Abstract
Background
Primary hyperparathyroidism (PHPT) affects mainly cortical bone. It is thought that parathyroid hormone (PTH) indirectly regulates the activity of osteoclasts by means of the osteoprotegerin/ligand of the receptor activator of nuclear factor-κβ (OPG/RANKL) system. Several studies have confirmed that OPG (osteoprotegerin) and RANKL (ligand of the receptor activator of nuclear factor-κβ) loci are determinants of bone mineral density (BMD) in the general population. The aim of this study is to analyze the relationship between fractures and BMD and the rs3102735 (163 A/G), rs3134070 (245 T/G) and rs2073618 (1181 G/C) SNPs of the OPG and the rs2277438 SNP of the RANKL, in patients with sporadic PHPT.
Methods
We enrolled 298 Caucasian patients with PHPT and 328 healthy volunteers in a cross-sectional study. We analyzed anthropometric data, history of fractures or renal lithiasis, biochemical determinants including markers for bone remodelling, BMD measurements in the lumbar spine, total hip, femoral neck and distal radius, and genotyping for the SNPs to be studied.
Results
Regarding the age of diagnosis, BMI, menopause status, frequency of fractures or renal lithiasis, we found no differences between genotypes in any of the SNPs studied in the PHPT group. Significant lower BMD in the distal radius with similar PTH levels was found in the minor allele homozygotes (GG) compared to heterozygotes and major allele homozygotes in both OPG rs3102735 (163 A/G) and OPG rs3134070 (245 T/G) SNPs in those with PHPT compared to control subjects. We found no differences between genotypes of the OPG rs2073618 (1181 G/C) SNP with regard to BMD in the PHPT subjects. In the evaluation of rs2277438 SNP of the RANKL in PHPT patients, we found a non significant trend towards lower BMD in the 1/3 distal radius and at total hip in the minor allele homocygotes (GG) genotype group versus heterocygotes and major allele homocygotes (AA).
Conclusions
Our study provides the first evaluation of the relationship between SNPs of the OPG/RANK system and sporadic PHPT. Subjects with PHPT and minor homocygote genotype (GG) for the OPG rs3102735 (163 A/G) and OPG rs3134070 (245 T/G) SNPs have lower BMD in the distal radius, and this association does not appear to be mediated by differences in PTH serum levels.
Similar content being viewed by others
Background
Primary hyperparathyroidism (PHPT) is a common endocrine disorder, usually sporadic and asymptomatic, characterized by inappropriate hypersecretion of parathyroid hormone (PTH) and hypercalcemia. PHPT expression in bone involves mainly cortical bone such as the distal radius, with preservation of cancellous bone [1–8] which suggests that the response to high levels of PTH may differ depending on the skeletal structure [9], having anabolic effects in cancellous bone and catabolic actions at cortical sites. These findings in the pattern of bone mineral density (BMD) in PHPT are the opposite of those found in postmenopausal osteoporosis (OP). However it has been demonstrated that a group of patients with PHPT have lumbar OP that can improve after parathyroidectomy [10].
It is thought that PTH indirectly stimulates osteoclasts through neighbouring osteoblasts by inducing the ligand of the receptor activator of nuclear factor-κβ (RANKL) expression [7, 11]. RANK is expressed on the osteoclasts cell membrane and RANKL is a cytokine present on the membranes of osteoblasts and when binding with RANK, osteoclast differentiation, activation and survival are stimulated. RANK-RANKL interactions, and its effects, are prevented if RANKL binds with osteoprotegerin (OPG), a soluble receptor secreted by the osteoblasts. Genome wide association studies tested candidate genes for their association with bone mineral density (BMD) and found that the OPG gene is a determinant of fractures and BMD in both the spine and the hip [12, 13] and that RANKL locus influences BMD in both the spine and the hip also [13].
A number of studies analyzing the association between different single nucleotide polymorphisms (SNPs) of the OPG-RANK-RANKL system genes and bone density have been carried out in the osteoporotic and general populations, including a recent meta-analysis [14], but none of these studied sporadic PHPT. SNPs rs3102735 (163 A/G) [15–17] and rs3134070 (245 T/G) [15, 18] of the OPG have been studied, showing lower BMD or higher frequency of fractures related to the minor allele (G) at different bone sites, mainly in postmenopausal women. Regarding the rs2073618 (1181 G/C) SNP of the OPG, the minor allele (C) has been related to higher BMD [15, 18–24]. Other studies could not demonstrate these associations [25–28]. Among the SNPs of the RANKL studied, the minor allele (G) of the rs2277438 has been related to a higher femoral neck compression strength index [29] but not to BMD [19, 29, 30]. The aim of this study is to analyze the relationship between BMD and fractures and the three aforementioned SNPs of the OPG and the rs2277438 SNP of the RANKL, in patients with sporadic primary hyperparathyroidism, a model of chronic PTH hyperstimulation of the skeleton.
