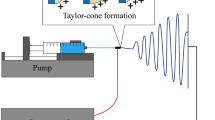
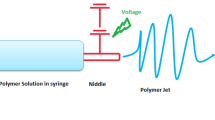
Abstract
Electrospun nanofibers have been extensively studied in the biomedical field, including the controlled release of drugs, bionics, cell scaffolds, hemostasis, wound healing, and tissue engineering because of their high porosity, large surface area-to-volume ratio, and programmable features. In recent years researchers have continuously broadened the structural design of electrospun nanofibers, which have evolved from one-dimensional to three-dimensional structures, in order to diversify their function. These properties enable nanofibers to structurally and functionally mimic natural extracellular matrix (ECM), thereby obtaining a favorable physiological microenvironment for both wound healing and hemostasis due to improved blood coagulation and concentration. A comprehensive review summarizing the recent research progress of the structural and functional design of electrospun nanofibers for hemostasis and wound healing, on the other hand, is lacking. This review summarizes electrospun nanofibers used for hemostasis and wound healing, with a focus on structural design and modification strategies. The wide application of electrospun nanofibers in hemostasis and wound healing is clarified using a special structural and innovative design for electrospinning. The advantages and limitations of electrospun nanofibers with various structural forms are also discussed, as are the main challenges and future development directions for the development of structurally specific electrospun nanofibers for hemostasis and wound healing.


Copyright 2018, Elsevier


Copyright 2020, Elsevier

Copyright 2021, Elsevier. b Schematic illustration of the (SF/PCL)/PVA-LBL coaxial fibers loaded with BMP2 and CTGF. Reproduced with permission of [83], Copyright 2019, American Chemical Society. c Viability, distribution, morphology and cytoskeleton development of endothelial cells in bioactive nanofiber scaffolds fabricated by concurrent emulsion electrospinning and coaxial cell electrospraying. Reproduced with permission of [86], Copyright 2021, Elsevier. d Schematic diagram of the additional routes for drug molecules entering into the dissolution media from the reservoir and SEM image of the residue depots after exhaustion of loaded KET. Reproduced with permission of [88], Copyright 2020, Elsevier. e Schematic diagram of triaxial electrospinning and the diffusion mechanism of the two drugs in triaxial electrospun nanofibers. Reproduced with permission of [89], Copyright 2019, American Chemical Society.

Copyright 2021, Taylor & Francis. b Schematic illustration of the preparation and application of PLGA nanofibers coated layer-by-layer with quaternized chitin and FGF2 containing hyaluronic acid. Reproduced with permission of [104]. Copyright 2019, Elsevier

Copyright 2018, Elsevier. b Schematic illustration of PCL/collagen nanofibers using tannic acid as the binding site to prepare fixed micelles. Reproduced with permission of [111], Copyright 2018, Wiley–VCH

Copyright 2019, Springer Nature. b Schematic illustration showing the three-dimensional nanofiber gelatin sponge for rapid hemostasis and the mechanism of hemostatic action. Reproduced with permission of [126], Copyright 2021, Wiley–VCH. c Schematic illustration of the direction of the three-dimensional chitosan (CS)/PVA nanofiber sponge and morphological changes of the CS/PVA nanofiber membrane before and after expansion. d Schematic representation of the recovery of CS/PVA nanofiber sponges after different degrees of compression. Reproduced with permission of [127], Copyright 2019, Elsevier

Copyright 2017, Elsevier. b Schematic illustration of the process of electrospraying chitosan nanoparticles containing epinephrine onto the surface of gauze and then depositing gelatin for electrospinning. Reproduced with permission of [129], Copyright 2021, Elsevier

Copyright 2019, Elsevier. b PCL and PLA nanofiber scaffolds spin-coated with CaCuSi4O10 nanoparticles design strategy inspired by Chinese sesame sticks. Reproduced with permission of [132], Copyright 2019, Elsevier

Copyright 2018, Wiley–VCH. b Schematic diagram of the sweat output pathway of traditional cotton textiles and Janus textiles, representation and SEM image of Janus textiles. Reproduced with permission of [150], Copyright 2019, Wiley–VCH. c Scheme showing the capillary forces driving unidirectional water transport when water is dripped on the hydrophobic side: (1) hydrophobic layer, (2) mixed layer, and (3) hydrophilic layer. (4) Scheme of the cross-membrane transport force when dripping water on the hydrophilic layer. Reproduced with permission of [151], Copyright 2022, Elsevier. d PCL-gelatin/PCL-PFMA nanofibers with unidirectional drainage and antiadhesion properties. Reproduced with permission of [152], Copyright 2021, Elsevier

Copyright 2013, American Chemical Society. b The potential mechanism of three-dimensional scaffolds consisting of radially aligned nanofibers and vertically aligned nanofibers for diabetic wound healing. c Schematic illustrating the application of three-dimensional scaffolds consisting of radially aligned nanofibers in stages 1 & 2 diabetic foot ulcer (DFU) healing. d Schematic illustrating the application of three-dimensional scaffolds consisting of radially aligned nanofibers in stages 3 & 4 diabetic foot ulcer (DFU) healing. Reproduced with permission of [156], Copyright 2020, Elsevier

Copyright 2021, Wiley–VCH

Copyright 2017, Wiley–VCH. b The color change of nanofibers when they are exposed to aqueous media with different pH values and after two hours of incubation in aqueous media with different pH values. Reproduced with permission of [170], Copyright 2019, MDPI

