Abstract
Background
Cathepsin X is a cysteine protease involved in mechanisms of malignant progression. It is secreted from tumour cells as a proenzyme and may serve to predict the disease status and risk of death for cancer patients. In a previous, pilot, study on 77 colorectal patients we demonstrated the correlation of higher serum levels with shorter overall survival.
Methods
264 patients with colorectal cancer were included in a prospectively accrued multi-centre observational cohort study with the aim of testing novel biomarkers. Blood samples were collected before preoperative large bowel endoscopy and total cathepsin X was measured in sera by ELISA. As a control group we selected at random 77 subjects who had no findings at endoscopy and reported no co-morbidity.
Results
The mean level of cathepsin X in cancer patients did not differ from the control levels (23.4 ng/ml ± 6.4 SD vs. 18.8 ng/ml ± 11.4 SD, p > 0.05) and there was no association with age, gender, disease stage, tumour location or CEA. In univariate analysis no association between cathepsin X levels and overall survival was demonstrated for the entire set of patients, however, cathepsin X was associated with survival in a group of patients with local resectable disease (stages I-III) (HR = 1.69, 95% CI: 1.03-2.75, p = 0.03). For this group, multivariate Cox regression analysis showed an association (HR = 3.13, 95% CI: 1.37-7.18, p = 0.003) between high cathepsin X levels and shorter overall survival for patients who did not receive chemotherapy, whereas, for patients who received chemotherapy, there was no association between cathepsin X and survival (HR = 0.51, 95% CI: 0.20-1.33, p = 0.88).
Conclusions
Association of cathepsin X levels with overall survival was not confirmed for an entire set of 264 colorectal patients, but for patients in stages I-III with local resectable disease. The significant association of cathepsin X with survival in a group of patients who received no chemotherapy and the absence of this association in the group who received chemotherapy, suggest the possible predictive value for response to chemotherapy. The results have to be confirmed in a further prospective study.
Similar content being viewed by others
Background
Discovery of molecules associated with colorectal cancer (CRC) whose levels may provide predictive and prognostic information, is important in order to improve treatment, optimize the quality of life and prolong survival of the patients. Several serum tumour markers are currently under evaluation, however, carcinoembryonic antigen (CEA) is the only one in regular clinical use for determining prognosis, for surveillance following curative resection and for monitoring therapy in advanced disease [1–4].
Cysteine cathepsins, a group of lysosomal cysteine proteases, have been reported as being involved in the development and progression of cancer. They are used as targets for developing new antitumour therapies. Their expression, protein and activity levels [5–7] have also been associated with prediction of prognosis in various cancer types, including CRC [8, 9]. Cathepsins B and L, in particular, are the most extensively studied cysteine cathepsins in colorectal diseases. In colorectal carcinoma they are generally overexpressed and their ability to degrade the components of extracellular matrix has been related to tumour invasion, migration and metastasis [8, 10]. Endogenous inhibitors of cysteine proteases, the cystatins, insufficiently impair the tumour associated activity of cathepsins; their lower levels in tumours, higher enzyme:inhibitor ratio and lower stability of the complex have been associated with cancer aggressiveness and patient survival [8, 11].
In recent studies the potential of other cathepsins, exhibiting more specific functions and localisation than cathepsins B and L, have been evaluated. Cathepsin X (Cat X), for example, is expressed specifically in various cells of the immune system [12] and regulates important processes such as proliferation, maturation, migration and adhesion of immune cells, phagocytosis and signal transduction [13–15]. Elevated expression of Cat X has also been associated with the inflammatory processes in inflammatory related neurodegenerative disorders [16], Helicobacter pylori infection [17], multiple trauma [18] and tuberculosis [19]. However, there is increasing evidence that Cat X is also involved in malignant processes. The gene encoding Cat X is localized in chromosomal region 20q13, which is frequently amplified in several cancer types [20, 21]. In lung tumours, immunohistochemical analysis revealed strong staining of Cat X in infiltrated immune cells, but very weak staining in tumour cells [12]. On the other hand, increased expression of Cat X was found in both tumour and immune cells of prostate [22] and gastric cancer [17] and in the most aggressive phenotypes of malignant melanoma [23]. Further, breast cancer transgenic mice with Cat X deficiency had a prolonged tumour-free period for breast cancer, unlike wild type mice [24]. Moreover, Cat X deficiency leads to accelerated cell senescence, which is a powerful tumour suppressing mechanism [25]. Cat X may also promote tumour processes by mechanisms typical of immune cells, such as binding and activation of integrin receptors [15] and modulation of CXCL-12 chemokine [26] and gamma enolase [16].
