Abstract
Background
Activated drotrecogin alfa (human activated protein C, rhAPC), is produced by recombinant DNA technology, and purports to improve clinical outcomes by counteracting the inflammatory and thrombotic consequences of severe sepsis. Controversy exists around the clinical benefits of this drug and an updated economic study that considers this variability is needed.
Methods
A systematic literature review was performed using Medline, Embase and the International Network of Agencies for Health Technology Assessment (INAHTA) databases to determine efficacy, safety and previous economic studies. Our economic model was populated with systematic estimates of these parameters and with population life tables for longer term survival information. Monte Carlo simulations were used to estimate the incremental cost-effectiveness ratios (ICERs) and variance for the decision analytic models.
Results
Two randomized clinical trials (RCTS) of drotrecogin alfa in adults with severe sepsis and 8 previous economic studies were identified. Although associated with statistical heterogeneity, a pooled analysis of the RCTs did not show a statistically significant 28-day mortality benefit for drotrecogin alfa compared to placebo either for all patients (RR: 0.93, 95% CI: 0.69, 1.26) or those at highest risk as measured by APACHE II ≥ 25 (RR: 0.90, 95% CI: 0.54, 1.49). Our economic analysis based on the totality of the available clinical evidence suggests that the cost-effectiveness of drotrecogin alfa is uncertain (< 59% probability that incremental cost-effectiveness ratio (ICER) life year gained (LYG) ≤ $50,000/LYG) when applied to all patients with severe sepsis. The economic attractiveness of this therapy improves when administered to those at highest risk as assessed by APACHE II ≥ 25 (93% probability ICER ≤ $50,000/LYG) but these results are not robust to different measures of disease severity.
Conclusion
The evidence supporting the clinical and economic attractiveness of drotrecogin alfa is not conclusive and further research appears to be indicated.
Similar content being viewed by others
Background
Sepsis is a complex syndrome with protean etiologies characterized by a systemic inflammatory and procoagulant response to an infection, and is considered severe in the presence of acute organ dysfunction [1]. Endogenous protein C activation attempts to counteract these manifestations of sepsis and drotrecogin alfa, a form of human activated protein C produced by recombinant DNA techniques [2] has been approved for the subgroup of patients with severe sepsis and the highest risk of death [3–5].
Controversy has plagued this drug since its 2001 FDA evenly divided approval vote [6, 7]. This controversy emanates from approval being based on a single randomized controlled trial (RCT) (PROWESS) and particularly on one subgroup analysis. Moreover concerns exist regarding drotrecogin alfa's cost-effectiveness and the inconsistent results observed in more recent studies. Several economic analyses have nevertheless suggested that the drug may be cost-effective but these early studies have generally not considered the totality of the efficacy evidence now available. Therefore we performed a systematic review of all published drotrecogin alfa evidence, as a prelude for an updated economic analysis to assist in difficult resource allocation decisions.
Methods
Literature search
A systematic literature search of RCTs and economic evaluations comparing drotrecogin alfa and placebo in adult and pediatric patients published in English or French was performed using Medline and Embase databases (search (MeSH) terms: (Drotrecogin OR Activated protein C OR Xigris) AND (Sepsis). The search included publications until Dec 31 2006. The reference lists of the publications identified were also searched for additional relevant publications.
Methods for economic analysis
Cost-effectiveness analysis ($/life year gained (LYG) was performed using a decision-tree model (see Figure 1) and a 20 year horizon, based on the approximate life-expectancy of our base case patient population. Drotrecogin alfa efficacy measures for our economic model were ascertained, when possible, by combining RCTs results from our systematic review in a random effects meta-analysis (RevMan V 4.2, Cochrane Collaboration, Oxford, England). Beta distributions for model parameters were derived from this information.
Simplified decision analytic model employed. *The individual survival probabilities between 28 days and 30 months were included in the model but are not shown in this figure. A similar tree was used in the bleeding and non-bleeding arms, and for the drotrecogin alfa and placebo arms (not shown). After the 30 months a Markov model was employed (determined by the node M).