Methods
The study population consisted of 298 Caucasian patients with sporadic PHPT. Cases were defined by chronic hypercalcemia (total and/or ionic calcium) plus high levels of PTH or plus inappropriately normal PTH levels which means hypercalcemia and PTH values at the upper limit of the normal range. We excluded the few cases of PHPT that were part of a Multiple Endocrine Neoplasia Syndrome. After cautiously reviewing the family clinical history we also excluded cases of possible hereditary isolated PHPT.
In order to study the allelic distribution in PHPT versus healthy population, 328 volunteers were enrolled through face to face and written requests from hospital workers, and through civic associations, religious groups and geriatric residences. Written informed consent was obtained from participants. This study was approved by the Ethics Committee of our hospital (University Hospital "Marqués de Valdecilla", Santander, Spain). Subjects with history of diseases known to affect skeletal homeostasis, with non-Spanish ancestry, or who were taking drugs which interfere with bone metabolism (biphosphonates, strontium ranelate, corticosteroids, anti-epileptics, estrogens, thiazides, hormonal replacement therapy with estrogens in women beyond 55 years old) were excluded. Fracture condition was defined by any traumatic or spontaneous fracture at any location except the nose, toe, head, jaw, skull and hands. The renal lithiasis condition was defined by any detected lithiasis by radiological exploration with or without symptoms.
Measurement of BMD
MD was quantified using X-ray absorptiometry (DXA, Hologic, Walthan, MA, USA) in the lumbar spine (L2-4), femoral neck, total hip and radius. The BMD measurement variation coefficient was 1.2% in lumbar spine, total hip locations and radius projection, and 1.4% in the femoral neck.
DNA Analysis
Genomic DNA was extracted from venous blood (buffy coat) by the Qiagen method (Hilden, Germany) and stored at -40°C until analyzed. Polymerase chain reaction (PCR) products were amplified in a 5 μl reaction following the instructions of the manufacturer in an Applied Thermal Cycler 9700 (Applied Biosystems). Cycling conditions consisted of an initial denaturation step at 95°C for 10 minutes and 48 cycles of denaturation at 92°C for 30 seconds and annealing for 1 minute at 60°C. After amplification, end-point fluorescence reading and allele identification were carried out with an ABI 7300 sequence detector (Applied Biosystems). Random samples (8% of the total samples) were analysed twice for quality control.
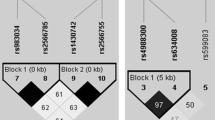
The SNPs to be analyzed, 163 A/G (rs3102735) located in promoter, 1181 G/C (rs2073618) located in exon I and 245 T/G (rs3134070) located in promoter of the OPG (chromosomal location 8q24, figure 1) [23] and the rs2277438 from the RANKL (13q14) located in a 5' untranslated region (UTR) [30] were chosen in relation to previous data in the literature suggesting possible associations with bone mass and functional significance. rs3102735 (163 A/G) is in strong linkage disequilibrium with other loci (rs3134070 (245 T/G), 950 T/C, and 6890 A/C) [22]. The OPG SNPs rs2073618 (1181 G/C) and rs3102735 (163 A/G) appear to be in different haplotype blocks (Figure 1). rs2073618 (1181 G/C) SNP of OPG causes the third amino acid of the signal peptide to change from lysine to asparagine. rs2277438 from the RANKL is a missense intronic substitution.
Polymorphisms in the proximal region of the OPG gene. The 245 T/G (rs3134070) and 163 A/G (rs 3102735) SNPs appear to be in the same haplotype block (Promoter) but in different block (Exon 1) from the 1181 G/C (rs 2073618) SNP. The linkage between SNP pairs is shown in the figures as D'. The numbers in the figures represent the Levontin distance (100 × D'); numbers are omitted from SNP pairs showing a D' of 100. The correlation (r2) between the OPG loci studied was: rs3102735-rs2073618 = 0.01; rs3102735-rs3134070 = 0.39; rs3134070-rs2073618 = 0.09.
Analysis of the OPG rs3102735(163 A/G), rs3134070 (245 T/G) and rs2073618 (1181 G/C) polymorphisms. Genotyping was performed with Custom Taqman® SNP Genotyping assays (Assay-by-Design) (Applied Biosystems, Warrington, Cheshire, UK) using allele discrimination-specific Taqman probes, labelled with VIC and FAM. Oligonucleotide primers were designed, based on the sequence of the OPG available in GenBank (AB008821). The following primer sequences were used. For rs3102735 (163 A/G) SNP: CATGAATGGGACCACACTTTACAAG (forward) and TGCTCTAGGGTTCGCTGTCT (reverse). For rs3134070 (245 T/G) SNP: CCCTAGAGCAAAGTGCCAAACT (forward) and AGCTTCCTACGCGCTGAAC (reverse). For rs2073618 (1181 G/C SNP): CCAAGCCCCTGAGGTTTCC (forward) and CCCAGGGACTTACCACGAG (reverse).