Reports evaluating the clinical relevance of Cat X are less numerous. Wang et al. demonstrated that increased Cat X mRNA levels in hepatocellular carcinomas correlate with advanced tumour stage [27]. In a pilot study our group showed significantly lower levels of active Cat X in sera of patients with inflammatory breast cancer than with non-inflammatory breast cancer [28]. However, no significant correlation with any clinico-pathological parameter could be demonstrated in epithelial ovarian cancer cyst fluid [29]. On the other hand, higher levels of Cat X (both pro-form and active mature form) in sera from 77 patients with CRC correlated significantly to shorter overall survival [30].
In the present study, the aim was to confirm the results of the pilot study with an extended cohort of CRC patients and, further, to evaluate the use of serum levels of total Cat X for selecting patients who could benefit from chemotherapy.
Methods
Patients
The study was approved by the Regional Ethical Committee of Copenhagen and The Danish Data Protection Agency. 264 subjects diagnosed with primary rectal or colon cancer and 77 controls were included in a multi-centre observational cohort study conducted at six Danish hospitals from October 2003 to December 2005. All gave informed consent to being included in the study. Cancer diagnoses were verified by histology of resected large bowel specimens. Patients who did not have the tumour removed for various reasons were classified according to the available clinical information and, as a result, exact tumour staging was not possible in 17 cases. As a control group, we selected subjects at random who had symptoms which could be due to CRC but exhibited no findings at endoscopy and reported no co-morbidity (healthy persons). Disease stage was based on the TNM-stage (International Union Against Cancer (UICC)). 26 patients with Stages I-III received adjuvant therapy whereas 33 patients with stage IV received palliative chemotherapy. It should be emphasized that patients were not randomized to adjuvant therapy and palliative chemotherapy but selected as part of the clinical decisions. Patient characteristics and applied first line therapies are given in Table 1.
Sampling
Blood samples were collected from healthy persons and from CRC patients just before preoperative large bowel endoscopy according to a validated standard operating procedure. Blood was collected at moderate tourniquet pressure, in 10 ml serum tubes (Vacutainer® Becton-Dickinson, Mountain View, CA, USA), and spun for 10 minutes at 3000 g and 4°C within 1 hour of collection. Samples were immediately stored at −80°C.
Cat X and CEA analysis
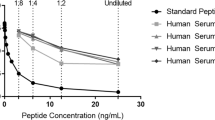
Human total Cat X was analysed by ELISA as described [12, 30]. Goat polyclonal antibody (R&D SYSTEMS®, Minneapolis, USA) and horseradish peroxidase-conjugated 3B10 mouse monoclonal antibody, both recognizing pro- and mature Cat X, were used. Pro-Cat X, used as a standard, was prepared and characterized as described [31]. Serum samples were diluted in a 1:2 ratio with 2% BSA in 10 mM phosphate buffer (pH 7.2), 150 mM NaCl and 0.05% Tween® 20 (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). The range of the calibration curve for Cat X extended from 1.0 to 32.5 ng/ml (see Additional file 1: Figure S1). To determine the linearity of the ELISA, serum samples were serially diluted in 2% BSA in 10 mM phosphate buffer to levels encompassing the range of the assay, and their linearity was evaluated by comparing the measured values with the calibration values. Dilutions of 4 serum samples showed a linear dose response parallel to the calibration curve in a dilution range 1:2 to 1:16 (see Additional file 2: Figure S2). To evaluate recovery rate, recombinant pro-Cat X at different concentrations was added to serum samples with known total Cat X concentrations. Recovery levels were determined by comparing the expected and observed concentrations. Recovery levels ranged from 87.0 to 100.6%. Mean recovery was 92.1%. The plot of expected vs. observed concentrations for Cat X ELISA is shown in (see Additional file 3: Figure S3). The intra-assay coefficient of variance (CV), determined by measuring 20 control replicates, was 6.5%. Inter-assay precision was derived by evaluating one high and one low control in duplicates in seven separate assays and was 7.9%. Detection limit of the assay, defined as the concentration corresponding to the mean absorbance of 10 replicates of zero standard plus 3 standard deviations (SDs) was determined to be 0.75 ng/ml.
