Abstract
A method has been advanced for the quantitative separation of the degradation channels of the electron-excitation energy of rare-earth activators, which is based on measurements of the duration of activator luminescence and of the absorption coefficient of OH-groups in concentration series of glasses. The optimal neodymium concentration has been established for obtaining the maximum gain in phosphate glasses. Preliminary lasing tests of the concentration series of phosphate and also silicate glass with a neodymium concentration of approximately 2.3 × 1020 ions cm–3 have been performed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nonradiative deactivation of the excited levels of rare-earth activators (REA) with a growth in the concentration of the latter and an enhancement of the excitation-energy migration is determined chiefly by two factors: the exchange of excitation energy for valence vibrations of impurity OH-groups and the quenching of the luminescence of the REA according to the cross-relaxation scheme. The physical mechanisms of these processes have been investigated in detail in [1, 2].
The present communication advances a method for the quantitative separation of each of the degradation channels indicated above for the electron-excitation energy. The method is based on measuring the duration T of the activator luminescence and the absorption coefficient of the OH-groups in concentration series of glasses, each of which includes a glass having a constant activator concentration and a variable concentration of the OH-groups. Moreover, the possibility is investigated of obtaining continuous-wave lasing on neodymium glasses with high and low cross sections of stimulated radiation over a wide range of concentrations.
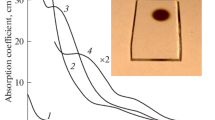
Figure 1 displays the dependences of 1/τ on the absorption coefficients KOH for glasses activated with Nd3+ and Yb3+–Er3+. From Fig. la, it is evident that for each fixed concentration N of neodymium, the dependence of 1/τ on KOH is linear. The slope angles of the straight lines increase with increasing N. The tangents of the slope angles made by the straight lines with the axis of ordinates characterize the quenching of neodymium luminescence by the OH-groups which is due to migration of the excitation along the activator ions to the OH-groups. The values of 1/τ intercepted by the straight lines on the axis of ordinates, less the probability 1/τ0 of radiative transitions, characterize the quenching of neodymium via the cross-relaxation scheme. Under these conditions, the value of 1/τ corresponding to the minimum activator concentration N ∼ 0.5 × 1020 ions cm–3 coincides with the radiative probability of 1/τ of neodymium which is equal to 2500 s–1 and has been calculated for barium-lead-phosphate glass of the GLS 22 type according to the Judd theory [3] and from the q/τ relationship.
Dependence of the probability of deactivation of the 4F3/2 level of neodymium (a) and the 4I13/2 level of erbium (b) on the absorption coefficient of OH-groups in phosphate glass at a wavelength of approximately 3.4 μm: (a) neodymium concentration N × 10–20 cm–3: (1) 0.56; (2) 1.12; (3) 2.24; (4) 3.37; (5) 4.3; (6) 9.3; (7) 12.95; (b) erbium concentration N × 10–19 cm–3: (1) 4.7; (2) 8; (3) 12; (4) 4.7; neodymium concentration N × 10–19 cm–3; (4) 2.
In the case of phosphate glasses that are doubly activated with Yb3+–Er3+ (Fig. 1b), the slope angles or the analogous straight lines likewise increase with a growth of erbium concentration, but the lines converge to one point on the axis of ordinates corresponding to the radiative probability of erbium which is equal to approximately 116 s–1. When neodymium is introduced into glass containing erbium (Fig. 1b, the straight line 4), the quenching of erbium by hydroxyl groups is weakened, which is evidenced by the decrease in the slope angle of the straight line 4 with respect to the axis of ordinates, but the intercept of the indicated straight line on the axis of ordinates is shifted in the direction of increasing 1/τ. The difference between the values of 1/τ, (for KOH = 0) for the straight lines 1–3 (Fig. lb) and the straight line 4 characterizes the probability of erbium quenching by neodymium in the absence of hydroxyl groups in the glass.
Figure 2 shows the concentration dependences of the probability of nonradiative deactivation of the 4F3/2 level of neodymium which were obtained from the data of Fig. 1a. Curve 1 (Fig. 2a) was plotted from points corresponding to the values of 1/τ – 1/τ0 for KOH = 0 and characterizes the quenching of neodymium via the cross-relaxation scheme. Curves 2–4 were obtained from the difference between the values of 1/τ taken from the dependence 1/τ = 1/(KOH) at the values KOH = 5, 10, 20 cm–1 and KOH = 0 for each fixed activator concentration and characterize the concentration quenching of neodymium for a specified water content of the glass.