Analysis of RANKL rs2277438 polymorphism. This SNP was assayed by validated Taqman SNP Genotyping Assay. TTGTTGGGGACATAAAGACTCTTGC[A/G]AGTATGAATTTTTTGTTCTTAAGTC (context sequence).
Linkage structure was established from the Hapmap Caucasian (CEU) population (http://www.Hapmap.org), analyzed with Haploview 4.2 software [31].
Biochemical analysis
Fasting venous blood samples, for general biochemical analysis and specific determinations, were obtained after 30 minutes of supine rest from an antecubital vein. Samples were centrifuged immediately and serum was stored at -40°C in multiple aliquots until assayed to avoid freeze cycles. Total calcium (in serum and urine) and alkaline phosphatase were measured by automated methods in an ADVIA 2000 (Siemens Corp., Tarrytown, NY, USA). Intra- and inter-assay variations were <2%. Ionized calcium was measured by calcium-selective electrodes automated in a Ciba Corning 634 Ca++/pH Analyzer (Ciba Corning Diag. Corp, Medfield, Massachusetts, USA). Intact PTH was determined by automated immunoassay in a Liason (DiaSorin, Stillwater, Minnesota, USA). Sensitivity of assay was 5 pg/mL. Intra- and inter-assay variations were <5 and <8% respectively. 25-hydroxy-vitamin D was measured by RIA after extraction (DiaSorin, Stillwater, Minnesota, USA). The minimum detectable concentration was estimated to be 1.5 ng/mL. Intra- and inter-assay precision were 9.4 and 10.8%. Bone alkaline phosphatase (BAP) was determined by immunoassay (Alkphase B kit, Metra Biosystems, Mountain View, CA, USA). The minimum detectable dose is 0.7 U/L. Intra- and inter-assay variations were 3.5 and 6.2% respectively. Specificity: bone: 100%; liver: 3-8%; intestine: 0.4%. Serum osteocalcin was measured by immunoradiometric assay (IRMA) (OSTEO-RIACT kit, CIS Bio international, Gif-sur-Yvette, France). The sensitivity of the osteocalcin was 0.4 ng/mL. Intra- and inter-assay variations are 2.0 and 4.4% respectively. Collagen type I N-terminal propeptide levels were measured by RIA (Orion Diagnostica, Espoo, Finland). Sensitivity of the propeptide is 2 μg/L. Intra- and inter-assay variations are 8.75 and 5% respectively. Crosslaps were measured by ELISA (Nordic Bioscience Diagnostics, Herlev Hovedgade, Denmark). Sensitivity of assay is 0.010 ng/mL; intra- and inter-assay variations were 5.1 and 6.6% respectively. All biochemical and DXA procedures were performed at time of diagnosis and before any specific treatment had been initiated.
Statistical analysis
The results were processed with the computer package SPSS (Statistical Package for Social Sciences, Chicago, IL, USA). Normal or non-normal distribution of the variables under study was tested with the Kolmogorov-Smirnov test. All data were expressed as mean ± SD (standard deviation of the mean), or the median and range for non-normal variables. Comparisons between PHPT patients and 114 age and gender matched controls from the 328 total control group were tested by Student t-test and Mann-Whitney U for normally and non-normally distributed parameters. Allele frequencies were estimated by counting, and the χ2 test was used to identify significant departure from the Hardy-Weinberg equilibrium and for allele frequencies related to control and PHPT patients as well. In order to test association between the SNPs and PHPT, an odds ratio between cases and controls was calculated in addition. Fracture and nephrolithiasis frequency in the different genotype groups was tested by χ2. All shown data were adjusted for age, body mass index (BMI) and sex. Differences in age, BMI, BMD and bone remodelling markers between genotype groups were examined using the One-way ANOVA or Kruskal-Wallis test. If global test results were significant, between-group differences were then tested after Bonferroni correction for multiple comparisons (with a cut-off p value of 0.0167) except for BMD 1/3 radius and osteocalcin. In these two cases we used the Mann-Whitney U test (with a confidence interval of 98.3%) between pairs of alleles because there was only one subject in GG group who had osteocalcin and BMD 1/3 radius studied.
Results
General characteristics, bone biochemical parameters (non normal distribution) and BMD measurements (normal distribution) of both patients and control subjects are shown in Table 1. The presence of PHPT was excluded in all of the control subjects by measuring calcium and PTH. There were no differences regarding age, sex and menopause status between groups. The 25-OH-Vit D status was similar in both groups. BMI was significantly higher in PHPT than in control group. As expected, we found significant high levels of bone biochemical parameters in the PHPT group compared with the control group and lower BMD in the PHPT patients than in the control subjects at the three studied sites.
Allelic frequencies did not deviate from Hardy-Weinberg equilibrium. Genotype frequencies for controls and primary hyperparathyroidism subjects showed no differences in any of the SNPs studied (Tables 2 and 3).
Regarding the age of diagnosis, BMI, menopause status, frequency of fractures or renal lithiasis, we found no differences between genotypes in any of the SNPs studied in the PHPT group (Additional File 1) nor in control subjects.