CEA was determined using the Abbott ARCHITECT® i2000 automated immunoassay system utilising an on-market two-step dual monoclonal immunoassay (ARCHITECT® CEA assay). The determination was run at the Abbott Centre of Excellence research laboratory in Munich, Germany [32]. The assay precision is stated to be less than 8%, the mean recovery 99.9% and the detection limit better than 0.5 ng/ml (ARCHITECT® CEA assay datasheet).
All biomarker determinations were performed blinded to the technicians and to the study endpoint.
Statistical analysis
Rank sum tests were used for test of location and Spearman’s rank correlation was used as a measure for association. Univariate and multivariate Cox regression models were used to assess the association of total Cat X and other clinicopathologic parameters with overall survival. Cat X was scored by its actual value on the log scale (base 2), which means that the hazard ratios are for a two fold difference in the marker level. The Cox model included an interaction term in order to test the hypothesis that the association of Cat X was not independent of chemotherapy received by the patient. Additionally, as in the pilot study, the median was used as a cut-off value in univariate analysis. CEA was dichotomized at 5 ng/ml. Model assessment of the proportionality assumption and linearity was done using Schoenfeld and martingale residuals as well as 10-fold internal cross validation [33]. The results of the model assessment confirmed the estimates. Graphical representations of survival probabilities were presented by Kaplan-Meier curves grouping continuous variables by their tertiles. P-values less than 5% were considered to be statistically significant. Statistical analysis was carried out using SAS (v9.2, SAS Institute, Cary, N.C., USA) and R (R Core Team (2013); R: A language and environment for statistical computing. R statistical Computing. Vienna. Austria. URL. http://www.R-project.org/).
The results of this project are reported in accordance with the REMARK guidelines [34].
Results
Levels of total Cat X and CEA and association with baseline variables
Total Cat X and CEA concentrations were measured in sera from 264 patients with CRC. Results and descriptive statistics are shown in Table 2. The mean level of total Cat X in 77 control samples was 18.8 ng/ml ± 11.4 SD. There were no significant differences in Cat X levels between patients and controls or between disease stages, cancer localisation and gender.
Also, Spearman’s rank correlation between total Cat X concentrations, age and CEA showed no correlation (r = 0.08, p = 0.22; r = 0.02, p = 0.73). Median CEA was 3.7 ng/ml (range: 0.5-5046 ng/ml).
Analysis of survival
In the univariate and multivariate Cox regression model, total Cat X was scored as a continuous variable on the log scale using base 2, which means that hazard ratios (HR) are for two fold differences. The median time from inclusion to end of study was 4.8 years (3.9-6.0).
Univariate analysis of Cat X, scored by its log transformed value in the entire set, showed no significant differences in overall survival (HR = 1.26; 95% CI: 0.82-1.92; p = 0.29), although high total Cat X levels were associated with poor survival. As the pilot study demonstrated a significant association between Cat X and overall survival, we additionally analysed the current study using the median as a threshold, as in the pilot study. Again, no significant difference was demonstrated (HR = 1.33; 95% CI: 0.95-1.86, p = 0.10). However, when patients in stages I-III were analysed in this way, the difference was significant (HR 1.69; 95% CI: 1.03-2.75; p = 0.03). Pre-specified multivariate analysis of time to death from all causes for patients in stages I-III, including the covariates gender, age (per 10 years), stage, tumour localisation, adjuvant chemotherapy, levels of CEA and total Cat X and interactions terms between chemotherapy and the biomarkers identified a subgroup of patients with a significant association with the outcome of the disease, as shown in Table 3.
The estimated HR in multivariate analysis for total Cat X was 3.13 (95% CI: 1.37-7.18) for patients who did not receive chemotherapy and 0.51 (95% CI: 0.20-1.33) for those who received chemotherapy (interaction term p = 0.016). The latter means that patients who did not receive chemotherapy and had high total Cat X serum levels, had a significantly shorter survival (p = 0.003) comparable to that of the whole group of patients who had not undergone chemotherapy. A weaker, but still significant, association with survival within the same group of patients was also observed for CEA (p = 0.026). However, interaction between Cat X and CEA for this group of patients not receiving chemotherapy could not be demonstrated (p = 0.73), suggesting that the two markers are additive (i.e. not synergistic). The generalized concordance index was 0.75 (standard error (se) = 0.04), thus demonstrating reasonable discrimination in this model.