Survival rates for the first 30 months from RCT(s) were available for drotrecogin alfa and placebo from the PROWESS study [8]. We assumed that this numerical difference in survival at 30 months persisted without any further increases or decreases in long term survival. Long term survival could be estimated from population life tables, corrected for the higher mortality risk in sepsis patients. Costs beyond three years were also considered to be similar between the two groups. Separate models were developed for the whole study population and the subgroups at highest risk of death. Complication rates were taken from a pooled analysis using data available from the RCTs.
Probabilistic sensitivity analyses using 10,000 Monte Carlo simulations were used to estimate the incremental cost-effectiveness ratios (ICERs) and variance (TreeAge Pro 2007). The economic analyses were performed from the public healthcare provider viewpoint. Multiple sensitivity analyses were performed using a different clinical outcome (quality-adjusted life-years (QALYs) with utilities measured with the EuroQoL-5D questionnaire [9], as well as varying time horizons and discount rates. Table 1 shows the parameters and characteristics of the models employed. Costs are provided in 2006 US dollars.
The presence of overlapping distributions in the effectiveness, i.e., non statistically significant or small differences between two comparators, or even negative effectiveness, results in instability in the calculation of ICERs and their confidence intervals thereby rendering their interpretation difficult [10, 11]. Therefore we have graphically presented the results from our probabilistic sensitivity analyses in the cost-effectiveness plane as this shows the proportion of simulations where drotrecogin alfa had a higher effectiveness and/or cost compared to placebo. Acceptability curves using net health benefits are also presented as this measure overcomes the difficulties in interpreting negative ICERs [12].
Results
Systematic literature review
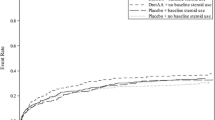
Our systematic literature search identified 2 adult randomized, double-blind, placebo-controlled trials [13, 14] (see Table 2) with long-term results available only for one [8]. Both RCTs were terminated prematurely, one for efficacy [13] and the other for futility [14]. Although the disease severity criteria for these two trials were slightly different, the pooling of these studies is justified since the same disease entity is being studied with the same research design using the same treatment protocol (see Table 2). The pooled 28-day efficacy results are shown in Figure 2. The totality of the available evidence indicates no statistically significant 28-day mortality reduction for drotrecogin alfa in patients with severe sepsis (RR, 0.93, 95% CI: 0.69, 1.26), even when stratified by disease severity (APACHE II ≥ 25, RR: 0.90, 95% CI: 0.54, 1.49). Despite the relative homogeneity in the 2 populations, there was presence of statistical heterogeneity in the overall results. However when the analyses were performed using the number of failed organs as a measure of disease severity or when in-hospital mortality was used as outcome (Figure 3), there was no longer evidence of statistical heterogeneity between the two RCTs.
Survival beyond 28 days or hospital discharge has been reported only for the PROWESS study [8]. No statistically significant mortality differences were observed in follow-up from 3 to 30 months in the whole cohort of patients and most reported subgroup analyses [8] except for the subgroup defined by an Acute Physiology and Chronic Health Evaluation (APACHE) II [15] score ≥ 25 (30-month survival: 45.6% vs 33.8% for drotrecogin alfa and placebo respectively, p = 0.001) [8]. In contrast, in the subgroup with ≥ 2 organ dysfunctions, which was the criterion used by the regulatory agency of the European Union to define higher disease severity [4], there was no statistically significant survival benefit with drotrecogin alfa over placebo [8], the same in other subgroups.
Because of its antithrombotic and profibrinolytic effects, bleeding complications may be anticipated with drotrecogin alfa [16]. Among the RCTs identified, the PROWESS reported 30 (3.5%) 28-day serious bleeding events with drotrecogin alfa and 17 (2%) with placebo (p = 0.06), in the ADDRESS study there were 51 (3.9%) and 28 (2.2%) such events respectively (p = 0.01) [14]. Pooling these two studies together using the inverse variance method yielded a 3.7% (± 1.06) rate of 28-day serious bleeding events with drotrecogin alfa and 2.1% (± 1.04) with placebo.
Despite only 2 RCTs, there were numerous secondary publications [17–30] involving subgroup analyses based on the PROWESS [13] trial. In addition 3 meta-analyses [31–33], and 9 economic analyses [34–42] were identified (Table 3). The calculated cost/life-year gained (LYG) for all patients varied between $US 8,500 and $US 33,300 and was generally slightly lower for higher risk patients (Table 3). However, most of these analyses modeled long term results by assuming that the PROWESS 28-day mortality results were sustained and durable which has been shown to be incorrect (see above).
