); 128.6 (C2’); 119.6 (C6); 108.6 (C3); 55.9 (CH2); 51.5 (CH3).
Anal. calcd for C14H13N3O: 70.29 H, 5.39, N, 17.57; found: C, 70.13, H, 5.51, N, 17.38.
2-(4’-Methoxyphenyl)-3-hydroxymethyl imidazo[1,2-a]pyrimidine 7e
Isolated as a yellow solid in 86% yield, mp 187 - 188ºC.
IR υ max cm-1: 3409 (OH); aprox 2900 (C-H); 1617 (C=C).
1H NMR (500MHz, DMSO-d6) δ: 8.95 (1H, dd, J5,6=6.5, J5,7=2.0, H5); 8.56 (1H, dd, J7,6=4.0, J7,5=2.0, H7) 7.82 (2H, d, J2’,3’=8.5, H2’,6’); 7.12 (1H, dd, J6,5=6.5, J6,7=4.0, H6); 7.08 (2H, dd, J3’,2’=8.5, H3’,5’); 5.62 (1H, s, OH); 4.9 (2H, s, CH2); 3.83 (3H, s, OCH3).
13C NMR (125MHz, DMSO-d6) δ: 159.2 (C4’); 149.9 (C7); 147.1 (C8a); 143.6 (C2); 133.3 (C5); 129.5 (C2’); 118.4 (C6); 114.1 (C3’); 108.3 (C3); 55.2 (CH2) 51.7 (OCH 3 ).
Anal. calcd for C14H13N3O2: 65.88 H, 5.09, N, 16.47; found: C, 65.66, H, 4.80, N, 16.29.
2-(3’,4’-Dimethoxyphenyl)-3-hydroxymethyl imidazo[1,2-a]pyrimidine 7f
Isolated as a brown solid in 30.4% yield, mp 200 - 201ºC.
IR υ max cm-1: 3397 (OH); 3083, 2994 and 2937 (C-H); 1615 (C=C).
1H NMR (500MHz, DMSO-d6) δ: 8.97 (1H, d, J5,6=4.0, H5); 8.56 (1H, d, J7,6=2.0, H7); 7.49 (1H, d, J2’,6’=2.0, H2’); 7.42 (1H, d, J6’,5’=8.0, H6’); 7.09 (2H, m, H5’,6); 5.46 (1H, s, OH); 4.93 (2H, s, CH2); 3.8 (6H, s, OCH3).
13C NMR (125MHz, DMSO-d6) δ: 149.9 (C7); 148.8 (C4’); 148.7 (C3’); 147.0 (C8a); 143.7 (C2); 133.3 (C5); 126.4 (C1’); 120.7 (C6’); 118.6 (C3); 111.8 (C2’ and C5’); 108.6 (C6), 55.5 and 55.4 (OCH3); 51.7 (CH2).
Anal. calcd for C15H15N3O3: 63.15 H, 5.26, N, 14.73; found: C, 63.01, H, 5.17, N, 14.44.
2-(4’-Nitrophenyl)-3-hydroxymethyl imidazo[1,2-a]pyrimidine 7g
Isolated as a yellow solid in 72% yield, mp 213 - 214ºC.
IR υ max cm-1: 3376 (OH); 2933 (C-H); 1675 (C=C).
1H NMR (300MHz, DMSO-d6) δ: 8.64 (1H, t, J5,6=6.9, J5,7=1.2, H5); 8.34 (2H, dd, J2’,3’=8.7, H3’); 8.15 (2H, d, J2’,3’=8.7, H2’); 7.1 (1H, dd, J6,8=1.2, J7,8=9.0, H8); 6.15 (1H, dd, J5,7=1.2, J6,7=6.6, J7,8=9.0, H7); 5.59 (1H, d, J6,8=1.2, H8); 4.9 (1H, t, J5,7=1.2, J6,7=6.6, J7,8=9.0, H7); 3.32 (1H, t, J5,6=6.9, J6,7=6.6, J6,8=1.2, H6); 2.9 (1H, d, J=5.1, OH); 2.4 (2H, d, J=5.1, CH2).