Within the group of patients who received therapy there was no significant difference in overall survival between patients with low and high total Cat X. These results suggest the potential of total Cat X to predict the response to chemotherapy. Kaplan-Meier curves provide a similar result for patients with stages I-III, who did not receive chemotherapy (Figure 1A). Patients with total Cat X levels in the third tertile exhibited a significantly shorter overall survival than those from the first and second tertiles (p = 0.01). When Kaplan-Meier estimates of survival are stratified to stage III, again patients who did not receive chemotherapy with Cat X in the third tertile showed significantly shorter overall survival than those with low Cat X, whereas stage III patients who received chemotherapy exhibited no significant difference with regard to Cat X levels (Figure 1B). Inclusion of specific first line chemotherapies in multivariate analyses did not demonstrate significant interactions between specific therapy and Cat X (data not shown). Therapies involving 5-Fluorouracil (5-FU), Oxaliplatin and Capecitabine were tested in stages I-III and stage IV. However this study is not designed or powered to address this question.
Overall survival curves according to the total Cat X values in serum from patients with CRC. The number of patients at risk at 0, 24 and 48 months is shown below the axis for each stratum and the number of deaths (events) is shown to the left. The difference between the strata was tested using the log rank test. (A) Kaplan-Meier estimates of survival for patients with stages I-III who did not receive chemotherapy. Cat X has been grouped using tertiles (denoted 0, 1, 2) (B) Kaplan-Meier estimates of survival for patients with stage III who, 0: did not receive chemotherapy and had low Cat X, 1: did not receive chemotherapy and had high Cat X, 2: received chemotherapy and had low Cat X, 3: received chemotherapy and had high Cat X.
No significant effect of Cat X on survival could be demonstrated for stage IV, nor for univariate (HR = 1.02; 95% CI: 0.54-1.93; p = 0.95) or multivariate (HR = 0.68; 95% CI: 0.34-1.39; p = 0.29) analysis. Including patients not staged, a similar result was found (data not shown). 33 (48%) patients with stage IV received palliative chemotherapy but no significant interaction between total Cat X and chemotherapy could be demonstrated (p = 0.21).
Discussion
Colorectal cancer is the second leading cause of cancer-related death in Europe and the US. About half of the patients develop recurrent disease within 3 to 5 years of resection of the primary tumour, and they are candidates for systemic chemotherapy. The availability of validated biological markers for selection of chemotherapy and prediction of treatment efficacy, together with monitoring, may increase patient survival. In this study we identified serum Cat X as a potential tumour marker in CRC, based on the demonstrated correlation of its high levels with shorter patient overall survival and the association with the adjuvant chemotherapy.
In our previous (pilot) study on 77 CRC patients [30] we found that levels of total Cat X in sera from patients with CRC were not significantly higher than those in groups of healthy persons, patients with adenomas or patients with non-neoplastic findings, all matched with CRC patients for age and gender. The mean level of total Cat X, determined in sera from the 264 CRC patients included in this confirmation study, also does not differ statistically from that in healthy persons (mean 23.4 ng/mL ± 6.4 SD vs. 18.8 ng/ml ± 11.4 SD, p > 0.05). As in the pilot study, there is no significant association with patient age or gender, tumour stage or location and CEA. In a univariate Cox regression model, the levels of total Cat X evaluated in this study did not confirm the significant relation to overall survival found in the pilot study [30], although the higher levels were associated with poor survival. When, as in the pilot study, the median was used as a cut off value, a similar result was obtained for the entire set of patients whereas, for patients with local resectable disease (stages I-III), the relation to overall survival was significant. In contrast, for patients with metastatic disease (stage IV), there was no relation between Cat X and overall survival. In multivariate analysis, including patients with stages I-III, a strong association (HR = 3.13) between high total Cat X levels and shorter overall survival was observed for the group of patients who did not receive adjuvant chemotherapy whereas, within the group of patients who received chemotherapy, there was no association between total Cat X and survival (HR = 0.51). Patient age, stage and CEA also contributed to the prognosis in multivariate analysis, whereas gender and location were not significant. The significant interaction between Cat X and adjuvant chemotherapy suggests its possible predictive value, since the data suggest that patients with high levels of Cat X benefit from the therapy.