Current economic analysis
Our decision analytic model is shown in Figure 1 and the parameter and cost estimates are given in Tables 4, 5, 6. The totality of the evidence for all patients revealed considerable overlapping of the survival estimates in treated and placebo patients resulting in approximately 27% of the simulations with a negative ICER (i.e. more expensive but less effective) for drotrecogin alfa compared to placebo (see Figure 4). The model using APACHE II >= 25 as a severity measure showed a lower chance, approximately 3%, of a negative ICER (Figure 5).
Due to this instability in ICERs, we have elected to concentrate on acceptability curves that show the proportional benefit at varying willingness to pay (WTP) thresholds. Figures 6 and 7 show the net benefits acceptability curve obtained for all patients and those with APACHE II ≥ 25 respectively. In the model for all patients, there was a 48% chance that the ICER will be ≤ $30,000/LYG, and 59% chance that it will be ≤ $50,000 (Figure 6). Considering only those patients with APACHE II ≥ 25, there is an 89% chance of an ICER ≤ $30,000/LYG, and a 93% chance of an ICER ≤ $50,000/LYG (Figure 7). Since drotrecogin alfa had a higher cost compared to placebo due to its acquisition costs, the drug was never the dominant strategy (defined by a higher effectiveness and lower costs) in our analyses. Table 7 presents the incremental cost and life-years gained with drotrecogin alfa compared to placebo in our models.
Sensitivity analyses using QALYs as the measure of effectiveness did not change the results appreciably. Similar results were obtained with varying time horizons (10–30 years) and discount rates (data not shown). Importantly in the high risk group when defined by ≥ 2 organ system failures the probability that the ICER for drotrecogin alfa compared to placebo was ≤ $50,000 was only 52%, testifying to the lack of robustness in this subgroup economic analysis.
Discussion
Our systematic review identified 2 RCTs, 3 meta-analyses, and 9 economic analyses comparing drotrecogin alfa and placebo in adults. Our results question the short term survival benefit of drotrecogin alfa and are in agreement with two previous meta-analyses [31, 32]. While much clinical attention has been previously focused on the mortality benefit seen in the PROWESS APACHE II ≥ 25 subgroup, our analysis shows that when the totality of the evidence is examined uncertainty exists as to this benefit. Supporting this conclusion is the lack of benefit in the high risk group when assessed by a different measure (≥ 2 organs dysfunction) and diminishing benefits over time. Due to the difficulties in determining the APACHE II scores [15], the European Union regulatory authorities have preferred multiple organ dysfunction as a measure of disease severity and for labeling indications [43]. Other investigators have also failed to demonstrate any survival advantage with drotrecogin alfa at hospital discharge in other high risk groups, including the need for vasopressor support, APACHE II score between 30 and 53, and protein C deficiency [28]. The multiple subgroup analyses, both pre-specified and not, of the PROWESS data [17–30] increases the possibility that the APACHE II subgroup results may represent a false positive finding.
In contrast to our results, a company-sponsored meta-analysis has reported a statistically significant reduction in the 28-day all-cause mortality in all patients with drotrecogin alfa compared to placebo [33]. However, this included studies involving indirect comparisons with different sepsis drugs and without contemporaneous controls [33]. Variations in patient entry criteria, treatments received, lack of randomization, residual confounding and the potential for calendar time bias undermines the validity of these results.
Our economic analyses showed that drotrecogin alfa may not be cost-effective in all sepsis patients due to uncertainties in the survival benefits when all available evidence is considered. Our economic conclusions differ from most previous publications, which have assumed that the large 28-day mortality advantage in PROWESS would be sustained and which has not been confirmed either in a long term follow-up or in another RCT. Our conservative estimate of cost-effectiveness, while in contradiction to other published analyses, nevertheless more adequately models the known long-term efficacy data for this drug.
