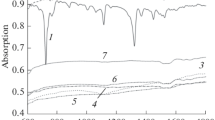
Abstract
The morphology of the surface of thin films of uranium bis-phthalocyanine and its pyrolysed derivatives is analyzed by atomic-force microscopy. During the pyrolysis of samples in an inert atmosphere (400–800°C), the transition from a crystalline to amorphous carbon structure with immobilized metal atoms is observed in uranium bis-phthalocyanine thin films deposited onto a substrate. It is found that at temperatures above 1000°C the pyrolysis is accompanied by the aggregation of nanoscale particles and the formation of ultraporous matrices with a high specific surface area (∼102 m2/g).
Similar content being viewed by others
References
J.-L. Desvaux, in Proc. International Conference on Radioactive Waste Management and Environmental Remediation ICEM'97 (ASME, Singapore, 1997), p.813.
Yu. V. Glagolenko, E. G. Dzekun, E. G. Drozhko, et al., Vopr. Radiats. Bezop., No. 2, 3 (1996).
Australian Nuclear Science and Technology Organization. http://www.ansto.gov.au/BusinessServices/ANSTOSynroc/index.htm.
D. G. Bennett, J. J. W. Higgo, and S. M. Wickham, Review of Waste Immobilization Matrices (Galson Science, Oakham, 2001).
I. A. Andryushin and Yu. A. Yudin, Management of Radioactive Waste and Nuclear Fuels Radioactive Waste: Review of Problems (Russian Federal Nuclear Center -All-Russian Research Institute of Experimental Physics, Sarov, 2010) [in Russian].
S. V. Stefanovskii, Yu. M. Kulyako, S. V. Yudintsev, et al., Vopr. Radiats. Bezop., No. 1, 15 (2002).
V. I. Tikhonov, P. N. Moskalev, and V. K. Kapustin, in Proc. 11th International Conference on Environmental Remediation and Radioactive Waste Management ICEM-2007 (Bruges, 2008), Rep. No. 7084.
V. I. Tikhonov, V. K. Kapustin, and P. N. Moskalev, RF Patent No. 2343575 (2007).
V. I. Tikhonov, V. K. Kapustin, V. T. Lebedev, et al., Radiochemistry 58 (5), 545 (2016).
V. Yu. Bairamukov, D. V. Lebedev, and V. I. Tikhonov, in Proc. International Multidisciplinary Microscopy Congress, Antalya, 2013, Springer Proceedings in Physics (Springer Int., 2014), Vol. 154, p.189.
D. Graw, Diphthalocyaninato-thorium(IV) and -uranium(IV), D. F. Lux, D. Dempf, D. Graw, Eds., Angew. Chem. Internat. 7 (10), 819 (1968).
A. E. Sovestnov, V. K. Kapustin, V. I. Tikhonov, et al., Phys. Solid State 56 (8), 1673 (2014).
I. S. Kirin, P. N. Moskalev, and Yu. A. Makashev, Russ. J. Inorg. Chem., No. 10, 1065 (1965).
V. M. Lebedev, V. T. Lebedev, D. N. Orlova, et al., J. Surf. Invest.: X-ray, Synchrotron Neutron Tech. 8 (5), 1002 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.Yu. Bairamukov, V.T. Lebedev, V.I. Tikhonov, 2018, published in Poverkhnost’, 2018, No. 2, pp. 87–91.
Rights and permissions
About this article
Cite this article
Bairamukov, V.Y., Lebedev, V.T. & Tikhonov, V.I. Atomic-Force-Microscopy Analysis of Uranium Bis-Phthalocyanine and Its Pyrolysed Derivatives. J. Surf. Investig. 12, 170–174 (2018). https://doi.org/10.1134/S1027451018010226
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451018010226