Several mechanisms have been suggested that could link higher activity and/or concentration of Cat X with the progression of malignant disease. Cat X can be present in sera from healthy persons due to constitutive secretion by leukocytes during normal physiological immune cell activation and turnover [18]. Increased secretion of pro-Cat X could reflect the overexpression of Cat X in tumour and tumour associated immune cells, as observed in several cancer types. Secreted pro-Cat X most probably remains in the extracellular space, however, it can be activated by membrane bound cathepsin L [35] or surface heparan sulphate proteoglycans [13]. The activation by cathepsin L can also take place in endosomal/lysosomal vesicles during their translocation toward the plasma membrane [15, 35]. Pro-Cat X can modulate cell adhesion and motility through interaction of its pro-region with heparan sulphate proteoglycans [13] and integrin receptors [36–38], while active Cat X can regulate cell functions through cleavage of C-terminal amino acids of chemokine CXCL-12 [26], beta-2 chain of integrin receptors [36], gamma-enolase (neuronal specific enolase - NSE) [16] and profilin [39, 40]. Therefore, both forms could promote tumour progression processes, resulting in enhanced adhesion and migration of tumour cells, induced epithelial-mesenchymal transition [27] and decreased cell senescence [25]. Results obtained on transgenic animals further confirm the active role of Cat X in tumour progression. It was suggested that Cat X, not being dependent on the degradation of extracellular matrix, a typical event for cathepsin B, may compensate the malignant effects of cathepsin B [24, 41] by changing the migration mode of tumour cells. Only mice with excluded expression of both cathepsin B and Cat X exhibited significantly lower tumour growth and metastasis formation. The relation between Cat X levels and response to therapy has not been reported, however, anticancer drugs may interfere with Cat X targets and processes, also regulating its expression and secretion.
From previous studies it is not clear whether Cat X is involved in early or late stages of cancer. The results by Sevenich et al. [24], using a PymT-induced metastatic breast cancer mouse model, showed that Cat X was related to the initial stages of the malignant process and less with tumour progression and metastasis. On the other hand, Hidaka et al. showed that Cat X could be involved in the later stages of cancer, since amplification of the region encoding for Cat X correlated with the metastatic potential and tumour progression in CRC [20]. A similar possibility was explored by Wang et al.[27], who showed that increased Cat X expression strongly correlated with advanced clinical stage, and induced metastatic potential and shorter overall survival of patients with hepatocellular carcinoma. Moreover, it was recently demonstrated that, in adenocarcinoma of the pancreatic duct, S100P-binding protein inversely regulates Cat X expression – lower levels of S100P-binding protein increased Cat X, cell adhesion and metastatic potential of pancreatic cancer cells [42]. Our findings are in accord with those of a recent study on lung cancer patients by Zhang et al., which also shows that higher Cat X serum levels are associated with shorter patient survival. However, lung cancer patients had significantly higher Cat X levels than healthy controls, which was not the case in our study [43]. Our combined results support the studies revealing the association of Cat X expression with the progression of CRC, since they provide the correlation of high levels of Cat X with shorter overall survival of CRC patients.
Conclusions
Our study confirms the previous results relating higher Cat X levels to progression of cancer and shorter overall survival of CRC patients. Although total Cat X serum levels did not differ between the entire group of patients and controls, a significant difference was found in the group of patients who did not receive adjuvant chemotherapy: patients with high total Cat X serum levels had significantly shorter overall survival than those with low levels. For patients who received adjuvant chemotherapy no difference in overall survival was observed between those groups with low and high total Cat X. Thus, total Cat X could be useful as a predictive, blood-based tumour marker that may allow selection of patients who could benefit from adjuvant chemotherapy. However, the results must be confirmed in a prospective clinical study.
Abbreviations
- Cat X:
-
Cathepsin X
- CEA:
-
Carcinoembryonic antigen
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- CV:
-
Coefficient of variance
- CXCL-12:
-
C-X-C motif chemokine 12
- 5-FU:
-
5-Fluorouracil
- HR:
-
Hazard ratio
- PymT:
-
Polyomavirus middle T oncogene
- SD:
-
Standard deviation.
References
Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, Babaian R, Bast Jr. RC, Dowell B, Esteva FJ, Haglund C, Harbeck N, Hayes DF, Holten-Andersen M, Klee GG, Lamerz R, Looijenga LH, Molina R, Nielsen HJ, Rittenhouse H, Semjonow A, Shih Ie M, Sibley P, Soletormos G, Stephan C, Sokoll L, Hoffman BR, Diamandis EP: National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008, 54: e11-e79. 10.1373/clinchem.2008.105601.
Booth RA: Minimally invasive biomarkers for detection and staging of colorectal cancer. Cancer Lett. 2007, 249: 87-96. 10.1016/j.canlet.2006.12.021.