Although the cost effectiveness of drotrecogin alfa improves when restricted to treating those at the highest risk, caution must be exercised in attempting to justify treatment only to a specific subgroup. Severe sepsis is a very complex syndrome [44], therefore, even in the RCTs it cannot be ruled out that some of the multiple measured and unmeasured baseline confounders may be unequally balanced with repeated subgroup analyses. Basing treatment on disease severity is problematic for several other reasons; not the least that the pooled data do not show a statistical benefit for very high risk, whether assessed by APACHE II or multiple organ failure score; second, APACHE II does not take into account important measures of severity in severe sepsis patients such as white blood cell count, number of days in hospital and ICU before the diagnosis of severe sepsis. [15].
The main limitation of our economic analyses centers on the uncertain estimate of clinical efficacy. While this been approached systematically the short term results are derived from only 2 trials with evidence of statistical heterogeneity between them and the long term results come from a single study. However as we have argued, the clinical similarities between the patient populations as well as the standardized active therapy suggests that the best estimate of clinical efficacy arises from the pooled estimate. Moreover the robustness of our model in different sensitivity analyses provides further assurance.
Conclusion
The debate on the place of drotrecogin alfa persists as judged by the numerous recently published editorials and comments [6, 7, 43, 45–58]. Hopefully our systematic and transparent economic model will assist clinicians and decision makers in assessing the current efficacy of drotrecogin alfa and its cost-effectiveness. At present, our analysis does not support the cost-effectiveness of drotrecogin alfa in all severe sepsis patients but suggests that a targeted approach to the very high risk patients may be appropriate while awaiting additional evidence.
Abbreviations
- APACHE:
-
acute physiology and chronic health evaluation
- CI:
-
confidence interval
- FDA:
-
Food and Drug Administration
- ICER:
-
incremental cost-effectiveness ratio
- LYG:
-
life-years gained
- QALY:
-
quality-adjusted life-years
- RCT:
-
randomized control trials
- RR:
-
relative risk
- WTP:
-
willingness to pay
References
Lyseng-Williamson KA, Perry CM: Drotrecogin alfa (activated). Drugs. 2002, 62: 617-30. 10.2165/00003495-200262040-00006. discussion 631-2
Food and Drug Administration: FDA Clinical Review. Drotrecogin alfa (activated). [Recombinant human activated protein C (rhAPC)] XIGRIS (TM). 2001, Last access: August 2006, [http://www.fda.gov/cder/biologics/review/droteli112101r1.pdf] . Parts 1 and 2
United States Food and Drug Administration. Drotrecogin alfa (activated) letter of approval. November 21st 2001. Last access: July 2006, [http://www.fda.gov/cder/foi/appletter/2001/droteli112101L.pdf]
European Medicines Agency. Xigris product information. [http://www.emea.eu.int/humandocs/Humans/EPAR/xigris/Xigris.htm]
Health Canada. Notice of Compliance - Xigris - 31-01-2003. Last access: July 2006, [http://www.nocdatabase.ca/]
Mackenzie AF: Activated protein C: do more survive?. Intensive Care Med. 2005, 31: 1624-6. 10.1007/s00134-005-2829-4.
Smith B: Drotrecogin alfa: too early to give it early. Emerg Med Australas. 2005, 17: 179-80. 10.1111/j.1742-6723.2005.00713.x.
Angus DC, Laterre PF, Helterbrand J, Ely EW, Ball DE, Garg R, Weissfeld LA, Bernard GR: The effect of drotrecogin alfa (activated) on long-term survival after severe sepsis. Crit Care Med. 2004, 32: 2199-206. 10.1097/01.CCM.0000114816.62331.08.
Drabinski A, Williams G, Formica C: Observational evaluation of health state utilities among a cohort of sepsis patients. Value in Health. 2001, 4: 128-9. 10.1046/j.1524-4733.2001.40202-165.x.
Baltussen RMPM, Hutubessy RCW, Evans DB, Murray CJM: Uncertainty in cost-effectiveness analysis. International Journal of Technology Assessment in Health Care. 2002, 18: 112-9.
Glick HA, Briggs AH, Polsky D: Quantifying stochastic uncertainty and presenting results of cost-effectiveness analyses. Expert Rev. Pharmacoeconomics Outcome Res. 2001, 1: 25-36. 10.1586/14737167.1.1.25.
