Abstract
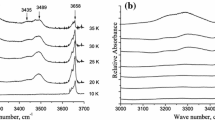
The topology and energetic characteristics of hydrogen bonded clusters in methanol and methanol-heptane mixtures are investigated by IR spectroscopy over a wide temperature range. The average number of hydrogen bonds per methanol molecule was calculated. Based on an analysis of changes in the spectral parameters of O...H intramolecular bonds, conclusions on structural rearrangements in the mixture under study at elevated temperatures are drawn.
Similar content being viewed by others
References
S. J. Barlow, G. V. Bondarenko, Yu. E. Gorbaty, T. Yamaguchi, and M. J. Poliakoff, Phys. Chem. A 106, 10452 (2002).
M. Sokolova, S. J. Barlow, G. V. Bondarenko, Yu. E. Gorbaty, and M. J. Poliakoff, Phys. Chem. A 110, 3882 (2006).
Yu. E. Gorbaty and G. V. Bondarenko, SKF-TP 2(2), 5 (2007).
Yu. E. Gorbaty and G. V. Bondarenko, Vestn. Otd. Nauk Zemle RAN 1(26), 1 (2008).
T. Yamaguchi, C. J. Benmore, and A. K. Soper, J. Chem. Phys. 112, 8976 (2000).
S. P. Krishtal’, Candidate’s Dissertation in Chemistry (IKhR RAN, Ivanovo, 2004).
S. Krishtal, M. G. Kiselev, A. M. Kolker, and A. Idrissi, Theor. Chem. Acc. 117, 297 (2007).
D. V. Ivlev, A. A. Dyshin, M. G. Kiselev, and A. M. Kolker, Russ. J. Phys. Chem. A 84, 2077 (2010).
N. Asahi and Y. Nakamura, J. Chem. Phys. 109, 9879 (1998).
D. Dellis, M. Chalaris, and J. Samios, J. Phys. Chem. B 109, 18575 (2005).
G. V. Bondarenko and Yu. E. Gorbaty, Geochim. Cosmochim. Acta 61, 1413 (1997).
D. V. Ivlev, A. A. Dyshin, M. G. Kiselev, and O. V. Eliseeva, SKF-TP 2, 70 (2007).
L. Stavely and P. Taylor, J. Chem. Soc. 65, 200 (1956).
J. L. E. Chevalier, P. J. Petrino, and Y. M. J. Gaston-Bonhome, Chem. Eng. Data 35, 206 (1990).
I. A. Luk’yanchikova, Candidate’s Dissertation in Chemistry (IKhNR RAN, Ivanovo, 1995).
D. V. Ivlev and M. G. Kiselev, Russ. J. Phys. Chem. A 75, 71 (2001).
Yu. E. Gorbaty, SKF-TP 2(1), 40 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Dyshin, R.D. Oparin, M.G. Kiselev, 2012, published in Sverkhkriticheskie Flyuidy: Teoriya i Praktika, 2012, Vol. 7, No. 2, pp. 67–74.
Rights and permissions
About this article
Cite this article
Dyshin, A.A., Oparin, R.D. & Kiselev, M.G. Order structure of methanol along the 200-bar isobar in the temperature range of 60–320°C according to IR spectroscopy. Russ. J. Phys. Chem. B 6, 868–872 (2012). https://doi.org/10.1134/S1990793112080106
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793112080106