Dependence of the deactivation probability W of the 4F3/2 level of neodymium on Nd3+ concentration (a) and on the square of the concentration (b): (a) (1) KOH = 0, W = \(\frac{1}{\tau }\) – \(\frac{1}{{{{\tau }_{0}}}}\); (2) KOH = 5 cm–1; (3) KOH = 10 cm–1; (4) KOH = 20 cm–1; (2–4) W = \(\frac{1}{\tau }\) – \(\frac{1}{{{{\tau }_{1}}}}\), where τ is the measured lifetime of Nd (μs), τ0 is the radiative lifetime of Nd (μsec), τ1 is the lifetime of Nd (μs) for KOH = 0 (Fig. 1a); (b) KOH = 0. The absorption coefficients were calculated using common logarithms.
The probability of quenching via the cross-relaxation scheme depends quadratically on the Nd3+ concentration, although for values of N ≤ (3–4) × 1020 ions cm–3 for which the migration of the excitation energy is still low, this dependence may be approximated as a linear dependence (curve 1, Fig. 2a) [4]. Quenching of neodymium luminescence by water (Fig. 2a, curves 2–4), on the contrary, corresponds to a linear dependence of W on N which for N ≤ (3–4) × 1020 ions cm–3 and a reduction of the excitation-energy migration along neodymium ions goes over into a quadratic dependence. As a whole, it follows from Fig. 2a that in glass having a value of KOH ∼ 5 cm–1, the quenching of neodymium luminescence by hydroxyl groups predominates over quenching via the cross-relaxation scheme for an activator concentration of up to approximately 7 × 1020 ions cm–3. At higher activator concentrations, cross-relaxation becomes predominant.
The revealed regularities allowed establishment of an optimal neodymium concentration for obtaining the maximum gain Kam for dehydrated glasses and determining its dependence on their content of OH‑groups. Figure 3 shows the dependence of the quantity Nq, which is proportional to the gain (for a pumping duration that is comparable with τNd), on neodymium concentration in the investigated glass and in vitreous lanthanum pentaphosphate which has the lowest concentration quenching of the well-known glasses [5]. From Fig. 3, it follows that the maximum Kam is realized in all the glasses for N ∼ 8 × 1020 ions cm–3. In the glass having KOH = 5 cm–1, the gain is reduced by a factor of 1.5 compared with the dehydrated glass, and its dependence on activator concentration is weakened. In the glass having the composition La2O3⋅5P2O5 with KOH = 1 cm–1, an increase in neodymium concentration from 8 × 1020 to 25 × 1020 ions cm–3 has practically no effect on the value of Kam.
Thus, a neodymium concentration N = 8 × 1020 ions cm–3 ensures the maximum gain in glasses having both conventional and reduced concentration quenching. For the condition requiring total utilization of the pumping light, this concentration is optimal for active elements having a diameter of 1–5 mm, and evidently for activated fibers. A further increase in neodymium content leads merely to a growth of the absorption in the region of 1.06 μm, which for N = 5.7 × 1020 O ions cm–3 ( GLS–24) glass already amounts to 6 × 10–4 cm–l [6]. Only in the case of activated films, where high activator absorption coefficients are required, does the introduction of ultrahigh neodymium concentrations become justified.
However, even here, one should take into account the fact that the cross sections σ of the stimulated emission in the La2O3⋅5P2O5 glass and, for example, in the investigated barium-lead-phosphate glass of the GLS-22 type amount to 2.3 × 10–20 and 3.2 × 10–20 cm2, respectively [3, 6], according to the spectroscopic procedure for determining this cross section. Therefore, the advantage in quantum yield may not compensate the low value of σ.
Based on the concentration-quenching results obtained, a phosphate glass KGSS-083 was developed which allows the introduction of high concentrations of neodymium in place of lanthanum up to approximately 13 × 1020 ions cm–3 and has good technological properties. The processes of nonradiative deactivation of the 4F3/2 level in these glasses are relatively weak, which makes them comparable to crystals which are being searched for in order to create miniature lasers. The glass has the following characteristics: refractive index nc = 1.5807, nF = 1.5904; number of' neodymium ions per cubic centimeter 12.7 × 1020; absorption index K586 ∼ 1.63, K874 ∼ 0.300; stimulated-emission cross section (spectroscopic measurements) approximately 3 × 10–20 cm2; duration or luminescence attenuation approximately 110 μsec; optical stress coefficient 1.4 nm cm kg–1; nonactive absorption K1.06 = 0.002 cm–1. Under production conditions, a concentration series has been synthesized on the basis of the given glass with a neodymium concentration or 2, 3, 4, 5, 6, 8, 10, and 12.7 ions cm–3.