13C NMR (125MHz, DMSO-d6) δ: 152.7 (C5); 148.6 (C8a); 148.06 (C1); 142.3 (C3); 141.6 (C3’); 135.2 (C4’); 125.0 (C2); 130.3 (C2’); 125.2 (C7); 122.6 (C8); 110.3 (C6); 52.88 (CH2).
Anal. calcd for C13H10N4O3: 57.77 H, 3.70, N, 20.74; found: C, 57.56, H, 3.85, N, 20.62.
Conclusion
2-Aryl-3-hydroxymethyl imidazo[1,2-a]pyridines and 2-aryl-3-hydroxymethyl imidazo[1,2-a]pyrimidines emitted light at long wavelengths more intensely than the respective non-substituted imidazo[1,2-a]pyridine and pyrimidine fluorophores. The hydroxymethyl group was an enhancer of the fluorescence intensity of the 2-aryl imidazo[1,2-a]azines, although in some cases it caused a decrement of the luminescent activity. These findings indicate that an interplay of the electronic character of the phenyl substituents with the hydroxymethyl moiety is most probably operating. The thermogravimetric analysis on selected most fluorescent imidazo[1,2-a]azines, indicated that they are thermally stable up to 280°C. Other studies aimed to decide if the herein investigated compounds are suitable as effective fluorescent dyes in biological applications are currently underway.
References
Dusza HP, Albright JD: U.S. 5, 037, 980(Cl. 544–281, CO 7D 487/ 04), 06 Aug. 1991 Appl. 182, 650, 18 Avr. 1988. Chem Abstr. 1991, 115: 256202q-
Okabe T, Bhooshan B, Novinson T, Hillyard IW, Garner GE, Robins RK: Dialkyl bicyclic heterocycles with a bridgehead nitrogen as purine analogs possessing significant cardiac inotropic activity. J Heterocyclic Chem. 1983, 20: 735-751. 10.1002/jhet.5570200345.
Stachle H, Kummer W, Koppe H: Ger. Offen. 1974, 2, 234, 622 (Cl. CO 7D), 31 Jan. Appl. 22, 34622. 1, 14 Jun. 1972. Chem Abstr. 1974, 80: 120993z-
Stachle H, Kummer W, Koppe H: Ger. Offen. 1983, DE 3, 124, 718(Cl. CO 7D 487/04), 13 Jan. Appl P 24 Jun. 1981. Chem. Abstr. 1983, 114: 126153u-
Stachle H, Kummer W, Koppe H: Ger. Offen. 1972, 2, 109, 524(Cl. CO7D), 07 Sep. Appl. 21 09524 9, 01 Mar.1971. Chem Abstr. 1972, 77: 164750 k-
Yashiano T, Tadashi M: Eur. Pat. 1985, Appl. EP 163, 240 (Cl. CO 7D 487/ 04), 04 Dec, JP. Appl. 84/ 104, 257, 22 Mai.1984. Chem Abstr. 1986, 104: 186438 t-
El Kazzouli S, Griffon Du Bellay A, Berteina-Raboin S, Delagrange P, Caignard D-H, Guillaumet G: Design and synthesis of 2-phenylimidazo[1,2-a]pyridines as a novel class of melatonin receptor ligands. Eur J Med Chem. 2011, 46: 4252-4257. 10.1016/j.ejmech.2011.06.030.
Gong Y-D, Cheon HG, Lee T, Kang NS: A novel 3-(8-chloro-6-(trifluoromethyl)imidazo[1,2-a]pyridine-2-yl)phenyl acetate skeleton and pharmacophore model as glucagon-like peptide 1 receptor agonists. Bull Korean Chem Soc. 2010, 31: 3760-3764. 10.5012/bkcs.2010.31.12.3760.
Koubachi J, El Kazzouli S, Berteina-Raboin S, Mouaddib A, Guillaumet G: Synthesis of polysubstituted imidazo[1,2-a]pyridines via microwave-assisted one-pot cyclization/Suzuki coupling/palladium-catalyzed heteroarylation. J Org Chem. 2007, 72: 7650-7655. 10.1021/jo0712603.