Relationship between the different SNPs studied and BMD and bone remodelling markers
SNP 163 A/G OPG (rs 3102735)
In PHPT patients, BMD at the 1/3 distal radius was significantly lower in the minor allele homozygote group (GG) versus the other two groups (p = 0.039); (GG-AA p = 0.038; GG-AG p = 0.037) (Table 4). There were no differences between the major allele homozygote and heterozygote group. Analysis of BMD in the lumbar spine or hip did not show any difference between genotypes. PTH, total and ionized calcium, P1NP, β Crosslaps, osteocalcin and bone alkaline phosphatase (BAP) showed a trend (not statistically significant) to higher levels in the minor allele homozygote group versus the other two groups. There were no differences in vitamin D levels among the three groups (data not shown except for PTH (Table 4, Additional File 2). These results were not different in the subgroup of women and we found no difference in their menopausal status between the three genotype groups.
In the control group, we did not find any difference between genotypes regarding BMD at three sites (Additional File 3).
SNP 245 T/G OPG (rs3134070)
In PHPT patients, BMD in the 1/3 distal radius was significantly lower in the minor allele (GG) homozygote group versus the other two groups despite its low frequency (p = 0.003); (GG-TT p = 0.003 and GG-TG p = 0.01) (Table 4). Analysis of BMD in the lumbar spine or hip did not show any difference between genotypes. Total calcium (mg/dL) GG = 12.3 ± 0.9; TT = 10.7 ± 0.7; TG = 10.9 ± 1.1 (p = 0.011); GG-TT p = 0.012 and GG-TG p = 0.032, β Crosslaps (ng/mL) GG = 1.770 ± 2.033; TT = 0.816 ± 0.512; TG = 0.726 ± 0.356 (p = 0.033); GG-TT p = 0.035 and GG-TG p = 0.029 and osteocalcin (ng/mL) GG = 175.0; TT = 30.4 ± 19.0; TG = 30.6 ± 18.2 (p < 0.001); GG-TT p < 0.001 and GG-TG p < 0.001 levels were significantly higher in the minor homozygote allele group than in the other two groups (Additional File 2). These results were not different in the subgroup of women and we found no difference in their menopausal status between the three genotype groups.
In the control group, we did not find any difference between genotypes regarding BMD in the three sites (Additional File 3).
SNP 1181 G/C OPG (rs 2073618)
In PHPT patients, BMD at all sites and bone remodelling markers were similar in the three genotype groups (Additional File 2). These results were not different in the subgroup of women and we found no difference in their menopausal status between the three genotype groups.
In the control group, we found higher levels of BMD (g/cm2) in CC subjects vs in GG (0.983 ± 0.170 vs 0.872 ± 0.155; p = 0.028) in the lumbar spine (Additional File 3).
SNP RANKL rs2277438
In PHPT patients, we found a non significant trend towards lower BMD in the 1/3 distal radius (g/cm2) (AA = 0.622 ± 0.105; AG = 0.610 ± 0.087; GG = 0.543 ± 0.074; p = 0.835), to lower BMD at total hip (g/cm2) (AA = 0.841 ± 0.132; AG = 0.821 ± 0.160; GG = 0.791 ± 0.118; p = 0.502) and to higher levels of BAP (U/L) (AA = 33.5 ± 17.3; AG = 29.9 ± 13.0; GG = 43.7 ± 8.4; p = 0.122) in the minor allele homozygote group (GG) versus the other two groups (Additional File 2). These results were not different in the subgroup of women and we found no difference in their menopausal status between the three genotype groups.
In the control group, we did not find any differences between genotypes regarding BMD at three sites (Additional File 3).
Due to the low frequency of one of the homozygous genotypes in the OPG rs3102735 (163 A/G), OPG rs3134070 (245 T/G) and RANKL rs2277438, we grouped the heterozygote and the less frequent homozygous subjects. We did not find any differences between the groups regarding BMD in all sites.
Discussion
As expected, subjects in the PHPT group had high levels of calcium, PTH, bone remodelling markers and low BMD at the three studied sites. We found BMI corresponding to overweight in the PHPT group consistent with previous studies [32, 33] although the nature of this relationship remains uncertain. We found no differences in vitamin D levels between patients and control subjects, as Vignali had also found in postmenopausal PHPT women [34], and, in contrast with previous data [35] showing lower vitamin D levels in PHPT patients than in control subjects, we did not find such a difference between the study groups although we did not evaluate seasonal factors as the aforementioned study did. OPG serum levels were three times higher in our PHPT patients than in a previous study on men and women aged 62 with PHPT [36] and our PHPT subjects had lower levels than a group of postmenopausal women [37]. Despite there being no established reference values, OPG serum levels appear to be higher in postmenopausal women than in other conditions as PHPT.
There were no differences in the allelic distribution between PHPT and control groups, this means that none of the studied SNPs appears to be a genetic factor which predisposes for PHPT.
