Abstract
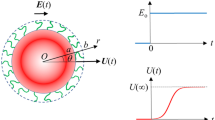
We study theoretically the phoretic motion of a spheroidal particle, which generates solute gradients in the surrounding unbounded solvent via chemical reactions active on its surface in a cap-like region centered at one of the poles of the particle. We derive, within the constraints of the mapping to classical diffusio-phoresis, an analytical expression for the phoretic velocity of such an object. This allows us to analyze in detail the dependence of the velocity on the aspect ratio of the polar and the equatorial diameters of the particle and on the fraction of the particle surface contributing to the chemical reaction. The particular cases of a sphere and of an approximation for a needle-like particle, which are the most common shapes employed in experimental realizations of such self-propelled objects, are obtained from the general solution in the limits that the aspect ratio approaches one or becomes very large, respectively.
Similar content being viewed by others
References
R.F. Ismagilov, A. Schwartz, N. Bowden, G.M. Whitesides, Angew. Chem., Int. Ed. 41, 652 (2002)
W.E. Paxton, K.C. Kistler, C.C. Olmeda, A. Sen, S.K. St. Angelo, Y. Cao, T.E. Mallouk, P.E. Lammert, V.H. Crespi, J. Am. Chem. Soc. 126, 13424 (2004)
J.M. Catchmark, S. Subramanian, A. Sen, Small 1, 1 (2005)
J.R. Howse, R.A.L. Jones, A.J. Ryan, T. Gough, R. Vafabakhsh, R. Golestanian, Phys. Rev. Lett. 99, 048102 (2007)
A. Erbe, M. Zientara, L. Baraban, C. Kreidler, P. Leiderer, J. Phys.: Condens. Matter 20, 404215 (2008)
L. Baraban, C. Kreidler, D. Makarov, P. Leiderer, A. Erbe, arXiv:0807.1619v1
W.E. Paxton, S. Sundararajan, T.E. Mallouk, A. Sen, Angew. Chem., Int. Ed. 45, 5420 (2006)
R. Golestanian, T.B. Liverpool, A. Ajdari, Phys. Rev. Lett. 94, 220801 (2005)
N. Bala Saidulu, K.L. Sebastian, J. Chem. Phys. 128, 074708 (2008)
W.E. Paxton, A. Sen, T.E. Mallouk, Chem. Eur. J. 11, 6462 (2005)
G. Rückner, R. Kapral, Phys. Rev. Lett. 98, 150603 (2007)
R. Golestanian, T.B. Liverpool, A. Ajdari, New J. Phys. 9, 126 (2007)
J.L. Anderson, Annu. Rev. Fluid Mech. 21, 61 (1989)
M.N. Popescu, S. Dietrich, G. Oshanin, J. Chem. Phys. 130, 194702 (2009)
F. Juelicher, J. Prost, Eur. Phys. J. E 29, 27 (2009)
As noticed in the Introduction, in doing so one is bound by a number of assumptions which are either already present in the classical theory of phoresis or arise as a result of the mapping of such “active” surface particles into the framework of a theory developed to describe the case of inert particles immersed in a pre-defined, externally controlled concentration gradient. These assumptions are discussed in detail in ref. Popescu_2009. For the purpose of the present work, we simply assume that such a mapping is possible and thus we subscribe to these assumptions.
A.B. Pawar, I. Kretzschmar, Langmuir 24, 355 (2008)
A.B. Pawar, I. Kretzschmar, Langmuir 25, 9057 (2009)
T. Ohta, T. Ohkuma, Phys. Rev. Lett. 102, 154101 (2009)
J. Happel, H. Brenner, Low Reynolds Number Hydrodynamics (Noordhoff International, Leyden, 1973), chapts. 4-26, 4-27, 4-30, and 4-31
A. Einstein, On the Movement of Small Particles Suspended in a Stationary Liquid Demanded by the Molecular-Kinetic Theory of Heat, in Investigations on the theory of the Brownian motion, edited by R. Fürth, translated by A.D. Cowper (Dover, New York, 1956)
A. Ajdari, L. Bocquet, Phys. Rev. Lett. 96, 186102 (2006)
Besides the translation described by $\mathbf{V}$, in the most general case a term accounting for a rigid-body rotation of the particle with angular velocity $\bm{\Omega}$ should also be considered. However, this angular velocity turns out to be identically zero in most cases in which the particle has homogeneous surface properties Anderson_1989,Morrison_1970. The azimuthal symmetry of our system and the additional assumption that the properties of the catalyst-covered surface are similar to those of the inert part (as far as the particle-solute effective interaction is concerned) ensures that we are dealing with such a case
F.A. Morrison jr., J. Colloid Interface Sci. 34, 210 (1970)
G.R. Willmott, Phys. Rev. E 79, 066309 (2009)
G.R. Willmott, Phys. Rev. E 77, 055302(R) (2008)
J.L. Anderson, J. Colloid Interface Sci. 105, 45 (1985)
M.C. Fair, J.L. Anderson, J. Colloid Interface Sci. 127, 388 (1989)
J. Happel, H. Brenner, Low Reynolds Number Hydrodynamics (Noordhoff International, Leyden, 1973), chapt.3-5, pp. 85-87
R. Golestanian, Phys. Rev. Lett. 102, 188305 (2009)
M. Abramowitz, I.A. Stegun, Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables (Dover, New York, 1965), p. 752
H.F. Bauer, J. Thermal Anal. 35, 1571 (1989)
W.R. Smythe, Static and Dynamic Electricity (McGraw-Hill, New York, 1968), chapts. 5.21–5.28
E.W. Hobson, The Theory of Spherical and Ellipsoidal Harmonics (Chelsea, New York, 1965), chapt. II
M. Abramowitz, I.A. Stegun, Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables (Dover, New York, 1965), p. 332
R.E. Collin, Field Theory of Guided Waves (McGraw-Hill, New York, 1960), pp. 553–570
F. Pomer, J. Navasquillo, J. Electrostatics 22, 309 (1989)
The numerical calculations have been performed using the software Mathematica (version 7.01), for which the Legendre function $Q_{\ell}(x)$ for arguments $x > 1$ or $x \in \mathbb{C} \setminus \mathbb{R}$ is implemented as “the Legendre function Q of type 3” LegendreQ$[\ell,0,x,3]$
M. Abramowitz, I.A. Stegun, Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables (Dover, New York, 1965), p. 774
H. Brenner, Chem. Eng. Sci. 19, 703 (1964)
M. Abramowitz, I.A. Stegun, Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables (Dover, New York, 1965), p. 557
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popescu, M.N., Dietrich, S., Tasinkevych, M. et al. Phoretic motion of spheroidal particles due to self-generated solute gradients. Eur. Phys. J. E 31, 351–367 (2010). https://doi.org/10.1140/epje/i2010-10593-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epje/i2010-10593-3