Tomoda H, Hirano T, Saito S, Mutai T, Araki K: Substituent Effects on Fluorescent Properties of Imidazo[1,2-a]pyridine-Based Compounds. Bull Chem Soc Jpn. 1999, 72: 1327-1334. 10.1246/bcsj.72.1327.
Rackham DM: Spectroscopic Studies of Some Imidazo[1,2-a]pyridine and Imidazo[1,2-a]pyrimidine Derivatives. Appl Spectroscopy. 1979, 33: 561-563. 10.1366/0003702794925129.
Hodgkiss RJ, Middleton RW, Parrick J, Rami HK, Wardman P, Wilson GD: Bioreductive fluorescent markers for hypoxic cells: A study of 2-nitroimidazoles with 1-substituents containing fluorescent, bridgehead-nitrogen, bicyclic systems. J Med Chem. 1920, 1992: 35-
Kurushima T, Iwata S, Tanaka K: Application of (4-piperidinylfluorophenyl)imidazopyridine to a multiple fluorescence chemosensor. Nippon Kagakkai Koen Yokoshu. 2006, 86: 1473-
Hee-Yeon K, Seung-Gak Y, Jung-Han S, Chang-Ho L, Hee-Joo K: Imidazopyrimidine-based compound and organic light-emitting device employing organic layer including the same. 2008, US20080226945A1, 18 Sep
Xuhong Q, Fengyu L: Promoting effects of the hydroxymethyl group on the fluorescent signaling recognition of anions by thioureas. Tetrahedron Lett. 2003, 44: 795-799. 10.1016/S0040-4039(02)02671-0.
Kiyoshi T, Tohru K, Satoru I, Syunsuke S: Fluorescent behavior of 2-(3,4,5,6-tetrafluoro-2-hydroxyphenyl)imidazo-[1,2-a]pyridine in the presence of metal perchlorate. J Heterocycl Chem. 2007, 44: 303-307. 10.1002/jhet.5570440204.
Nilsson M, Haraldsson M, Henriksson S, Emond R, Savory E, Simpson I: Imidazopyridine Compounds. 2010, WO2010064020A1, 10 Jun
Anaflous A, Benchat N, Mimouni M, Abouricha S, Ben-Hadda T, El-Bali B, Hakkou A, Hacht B: Armed imidazo[1,2-a]pyrimidines (pyridines): Evaluation of antibacterial activity. Lett Drug Des Discov. 2004, 1: 224-229. 10.2174/1570180043398885.
Acknowledgements
We thank Consejo Nacional de Ciencia y Tecnología Mexico for financial support through project 49937. We are grateful to Dr. Armando Gómez Poyou, Instituto de Fisiología Celular, Universidad Nacional Autónoma de México for the fluorometric measurements. Special thanks to Miguel Angel Canseco Martínez, Investigación en Materiales, Universidad Nacional Autónoma de México for the thermogravimetric study. SVO thanks Consejo Nacional de Ciencia y Tecnología for graduate scholarship No. 257526.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they do not have competing interests.
Authors’ contributions
MVP observed high fluorescence activity when preparing 3-hydroxymethyl derivatives by reaction of the imidazo[1,2-a]pyridine with formaldehyde in an acidic media. HSZ proposed and designed the project. SVO confirmed the observation made by MVP, carried out the synthesis and characterization of all new compounds, and participated in both the qualitative and quantitative photophysical analysis. CPG, ARA and ECA contributed in the analysis of all spectral data and discussed with HSZ the course of the investigation. All authors read, made comments and approve the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Velázquez-Olvera, S., Salgado-Zamora, H., Velázquez-Ponce, M. et al. Fluorescent property of 3-hydroxymethyl imidazo[1,2-a]pyridine and pyrimidine derivatives. Chemistry Central Journal 6, 83 (2012). https://doi.org/10.1186/1752-153X-6-83
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1752-153X-6-83