The main purpose of this study was to analyze the relationship between BMD, or fractures, and three SNPs of the OPG and the rs2277438 SNP of the RANKL in PHPT. We did not find any differences in frequency of fractures between genotypes in control nor in PHPT subjects in all the SNPs studied according to data of previous studies of OPG rs3102735 (163 A/G) [27] and of OPG rs2073618 (1181 G/C) [27, 28] on postmenopausal women. These two studies neither did find any differences in BMD. A study of OPG rs2073618 (1181 G/C) described a 26% higher risk of hip fractures and 52% higher of femoral neck fractures in CC homozygote than GG homozygote women independent of BMD [24]. There are no fracture data related to RANKL rs2277438 SNP in the literature. The genetic variation that causes SNPs appear not to have main influence on a clinical event as relevant as fractures in PHPT and in non PHPT population.
Significantly lower BMD in the 1/3 distal radius with similar PTH levels were found in the minor homozygotes (GG) compared to heterozygotes and major allele homozygotes in both OPG rs3102735 (163 A/G) and OPG rs3134070 (245 T/G) SNPs in PHPT but not in control subjects. This could mean that these minor homozygote individuals suffer from more specific cortical BMD loss not mediated by PTH or creatinine serum levels in PHPT. An association between the G allele of the OPG rs3102735 (163 A/G) SNP and low BMD in the forearm, low heel broadband ultrasound attenuation (BUA) and low heel speed of sound (SOS) were described in Danish women with and without hip or forearm fractures [16]. In Hungarian women the GG genotype of the OPG rs3102735 (163 A/G) was associated with low hip BMD [17]. Nevertheless, Hsu reported that males with the GG genotype of the OPG rs3102735 (163 A/G) had very low risk of having extremely low BMD in the hip [30]. Regarding the OPG rs3134070 (245 T/G) SNP, an association between the GG genotype and low BMD has already been reported in the radius and femoral neck in postmenopausal women [18]. Several other studies failed to show this association between OPG rs3102735 (163 A/G) or OPG 245 T/G [19, 22, 27, 28, 38].
We did not find any difference between genotypes of the OPG rs2073618 (1181 G/C) SNP regarding BMD in the PHPT subjects, a finding in accordance with previous studies on menopausal women [27, 28]. However, we did find higher BMD in the lumbar spine in the CC than in the GG genotype group in the healthy control subjects, also in accordance with previous findings in the general or postmenopausal osteoporotic populations [15, 19, 21–24]. These findings could mean that the bone loss in PHPT, mainly cortical, and in the osteoporotic condition, mainly cancellous, is modulated by different genetic factors. Some possible factors could be the G allele of the OPG rs2073618 (1181 G/C) SNP favouring lumbar spine bone loss in osteoporosis, or the G allele of the OPG rs3134070 (245 T/G) and rs3102735 (163 A/G) favouring cortical bone loss in PHPT. But it should be also considered that the wrist is a non-weight bearing site and therefore is free of external factors or remodelling due to body weight or physical activity.
Consistent with studies in the non-PHPT population [19, 29, 30] we did not find any significant difference between genotypes of the RANKL rs2277438 SNP in PHPT or in the control group.
The main limitations of our study are the low frequency of the GG genotype in both the OPG rs3102735 (163 A/G) and rs3134070 (245 T/G) SNPs and that there was a trend (not statistically significant) to difference in age between the GG and the rest of the groups. Otherwise, our results are supported by the fact that they are not reproducible in control subjects and that these two SNPs are located in the same haplotype block (promoter).
Conclusion
Our study provides the first evaluation of the relationship between SNPs of the OPG/RANK system and sporadic PHPT. Subjects with PHPT and GG genotype for the OPG rs3102735 (163 A/G) and OPG rs3134070 (245 T/G) SNPs appear to be at risk of having a lower BMD in the distal radius, which is mainly cortical bone. This association did not appear to be mediated by any difference in PTH serum levels. Further studies on larger PHPT populations, with complementary functional evaluation, are needed to support our results.
Abbreviations
- P1NP:
-
Amino-terminal propeptyde type 1 Colagen
- BMI:
-
Body mass index BAP: Bone alkaline phosphatase
- BMD:
-
Bone mineral density
- IRMA:
-
Immunoradiometric assay
- RANKL:
-
Ligand of the receptor activator of nuclear factor-κβ OP: Osteoporosis
- OPG:
-
Osteoprotegerin
- PTH:
-
Parathyroid hormone
- PCR:
-
Polymerase chain reaction
- PHPT:
-
Primary hyperparathyroidism
- RANK:
-
Receptor activator of nuclear factor-κβ
- SNP:
-
Single nucleotide polymorphism
- ELISA:
-
Specific immunoassay
- SD:
-
Standard deviation of the mean
- SPSS:
-
Statistical Package for Social Sciences
- DXA:
-
X-ray absorptiometry.
References
Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV, Lindsay R, Clemens TL, Bilezikian JP: Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989, 3: 283-291.