Stinnett AA, Mullahy J: Net Health Benefits: A New Framework for the Analysis of Uncertainty in Cost-Effectiveness Analysis. Medical Decision Making. 1998, 18: S68-S80. 10.1177/0272989X9801800209.
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ: Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001, 344: 699-709. 10.1056/NEJM200103083441001.
Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, Francois B, Guy JS, Bruckmann M, Rea-Neto A, Rossaint R, Perrotin D, Sablotzki A, Arkins N, Utterback BG, Macias WL: Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005, 353: 1332-41. 10.1056/NEJMoa050935.
Johnston JA: Determinants of mortality in patients with severe sepsis. Med Decis Making. 2005, 25: 374-86. 10.1177/0272989X05278933.
Bernard GR, Macias WL, Joyce DE, Williams MD, Bailey J, Vincent JL: Safety assessment of drotrecogin alfa (activated) in the treatment of adult patients with severe sepsis. Crit Care. 2003, 7: 155-63. 10.1186/cc2167.
Laterre PF, Garber G, Levy H, Wunderink R, Kinasewitz GT, Sollet JP, Maki DG, Bates B, Yan SC, Dhainaut JF: Severe community-acquired pneumonia as a cause of severe sepsis: data from the PROWESS study. Crit Care Med. 2005, 33: 952-61. 10.1097/01.CCM.0000162381.24074.D7.
Macias WL, Vallet B, Bernard GR, Vincent JL, Laterre PF, Nelson DR, Derchak PA, Dhainaut JF: Sources of variability on the estimate of treatment effect in the PROWESS trial: implications for the design and conduct of future studies in severe sepsis. Crit Care Med. 2004, 32: 2385-91. 10.1097/01.CCM.0000147440.71142.AC.
Barie PS, Williams MD, McCollam JS, Bates BM, Qualy RL, Lowry SF, Fry DE: Benefit/risk profile of drotrecogin alfa (activated) in surgical patients with severe sepsis. Am J Surg. 2004, 188: 212-20. 10.1016/j.amjsurg.2004.06.008.
Ely EW, Laterre PF, Angus DC, Helterbrand JD, Levy H, Dhainaut JF, Vincent JL, Macias WL, Bernard GR: Drotrecogin alfa (activated) administration across clinically important subgroups of patients with severe sepsis. Crit Care Med. 2003, 31: 12-9. 10.1097/00003246-200301000-00002.
Dhainaut JF, Laterre PF, LaRosa SP, Levy H, Garber GE, Heiselman D, Kinasewitz GT, Light RB, Morris P, Schein R, Sollet JP, Bates BM, Utterback BG, Maki D: The clinical evaluation committee in a large multicenter phase 3 trial of drotrecogin alfa (activated) in patients with severe sepsis (PROWESS): role, methodology, and results. Crit Care Med. 2003, 31: 2291-301. 10.1097/01.CCM.0000085089.88077.AF.
Dhainaut JF, Laterre PF, Janes JM, Bernard GR, Artigas A, Bakker J, Riess H, Basson BR, Charpentier J, Utterback BG, Vincent JL: Drotrecogin alfa (activated) in the treatment of severe sepsis patients with multiple-organ dysfunction: data from the PROWESS trial. Intensive Care Med. 2003, 29: 894-903.
Ely EW, Angus DC, Williams MD, Bates B, Qualy R, Bernard GR: Drotrecogin alfa (activated) treatment of older patients with severe sepsis. Clin Infect Dis. 2003, 37: 187-95. 10.1086/375775.
Dhainaut JF, Yan SB, Joyce DE, Pettila V, Basson B, Brandt JT, Sundin DP, Levi M: Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004, 2: 1924-33. 10.1111/j.1538-7836.2004.00955.x.
Shorr AF, Bernard GR, Dhainaut JF, Russell JR, Macias WL, Nelson DR, Sundin DP: Protein C concentrations in severe sepsis: an early directional change in plasma levels predicts outcome. Crit Care. 2006, 10: R92-10.1186/cc4946.
Levy H, Laterre PF, Bates B, Qualy RL: Steroid use in PROWESS severe sepsis patients treated with drotrecogin alfa (activated). Crit Care. 2005, 9: R502-7. 10.1186/cc3778.