The lasing experiments were preliminary in character and had as their purpose confirmation of the possibility of continuous-wave lasing in a small volume using glasses activated with neodymium. Continuous-wave lasing in the presence of longitudinal pumping by a Spectra-Physics 171-01 Kr laser was obtained for all samples of the concentration series of phosphate glasses, and likewise for silicate glass with a neodymium concentration of approximately 2.3 × 1020 cm–3. In order to reduce the overheating of the samples with light, the 0.752 μm radiation line was mainly used for pumping. The absorption coefficient, on this wavelength varied from 2.0 to 12.7 cm–1 depending on neodymium concentration. In accordance with this, the thickness of the samples for the lasing experiments was chosen to range from 0.5 to 3 mm. The resonator, which was formed by two plane mirrors that were pressed directly against the surfaces of the samples, was retuned for each investigated sample. The reflection coefficient of the mirrors at the wavelength 1.06 μm amounted to approximately 98 and 99.5%. The transmission of the mirrors at the pumping wavelength amounted to approximately 70%. The lasing which was obtained was highly directional with a divergence of the order of several degrees and was registered by means of an image converter as well as by a Spectra-Physics 404 power meter on the decaying section of its spectral-sensitivity curve.
For samples having a neodymium concentration N = 8 × 1020 cm–3 and higher, lasing was obtained for pumping with an unfocused laser using a spot of approximately 1.5 mm on the sample. The lasing threshold and yield depended essentially on the tuning and were determined by the losses in the resonator. For samples having a lower concentration, the radiation from the pumping laser was focused by a lens up to approximately 300 μm. The continuous-wave character of the lasing, which was preserved for several hours without any signs of deterioration, allowed the lasing spectrum to be recorded on a SDL-1 spectrometer.
Figure 4 shows the luminescence and lasing spectra of a sample having N = 8 × 1020 cm–3. The positions and intensities of the narrow lines in the lasing spectrum turned out to be unstable in time as a consequence of the fluctuations of the resonator modes.
Thus, it has been demonstrated that continuous-wave lasing from Nd3+ on glasses in the presence of laser excitation may be obtained not only on high-concentration phosphate or tellurite glasses [7], but also on glasses having a low neodymium content, including silicate glasses, which have low stimulated-emission cross sections.
Change history
14 March 2024
An Erratum to this paper has been published: https://doi.org/10.1134/S1062873823390016
REFERENCES
Gapontsev, V.P., Sverchkov, Yu.E., Gromov, A.K., et al., Pis’ma Zh. Eksp. Teor. Fiz., 1979, vol. 29, p. 234.
Avanesov, A.G. , Basiev, T.T. , Voron’ko, Yu.K., et al., Preprint of the Phys. Inst, Russ. Acad. Sci., Moscow, 1979, no. 15.
Brachkovskaya, N.B., Grubin, A.A., Lunter, S.G., et al., Sov. J. Quantum Electron., 1975, vol. 6, no. 5, p. 534.
Lunter, S.G., Mironov, A.N., and Fedorov, Yu.K., Abstracts of Papers, Second All-Union Conference on Laser Optics, Leningrad, 1980, p. 226.
Alekseev, N.E., Gapontsev, V.P., Gromov, A.K., et al., Radiotekh. Elektron., 1978, vol. 23, no. 9, p. 1896.
Ageeva, L.E., Brachkovskaya, N.B., Lunter, S.G., et al., Sov. J. Quantum Electron., 1977, vol. 7, p. 1379.
Michel, J.C., Morin, D. , and Auzel, F., Rev. Phys. Appl., 1978, vol. 13, p. 859.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Modifications have been made to the dates of received, revised and accepted. Full information regarding the corrections made can be found in the erratum/correction for this article.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ekimov, A.I., Lunter, S.G., Mironov, A.N. et al. Spectroscopic and Lasing Properties of Highly Concentrated Glasses. Bull. Russ. Acad. Sci. Phys. 87, 1762–1765 (2023). https://doi.org/10.1134/S1062873823370013
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062873823370013