Parisien M, Cosman F, Mellish RWE, Schnitzer M, Nieves J, Silverberg SJ, Shane E, Kimmel D, Recker RR, Bilezikian JP, Lindsay R, Dempster DW: Bone structure in postmenopausal hyperparathyroid osteoporotic and normal women. J Bone Miner Res. 1995, 10: 1393-1399.
Dempster DW, Parisien M, Silverberg SJ, Liang XG, Schnitzer M, Shen V, Shane E, Kimmel DB, Recker R, Lindsay R, Bilezikian JP: On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endoc Metab. 1999, 84: 1562-1566. 10.1210/jc.84.5.1562.
Bollerslev J, Jansson S, Mollerup C, Nordenström J, Lundgren E, Tørring O, Varhaug JE, Baranowski M, Aanderud S, Franco C, Freyschuss B, Isaksen GA, Ueland T, Rosen T: Medical observation, compared with parathyroidectomy, for asymptomatic primary hyperparathyroidism: a prospective, randomized trial. J Clin Endoc Metab. 2007, 92: 1687-1692. 10.1210/jc.2006-1836.
Dempster DW, Müller R, Zhou H, Kohler T, Shane E, Parisien M, Silverberg SJ, Bilezikian JP: Preserved three-dimensional cancellous bone structure in mild primary hyperparathyroidism. Bone. 2007, 41: 19-24. 10.1016/j.bone.2007.03.020.
Rubin MR, Bilezikian JP, McMahon DJ, Jacobs T, Shane E, Siris E, Udesky J, Silverberg SJ: The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endoc Metab. 2008, 93: 3462-3470. 10.1210/jc.2007-1215.
Mosekilde L: Primary hyperparathyroidism and the skeleton. Clin Endocrinol. 2008, 69: 1-19. 10.1111/j.1365-2265.2007.03162.x.
Moosgaard B, Christensen SE, Vestergaard P, Heickendorff L, Christiansen P, Mosekilde L: Vitamin D metabolites and skeletal consequences in primary hyperparathyroidism. Clin Endocrinol. 2008, 68: 707-715. 10.1111/j.1365-2265.2007.03109.x.
Khan A, Bilezikian J: Primary Hiperparathyroidism: pathophysiology and impact on bone. Can Med Assoc J. 2000, 163: 184-187.
Ambrogini E, Cetani F, Cianferotti L, Vignali E, Banti C, Viccica G, Oppo A, Miccoli P, Berti P, Bilezikian JP, Pinchera A, Marcocci C: Surgery or surveillance for mild asymptomatic prymary hyperparathyroidism: a prospective, randomized clinical trial. J Clin Endocrinol Metab. 2007, 92: 3114-3121. 10.1210/jc.2007-0219.
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T: Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998, 95: 3597-3602. 10.1073/pnas.95.7.3597.
Richards JB, Rivadeneira F, Intuye M, Pastinen TM, Soranzo N, Wilson SG, Andrew T, Falchi M, Gwilliam R, Ahmadi KR, Valdes AM, Arp P, Whittaker P, Verlaan DJ, Jhamai M, Kumanduri V, Moorhouse M, van Meurs JB, Hofman A, Pols HA, Hart D, Zhai G, Kato BS, Mullin BH, Zhang F, Deloukas P, Uitterlinden AG, Spector TD: Bone mineral density, osteoporosis and osteoporotic fractures: a genome-wide association study. Lancet. 2008, 371: 1505-12. 10.1016/S0140-6736(08)60599-1.
Styrkasdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, Jonsdottir T, Saemundsdottir J, Center JR, Nguyen TV, Bagger Y, Gulcher JR, Eisman JA, Christiansen C, Sigurdsson G, Kong A, Thorsteinsdottir U, Stefansson K: Multiple genetic loci for bone mineral density and fractures. New Engl J Med. 2008, 358: 2355-2365. 10.1056/NEJMoa0801197.
Paternoster L, Ohlsson C, Sayers L, Vandenput L, Lorentzon M, Evans DM, Tobias JH: OPG and RANK polymorphisms are both associated with cortical bone mineral density: findings from a metaanalysis of the Avon Longitudinal Study of Parents and Children and Gothenburg Osteoporosis and Obesity Determinants cohorts. J Clin Endoc Metab. 2010, 95: 3940-3948. 10.1210/jc.2010-0025.
Langdahl BL, Carstens M, Stenkjaer L, Eriksen EF: Polymorphisms in the osteoprotegerin gene are associated with osteoporotic fractures. J Bone Miner Res. 2002, 17: 1245-1255. 10.1359/jbmr.2002.17.7.1245.
Jorgensen HL, Kusk P, Madsen B, Fenger M, Lauritzen JB: Serum osteoprotegerin (OPG) and the A163G polymorphism in the OPG promoter region are related to peripheral measures of bone mass and fracture odds ratio. J Bone Miner Metab. 2004, 22: 132-138. 10.1007/s00774-003-0461-3.