Cone LA, Stone RA, Jesser K, Nelson R: Clinical experience with drotrecogin alfa in treating gram-positive and -negative pathogens in patients with severe sepsis. J Investig Med. 2004, 52: 470-4.
Laterre PF, Levy H, Clermont G, Ball DE, Garg R, Nelson DR, Dhainaut JF, Angus DC: Hospital mortality and resource use in subgroups of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial. Crit Care Med. 2004, 32: 2207-18.
Opal SM, Garber GE, LaRosa SP, Maki DG, Freebairn RC, Kinasewitz GT, Dhainaut JF, Yan SB, Williams MD, Graham DE, Nelson DR, Levy H, Bernard GR: Systemic host responses in severe sepsis analyzed by causative microorganism and treatment effects of drotrecogin alfa (activated). Clin Infect Dis. 2003, 37: 50-8. 10.1086/375593.
Vincent JL, Angus DC, Artigas A, Kalil A, Basson BR, Jamal HH, Johnson G, Bernard GR: Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit Care Med. 2003, 31: 834-40. 10.1097/01.CCM.0000051515.56179.E1.
Wiedermann CJ, Kaneider NC: A meta-analysis of controlled trials of recombinant human activated protein C therapy in patients withsepsis. BMC Emerg Med. 2005, 5: 7-10.1186/1471-227X-5-7.
Friedrich JO: Drotrecogin alfa (activated) in severesepsis. N Engl J Med. 2006, 354: 94-6. 10.1056/NEJMc052759. author reply 94-6
Sashegyi A, Trzaskoma BL, Nelson DR, Williams MD, Macias W: International INtegrated Database for the Evaluation of severe sePsis and drotrecogin alfa (activated) THerapy: component trials and statistical methods for INDEPTH. Curr Med Res Opin. 2006, 22: 1001-12. 10.1185/030079906X104713.
Fowler RA, Hill-Popper M, Stasinos J, Petrou C, Sanders GD, Garber AM: Cost-effectiveness of recombinant human activated protein C and the influence of severity of illness in the treatment of patients with severe sepsis. J Crit Care. 2003, 18: 181-91. 10.1016/j.jcrc.2003.08.009. discussion 191-4
Riou Franca L, Launois R, Le Lay K, Aegerter P, Bouhassira M, Meshaka P, Guidet B: Cost-effectiveness of drotrecogin alfa (activated) in the treatment of severe sepsis with multiple organ failure. Int J Technol Assess Health Care. 2006, 22: 101-8.
Hjelmgren J, Persson U, Tennvall GR: Local treatment pattern versus trial-based data: a cost-effectiveness analysis of drotrecogin alfa (activated) in the treatment of severe sepsis in Sweden. Am J Ther. 2005, 12: 425-30. 10.1097/01.mjt.0000141605.27943.8d.
Davies A, Ridley S, Hutton J, Chinn C, Barber B, Angus DC: Cost effectiveness of drotrecogin alfa (activated) for the treatment of severe sepsis in the United Kingdom. Anaesthesia. 2005, 60: 155-62. 10.1111/j.1365-2044.2004.04068.x.
Betancourt M, McKinnon PS, Massanari RM, Kanji S, Bach D, Devlin JW: An evaluation of the cost effectiveness of drotrecogin alfa (activated) relative to the number of organ system failures. Pharmacoeconomics. 2003, 21: 1331-40. 10.2165/00019053-200321180-00004.
Neilson AR, Burchardi H, Chinn C, Clouth J, Schneider H, Angus D: Cost-effectiveness of drotrecogin alfa (activated) for the treatment of severe sepsis in Germany. J Crit Care. 2003, 18: 217-27. 10.1016/j.jcrc.2003.10.005.
Manns BJ, Lee H, Doig CJ, Johnson D, Donaldson C: An economic evaluation of activated protein C treatment for severe sepsis. N Engl J Med. 2002, 347: 993-1000. 10.1056/NEJMsa020969.
Angus DC, Linde-Zwirble WT, Clermont G, Ball DE, Basson BR, Ely EW, Laterre PF, Vincent JL, Bernard G, van Hout B: Cost-effectiveness of drotrecogin alfa (activated) in the treatment of severe sepsis. Crit Care Med. 2003, 31: 1-11. 10.1097/00003246-200301000-00001.