Nielsen HJ, Brunner N, Frederiksen C, Lomholt AF, King D, Jorgensen LN, Olsen J, Rahr HB, Thygesen K, Hoyer U, Laurberg S, Christensen IJ, Danish-Australian Endoscopy Study Group On Colorectal Cancer D, Danish Colorectal Cancer Cooperative G: Plasma tissue inhibitor of metalloproteinases-1 (TIMP-1): a novel biological marker in the detection of primary colorectal cancer. Protocol outlines of the Danish-Australian endoscopy study group on colorectal cancer detection. Scand J Gastroenterol. 2008, 43: 242-248. 10.1080/00365520701523439.
Nielsen HJ, Christensen IJ, Brunner N: A novel prognostic index in colorectal cancer defined by serum carcinoembryonic antigen and plasma tissue inhibitor of metalloproteinases-1. Scand J Gastroenterol. 2010, 45: 200-207. 10.3109/00365520903429406.
Turk V, Kos J, Turk B: Cysteine cathepsins (proteases)–on the main stage of cancer?. Cancer Cell. 2004, 5: 409-410. 10.1016/S1535-6108(04)00117-5.
Strojan P: Cysteine cathepsins and stefins in head and neck cancer: an update of clinical studies. Radiol Oncol. 2008, 42: 69-81.
Ardebili SY, Zajc I, Gole B, Campos B, Herold-Mende C, Drmota S, Lah TT: CD133/prominin1 is prognostic for GBM patient’s survival, but inversely correlated with cysteine cathepsins’ expression in glioblastoma derived spheroids. Radiol Oncol. 2011, 45: 102-115.
Kos J, Lah TT: Cysteine proteinases and their endogenous inhibitors: target proteins for prognosis, diagnosis and therapy in cancer (review). Oncology Rep. 1998, 5: 1349-1361.
Troy AM, Sheahan K, Mulcahy HE, Duffy MJ, Hyland JM, O’Donoghue DP: Expression of Cathepsin B and L antigen and activity is associated with early colorectal cancer progression. Eur J Cancer. 2004, 40: 1610-1616. 10.1016/j.ejca.2004.03.011.
Kuester D, Lippert H, Roessner A, Krueger S: The cathepsin family and their role in colorectal cancer. Pathol Res Pract. 2008, 204: 491-500. 10.1016/j.prp.2008.04.010.
Zore I, Krasovec M, Cimerman N, Kuhelj R, Werle B, Nielsen HJ, Brunner N, Kos J: Cathepsin B/cystatin C complex levels in sera from patients with lung and colorectal cancer. Biol Chem. 2001, 382: 805-810.
Kos J, Sekirnik A, Premzl A, Zavasnik Bergant V, Langerholc T, Turk B, Werle B, Golouh R, Repnik U, Jeras M, Turk V: Carboxypeptidases cathepsins X and B display distinct protein profile in human cells and tissues. Exp Cell Res. 2005, 306: 103-113. 10.1016/j.yexcr.2004.12.006.
Nascimento FD, Rizzi CCA, Nantes IL, Stefe L, Turk B, Carmona AK, Nader HB, Juliano L, Tersariol ILS: Cathepsin X binds to cell surface heparan sulfate proteoglycans. Arch Biochem Biophys. 2005, 436: 323-332. 10.1016/j.abb.2005.01.013.
Obermajer N, Jevnikar Z, Doljak B, Sadaghiani AM, Bogyo M, Kos J: Cathepsin X-mediated beta2 integrin activation results in nanotube outgrowth. Cell Mol Life Sci. 2009, 66: 1126-1134. 10.1007/s00018-009-8829-8.
Kos J, Jevnikar Z, Obermajer N: The role of cathepsin X in cell signaling. Cell Adh Migr. 2009, 3: 164-166. 10.4161/cam.3.2.7403.
Obermajer N, Doljak B, Jamnik P, Fonovic UP, Kos J: Cathepsin X cleaves the C-terminal dipeptide of alpha- and gamma-enolase and impairs survival and neuritogenesis of neuronal cells. Int J Biochem Cell Biol. 2009, 41: 1685-1696. 10.1016/j.biocel.2009.02.019.
Krueger S, Kalinski T, Hundertmark T, Wex T, Kuster D, Peitz U, Ebert M, Nagler DK, Kellner U, Malfertheiner P, Naumann M, Rocken C, Roessner A: Up-regulation of cathepsin X in Helicobacter pylori gastritis and gastric cancer. J Pathol. 2005, 207: 32-42. 10.1002/path.1820.
Nagler DK, Lechner AM, Oettl A, Kozaczynska K, Scheuber HP, Gippner-Steppert C, Bogner V, Biberthaler P, Jochum M: An enzyme-linked immunosorbent assay for human cathepsin X, a potential new inflammatory marker. J Immunol Methods. 2006, 308: 241-250. 10.1016/j.jim.2005.11.002.