Takács I, Lazáry Á, Kósa JP, Kiss J, Balla B, Nagy Z, Bácsi K, Speer G, Lakatos P: Allelic variations of RANKL/OPG signaling system are related to bone mineral density and in vivo gene expression. Eur J Endocrinol. 2010, 162: 423-431. 10.1530/EJE-09-0617.
Yamada Y, Ando F, Niino N, Shimokata H: Association of polymorphisms of the osteoprotegerin gene with bone mineral density in Japanese women but not men. Mol Genet Metab. 2003, 80: 344-349. 10.1016/S1096-7192(03)00125-2.
Arko B, Prezelj J, Kocijancic A, Komel R, Marc J: Association of the osteoprotegerin gene polymorphisms with bone mineral density in postmenopausal women. Maturitas. 2005, 51: 270-279. 10.1016/j.maturitas.2004.08.006.
Kim JG, Kim JH, Kim JY, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM: Association between osteoprotegerin (OPG), receptor activator of nuclear factor-JB (RANK), and RANK ligand (RANKL) gene polymorphisms and circulating OPG, soluble RANKL levels, and bone mineral density in Korean postmenopausal women. Menopause. 2007, 14: 913-918. 10.1097/gme.0b013e31802d976f.
Zhao H, Liu J, Ning G, Zhao YJ, Zhang LZ, Sun LH, Xu MY, Uitterlinden AG, Chen JL: The influence of Lys3Asn polymorphism in the osteoprotegerin gene of bone mineral density in Chinese postmenopausal women. Osteoporosis Int. 2005, 16: 1519-1524. 10.1007/s00198-005-1865-9.
Choi JY, Shin A, Park SK, Chung HW, Cho SI, Shin CS, Kim H, Lee KM, Lee KH, Kang C, Cho DY, Kang D: Genetic Polymorphisms of OPG, RANK, and ESR1, and Bone Mineral Density in Korean Postmenopausal Women. Calcified Tissue Int. 2005, 77: 152-159. 10.1007/s00223-004-0264-0.
García-Unzueta MT, Riancho JA, Zarrabeitia MT, Sañudo C, Berja A, Valero C, Pesquera C, Paule B, González-Macías J, Amado JA: Association of the 163A/G and 1181G/C osteoprotegerin polymorphism with bone mineral density. Horm Metab Res. 2008, 40: 219-224. 10.1055/s-2008-1046793.
Moffett SP, Oakley JI, Cauley JA, Lui LY, Ensrud KE, Taylor BC, Hillier TA, Hochberg MC, Li J, Cayabyab S, Lee JM, Peltz G, Cummings SR, Zmuda JM: Study of Osteoporotic Fractures Research Group. Osteoprotegerin Lys3Asn polymorphism and the risk of fracture in older women. J Clin Endoc Metab. 2008, 93: 2002-2008. 10.1210/jc.2007-1019.
Ohmori H, Makita Y, Funamizu M, Hirooka K, Hosoi T, Orimo H, Suzuki T, Ikari K, Nakajima T, Inoue I, Hata A: Linkage and association analyses of the OPG gene locus with human osteoporosis. J Hum Genet. 2002, 47: 400-406. 10.1007/s100380200058.
Wynne F, Drummond F, O'Sullivan K, Daly M, Shanahan F, Molloy MG, Quane KA: Investigation of the genetic influence of the OPG, VDR (Fok1) and COLIA1 Sp1 polymorphisms on BMD in the Irish population. Calcified Tissue Int. 2002, 71: 26-35. 10.1007/s00223-001-2081-z.
Ueland T, Bollerslev J, Wilson SG, Dick IM, Islam FM, Mullin BH, Devine A, Prince RL: No associations between OPG gene polymorphisms or serum levels and measures of osteoporosis in elderly Australian women. Bone. 2007, 40: 175-181. 10.1016/j.bone.2006.06.022.
Jurado S, Nogues L, Agueda L, Garcia-Giralt N, Urreizti R, Yoskovitz G, Pérez-Edo L, Saló G, Carreras R, Mellibovsky L, Balcells S, Grinberg D, Díez-Pérez A: Polymorphisms and haplotypes across the osteoprotegerin gene associated with bone mineral density and osteoporotic fractures. Osteoporosis Int. 2010, 21: 287-296. 10.1007/s00198-009-0956-4.
Dong SS, Liu XG, Chen Y, Guo Y, Wang L, Zhao J, Xiong DH, Xu XH, Recker RR, Deng HW: Association analyses of RANKL/RANK/OPG gene polymorphisms with femoral neck compression strength index variation in Caucasians. Calcified Tissue Int. 2009, 85: 104-112. 10.1007/s00223-009-9255-5.
Hsu YH, Niu T, Terwedow HA, Xu X, Feng Y, Li Z, Brain JD, Rosen CJ, Laird N, Xu X: Variation in genes involved in the RANKL/RANK/OPG bone remodelling pathway are associated with bone mineral density at different skeletal sites in men. Hum Genet. 2006, 118: 568-577. 10.1007/s00439-005-0062-4.