Green C, Dinnes J, Takeda AL, Cuthbertson BH: Evaluation of the cost-effectiveness of drotrecogin alfa (activated) for the treatment of severe sepsis in the United Kingdom. Int J Technol Assess Health Care. 2006, 22: 90-100.
Carlet J: Prescribing indications based on successful clinical trials in sepsis: a difficult exercise. Crit Care Med. 2006, 34: 525-9. 10.1097/01.CCM.0000198329.85851.8E.
Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, Payne E, Cuthbertson BH: Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris) for the treatment of severe sepsis in adults: a systematic review and economic evaluation. Health Technol Assess. 2005, 1-126.
Mackenzie AF: Conflict of opinion – is PROWESS real progress?. Intensive Care Med. 2006, 32: 610-2. 10.1007/s00134-006-0090-0.
Wiedermann CJ: When a single pivotal trial should not be enough-the case of drotrecogin-alfa (activated). Intensive Care Med. 2006, 32: 604-10.1007/s00134-006-0086-9.
Taylor B, Burns D, van de Wal BW, MBewu T, Keeton GR: Should health care money in South Africa be spent on drotrecogin alfa?. S Afr Med J. 2003, 93: 500-1.
Carlet J: A blind clinical evaluation committee should, in theory, make data of a randomized clinical trial stronger, not weaker. Intensive Care Med. 2004, 30: 994-10.1007/s00134-004-2245-1. author reply 995
Manocha S, Walley KR: Activated protein C: beyond 28 days. Crit Care Med. 2004, 32: 2348-9.
Vincent JL: Sepsis in the ICU: who needs progress?. Intensive Care Med. 2006, 32: 609-10.1007/s00134-006-0089-6.
Hodgson RE, Williams PG, Foden AP, Grolman D: Drotrecogin alfa (activated) in South African private hospital ICUs. S Afr Med J. 2003, 93: 474-5.
Levi M: Benefit of recombinant human activated protein C beyond 28-day mortality: there is more to life than death. Crit Care Med. 2003, 31: 984-5. 10.1097/01.CCM.0000055386.01914.0C.
Dellinger RP: Recombinant activated protein C: decisions for administration. Crit Care Med. 2006, 34: 530-1. 10.1097/01.CCM.0000199068.67569.B3.
Dellinger RP: Recombinant activated protein C: the key is clinical assessment of risk of death, not subset analysis. Crit Care Med. 2006, 10:
Morris PE: Meeting unmet needs in patients with sepsis: the role of drotrecogin alfa (activated). Am J Crit Care. 2003, 12: 94-7.
Eichacker PQ, Natanson C, Danner RL: Surviving sepsis – practice guidelines, marketing campaigns, and Eli Lilly. N Engl J Med. 2006, 355: 1640-2. 10.1056/NEJMp068197.
Friedrich JO, Adhikari NK, Meade MO: Drotrecogin alfa (activated): does current evidence support treatment for any patients with severe sepsis?. Crit Care. 2006, 10: 145-10.1186/cc4947.
Eichacker PQ, Natanson C: Recombinant human activated protein C in sepsis: inconsistent trial results, an unclear mechanism of action, and safety concerns resulted in labeling restrictions and the need for phase IV trials. Crit Care Med. 2003, 31: S94-6. 10.1097/00003246-200301001-00013.
Statistics Canada. Life tables, Canada andprovinces and territories 2000–2002. Last access: August 2006, [http://www.statcan.ca/english/freepub/84-537-XIE/tables.htm]
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2253/7/5/prepub
Acknowledgements
Dr. Brophy is a funded scholar of les Fonds de la Recherche en Santé du Québec (FRSQ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
JB designed the study. VC conducted the systematic literature search, reviewed the studies identified, and extracted the data from the eligible studies. JB reviewed the eligibility of the studies identified, and the data extraction. JB and VC carried-out the meta-analyses, economic evaluations and economic modeling. JB and VC prepared the manuscript. All authors read and approved the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Costa, V., Brophy, J.M. Drotrecogin alfa (activated) in severe sepsis: a systematic review and new cost-effectiveness analysis. BMC Anesthesiol 7, 5 (2007). https://doi.org/10.1186/1471-2253-7-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2253-7-5