Baker AR, Zalwango S, Malone LL, Igo RP, Qiu F, Nsereko M, Adams MD, Supelak P, Mayanja-Kizza H, Boom WH, Stein CM: Genetic susceptibility to tuberculosis associated with cathepsin Z haplotype in a Ugandan household contact study. Hum Immunol. 2011, 72: 426-430. 10.1016/j.humimm.2011.02.016.
Hidaka S, Yasutake T, Takeshita H, Kondo M, Tsuji T, Nanashima A, Sawai T, Yamaguchi H, Nakagoe T, Ayabe H, Tagawa Y: Differences in 20q13.2 copy number between colorectal cancers with and without liver metastasis. Clin Cancer Res. 2000, 6: 2712-2717.
Bar-Shira A, Pinthus JH, Rozovsky U, Goldstein M, Sellers WR, Yaron Y, Eshhar Z, Orr-Urtreger A: Multiple genes in human 20q13 chromosomal region are involved in an advanced prostate cancer xenograft. Cancer Res. 2002, 62: 6803-6807.
Nagler DK, Kruger S, Kellner A, Ziomek E, Menard R, Buhtz P, Krams M, Roessner A, Kellner U: Up-regulation of cathepsin X in prostate cancer and prostatic intraepithelial neoplasia. Prostate. 2004, 60: 109-119. 10.1002/pros.20046.
Rumpler G, Becker B, Hafner C, McClelland M, Stolz W, Landthaler M, Schmitt R, Bosserhoff A, Vogt T: Identification of differentially expressed genes in models of melanoma progression by cDNA array analysis: SPARC, MIF and a novel cathepsin protease characterize aggressive phenotypes. Exp Dermatol. 2003, 12: 761-771. 10.1111/j.0906-6705.2003.00082.x.
Sevenich L, Schurigt U, Sachse K, Gajda M, Werner F, Muller S, Vasiljeva O, Schwinde A, Klemm N, Deussing J, Peters C, Reinheckel T: Synergistic antitumor effects of combined cathepsin B and cathepsin Z deficiencies on breast cancer progression and metastasis in mice. Proc Natl Acad Sci U S A. 2010, 107: 2497-2502. 10.1073/pnas.0907240107.
Kraus S, Bunsen T, Schuster S, Cichon MA, Tacke M, Reinheckel T, Sommerhoff CP, Jochum M, Nagler DK: Cellular senescence induced by cathepsin X downregulation. Eur J Cell Biol. 2011, 90: 678-686. 10.1016/j.ejcb.2011.03.008.
Staudt ND, Aicher WK, Kalbacher H, Stevanovic S, Carmona AK, Bogyo M, Klein G: Cathepsin X is secreted by human osteoblasts, digests CXCL-12 and impairs adhesion of hematopoietic stem and progenitor cells to osteoblasts. Haematologica. 2010, 95: 1452-1460. 10.3324/haematol.2009.018671.
Wang J, Chen LL, Li Y, Guan XY: Overexpression of cathepsin Z contributes to tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. PLoS One. 2011, 6: e24967-10.1371/journal.pone.0024967.
Decock J, Obermajer N, Vozelj S, Hendrickx W, Paridaens R, Kos J: Cathepsin B, cathepsin H, cathepsin X and cystatin C in sera of patients with early-stage and inflammatory breast cancer. Int J Biol Markers. 2008, 23: 161-168.
Kolwijck E, Kos J, Obermajer N, Span PN, Thomas CM, Massuger LF, Sweep FC: The balance between extracellular cathepsins and cystatin C is of importance for ovarian cancer. Eur J Clin Invest. 2010, 40: 591-599. 10.1111/j.1365-2362.2010.02305.x.
Vizin T, Christensen IJ, Nielsen HJ, Kos J: Cathepsin X in serum from patients with colorectal cancer: relation to prognosis. Radiol Oncol. 2012, 46: 207-212.
Fonovic UP, Kos J: Efficient removal of cathepsin L from active cathepsin X using immunoprecipitation technique. Acta Chim Slov. 2009, 56: 985-988.
Nielsen HJ, Brunner N, Jorgensen LN, Olsen J, Rahr HB, Thygesen K, Hoyer U, Laurberg S, Stieber P, Blankenstein MA, Davis G, Dowell BL, Christensen IJ: Plasma TIMP-1 and CEA in detection of primary colorectal cancer: a prospective, population based study of 4509 high-risk individuals. Scand J Gastroenterol. 2011, 46: 60-69. 10.3109/00365521.2010.513060.