Barrett JC, Fry B, Maller J, Daly MJ: Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005, 15: 263-265.
Bolland MJ, Grey AB, Gamble G, Reid IR: Association between Primary Hyperparathyroidism and Increased Body Weight: A Meta-Analysis. J Clin Endoc Metab. 2005, 90: 1525-1530.
Siilin H, Rastad J, Ljunggren Ö, Lundgren E: Disturbances of calcium homeostasis consistent with mild primary hyperparathyroidism in premenopausal women and associated morbidity. J Clin Endoc Metab. 2008, 93: 47-53.
Vignali E, Viccica G, Diacinti D, Cetani F, Cianferotti L, Ambrogini E, Banti C, Del Fiacco R, Bilezikian JP, Pinchera A, Marcocci C: Morphometric vertebral fractures in postmenopausal women with primary hyperparathyroidism. J Clin Endoc Metab. 2009, 94: 2306-2312. 10.1210/jc.2008-2006.
Moosgaard B, Vestergaard P, Heickendorff L, Melsen F, Christiansen P, Mosekilde L: Vitamin D status, seasonal variations, parathyroid adenoma weight and bone mineral density in primary hyperparathyroidism. Clin Endocrinol. 2005, 63: 506-513. 10.1111/j.1365-2265.2005.02371.x.
Stilgren LS, Hegedüs LM, Beck-Nielsen H, Abrahamsen B: Osteoprotegerin levels in primary hyperparathyroidism: effect of parathyroidectomy and association with bone metabolism. Calcified Tissue Int. 2003, 73: 210-216. 10.1007/s00223-002-2100-8.
Mezquita-Raya P, de la Higuera M, García DF, Alonso G, Ruiz-Requena ME, de Dios Luna J, Escobar-Jiménez F, Muñoz-Torres M: The contribution of serum osteoprotegerin to bone mass and vertebral fractures in postmenopausal women. Osteoporosis Int. 2005, 16: 1368-1374. 10.1007/s00198-005-1844-1.
Arko B, Prezelj J, Komel R, Kocijancic A, Hudler P, Marc J: Sequence variations in the osteoprotegerin gene promoter in patients with postmenopausal osteoporosis. J Clin Endoc Metab. 2002, 87: 4080-4088. 10.1210/jc.2002-020124.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2350/12/168/prepub
Acknowledgements and funding
Financial disclosure: Bernardo Lavín is a researcher whose work is supported by the IFIMAV grant N° BFR 03/09.This study was partly supported by the IFIMAV (Instituto de Formación e Investigación Marqués de Valdecilla) N° API 06/12 and partly as well by the Carlos III Institute grant N° 03/0052
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
all authors have read and approved the final manuscript.
MP: made substantial contributions to conception, design and draft of the manuscript and in acquisition,
analysis and interpretation of data
MTG-U: was involved in drafting, conception and design of the manuscript.
AB: carried out molecular genetic studies
BP: carried out inmunoassays
BAL: carried out inmunoassays
CV: carried out the double X ray absorptiometries
JAR: revised the manuscript critically for important intellectual content
JAA: was involved in drafting the manuscript and gave final approval of the version to be published
Electronic supplementary material
12881_2011_898_MOESM1_ESM.DOC
Additional file 1: "Distribution Of Fractures And Lithiasis Frequency Among the Genotypes of the Snps Studied In Phpt Patients". This file contains two tables showing the distribution of fractures and lithiasis frequency among the genotype groups of the OPG 163 A/G rs3102735, OPG 245 T/G rs3134070, OPG 1181 G/C rs2073618 and RANKL rs2277438 in PHPT patients. (DOC 46 KB)
12881_2011_898_MOESM2_ESM.DOC
Additional file 2: "Comparison Of Biochemical Parameters Among The Genotype Groups of the Snps Studied In Phpt Subjects". This file contains several tables showing the comparison of biochemical parameters among the genotype groups of the OPG 163 A/G rs3102735, OPG 245 T/G rs3134070, OPG 1181 G/C rs2073618 and RANKL rs2277438 in PHPT patients. (DOC 126 KB)
12881_2011_898_MOESM3_ESM.DOCX
Additional file 3: "Bone Mineral Density Distribution Among The Genotypes of the Snps Studied In Control Subjects". This file contains a table showing the distribution of BMD levels among the three genotype groups of the OPG 163 A/G rs3102735, OPG 245 T/G rs3134070, OPG 1181 G/C rs2073618 and RANKL rs2277438 in the control subjects. (DOCX 14 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Piedra, M., García-Unzueta, M.T., Berja, A. et al. "Single nucleotide polymorphisms of the OPG/RANKL system genes in primary hyperparathyroidism and their relationship with bone mineral density". BMC Med Genet 12, 168 (2011). https://doi.org/10.1186/1471-2350-12-168
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2350-12-168