Harrell FE, Lee KL, Mark DB: Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996, 15: 361-387. 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4.
Altman DG, McShane LM, Sauerbrei W, Taube SE: Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. 2012, 9: e1001216-10.1371/journal.pmed.1001216.
Nagler DK, Zhang RL, Tam W, Sulea T, Purisima EO, Menard R: Human cathepsin X: A cysteine protease with unique carboxypeptidase activity. Biochemistry. 1999, 38: 12648-12654. 10.1021/bi991371z.
Obermajer N, Premzl A, Zavasnik Bergant T, Turk B, Kos J: Carboxypeptidase cathepsin X mediates beta2-integrin-dependent adhesion of differentiated U-937 cells. Exp Cell Res. 2006, 312: 2515-2527. 10.1016/j.yexcr.2006.04.019.
Obermajer N, Repnik U, Jevnikar Z, Turk B, Kreft M, Kos J: Cysteine protease cathepsin X modulates immune response via activation of beta2 integrins. Immunology. 2008, 124: 76-88. 10.1111/j.1365-2567.2007.02740.x.
Lechner AM, Assfalg-Machleidt I, Zahler S, Stoeckelhuber M, Machleidt W, Jochum M, Nagler DK: RGD-dependent binding of procathepsin X to integrin alphavbeta3 mediates cell-adhesive properties. J Biol Chem. 2006, 281: 39588-39597. 10.1074/jbc.M513439200.
Pecar Fonovic U, Jevnikar Z, Rojnik M, Doljak B, Fonovic M, Jamnik P, Kos J: Profilin 1 as a target for cathepsin X activity in tumor cells. PLoS One. 2013, 8: e53918-10.1371/journal.pone.0053918.
Skvortsova I: Profilin 1: do we have a novel proteome-found biomarker predicting response to anticancer therapy?. Proteomics. 2013, 13: 2069-2071. 10.1002/pmic.201300165.
Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, Peters C, Reinheckel T: Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006, 66: 5242-5250. 10.1158/0008-5472.CAN-05-4463.
Lines KE, Chelala C, Dmitrovic B, Wijesuriya N, Kocher HM, Marshall JF, Crnogorac-Jurcevic T: S100P-binding protein, S100PBP, mediates adhesion through regulation of cathepsin Z in pancreatic cancer cells. Am J Pathol. 2012, 180: 1485-1494. 10.1016/j.ajpath.2011.12.031.
Zhang X, Hou Y, Niu Z, Li W, Meng X, Zhang N, Yang S: Clinical significance of detection of cathepsin X and cystatin C in the sera of patients with lung cancer. Zhongguo Fei Ai Za Zhi. 2013, 16: 411-416.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/259/prepub
Acknowledgements
This work was supported by the Slovenian Research Agency (grants P4-0127, J4-0123 to J.K.). H.J.N. received financial support form The Danish Cancer Society, The Kornerup Fund, The Aage and Johanne Louis-Hansen Fund, The Aase and Ejnar Danielsen Fund, and The Kathrine and Vigo Skovgaard Fund. Authors thank prof. Roger Pain for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
TV designed the study, carried out the immunochemical analyses and drafted the manuscript. IJC analysed data, performed the statistical analysis and helped draft the manuscript. MW collected clinical data and participated in the evaluation of adjuvant chemotherapy. HJN participated in the design of the study and coordination of sampling and clinical evaluation. JK conceived the study, participated in coordination and helped draft the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
12885_2014_4417_MOESM2_ESM.tiff
Additional file 2: Figure S2: Linearity of the total Cat X ELISA. Dilution curves of four sera from CRC patients. Sera were serially diluted (1/2, 1/4, 1/8, 1/16). (TIFF 32 KB)
12885_2014_4417_MOESM3_ESM.tiff
Additional file 3: Figure S3: Analytical recovery. Observed total Cat X concentrations vs. expected total Cat X concentrations in serum samples from patients with CRC. Three different amounts of pro-Cat X were added to known amounts of total Cat X in two serum samples. (TIFF 22 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Vižin, T., Christensen, I.J., Wilhelmsen, M. et al. Prognostic and predictive value of cathepsin X in serum from colorectal cancer patients. BMC Cancer 14, 259 (2014). https://doi.org/10.1186/1471-2407-14-259
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-14-259