Copyright 2014, Royal Society of Chemistry. b Schematic illustration of the electric field-modified nanofibers for liver resection hemostasis. c The area of nanofibers with different metal cone diameters after in situ deposition and the size of the deposition area as a function of metallic cone diameter. Reproduced with permission of [178], Copyright 2019, Elsevier. d Schematic illustration of a coaxial long needle portable solution blow spinning device and SEM image of the fibers of the medical glue. e Porcine liver before in situ electrospinning and PCL nanofibers were spun onto the wound surface of the porcine liver in 90 s. Reproduced with permission of [179], Copyright 2020, Elsevier

Copyright 2021, Royal Society of Chemistry
Similar content being viewed by others
References
Liang Y, He J, Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021;15:12687–722.
Yang Y, Liang Y, Chen J, Duan X, Guo B. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioact Mater 2022;8:341–54.
Yu R, Zhang H, Guo B. Conductive biomaterials as bioactive wound dressing for wound healing and skin tissue engineering. Nano-Micro Lett 2021;14:1.
Guo B, Dong R, Liang Y, Li M. Haemostatic materials for wound healing applications. Nat Rev Chem 2021;5:773–91.
Qu J, Zhao X, Liang Y, Zhang T, Ma PX, Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018;183:185–99.
Liang Y, Li M, Yang Y, Qiao L, Xu H, Guo B. pH/glucose dual responsive metformin release hydrogel dressings with adhesion and self-healing via dual-dynamic bonding for athletic diabetic foot wound healing. ACS Nano 2022;16:3194–207.
Dong R, Guo B. Smart wound dressings for wound healing. Nano Today 2021;41:101290.
Liu X, Xu H, Zhang M, Yu D-G. Electrospun medicated nanofibers for wound healing: review. Membranes 2021;11:770.
Cui C, Sun S, Wu S, Chen S, Ma J, Zhou F. Electrospun chitosan nanofibers for wound healing application. Eng Regener 2021;2:82–90.
Juncos Bombin AD, Dunne NJ, McCarthy HO. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater Sci Eng C 2020;114:110994.
Li Y, Wang J, Wang Y, Cui W. Advanced electrospun hydrogel fibers for wound healing. Compos B 2021;223:109101.
Miguel SP, Sequeira RS, Moreira AF, Cabral CSD, Mendonça AG, Ferreira P, Correia IJ. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur J Pharm Biopharm 2019;139:1–22.
Wang J, Huang D, Yu H, Cheng Y, Ren H, Zhao Y. Developing tissue engineering strategies for liver regeneration. Eng Regen 2022;3:80–91.
Zhuge W, Liu H, Wang W, Wang J. Microfluidic bioscaffolds for regenerative engineering. Eng Regen 2022;3:110–20.
Dong Y, Zheng Y, Zhang K, Yao Y, Wang L, Li X, Yu J, Ding B. Electrospun nanofibrous materials for wound healing. Adv Fiber Mater 2020;2:212–27.
Zhong H, Huang J, Wu J, Du J. Electrospinning nanofibers to 1D, 2D, and 3D scaffolds and their biomedical applications. Nano Res 2021;15:784–804.
Ghosal K, Augustine R, Zaszczynska A, Barman M, Jain A, Hasan A, Kalarikkal N, Sajkiewicz P, Thomas S. Novel drug delivery systems based on triaxial electrospinning based nanofibers. React Funct Polym 2021;163:104895.
Rasouli R, Barhoum A, Bechelany M, Dufresne A. Nanofibers for biomedical and healthcare applications. Macromol Biosci 2019;19:1800256.
Nakielski P, Pierini F. Blood interactions with nano- and microfibers: recent advances, challenges and applications in nano- and microfibrous hemostatic agents. Acta Biomater 2019;84:63–76.
Lee F-Y, Lee D, Lee T-C, Chen J-K, Wu R-C, Liu K-C, Liu S-J. Fabrication of multi-layered lidocaine and epinephrine-eluting PLGA/collagen nanofibers: in vitro and in vivo study. Polymers 2017;9:416.
Mousavi SM, Zarei M, Hashemi SA, Ramakrishna S, Chiang W-H, Lai CW, Gholami A, Omidifar N, Shokripour M. Asymmetric membranes: a potential scaffold for wound healing applications. Symmetry 2020;12:1100.
Haik J, Kornhaber R, Blal B, Harats M. The feasibility of a handheld electrospinning device for the application of nanofibrous wound dressings. Adv Wound Care 2017;6:166–74.
Yan X, Yu M, Ramakrishna S, Russell SJ, Long Y-Z. Advances in portable electrospinning devices for in situ delivery of personalized wound care. Nanoscale 2019;11:19166–78.
Singh B, Shukla N, Kim J, Kim K, Park M-H. Stimuli-responsive nanofibers containing gold nanorods for on-demand drug delivery platforms. Pharmaceutics 2021;13:1319.
Pal P, Das B, Dadhich P, Achar A, Dhara S. Carbon nanodot impregnated fluorescent nanofibers for in vivo monitoring and accelerating full-thickness wound healing. J Mater Chem B 2017;5:6645–56.
Koehler J, Brandl FP, Goepferich AM. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur Polym J 2018;100:1–11.
Zhou Y, Chyu J, Zumwalt M. Recent progress of fabrication of cell scaffold by electrospinning technique for articular cartilage tissue engineering. Int J Biomater 2018;2018:1953636.
Joseph B, Augustine R, Kalarikkal N, Thomas S, Seantier B, Grohens Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater Today Commun 2019;19:319–35.
Yu H, Chen X, Cai J, Ye D, Wu Y, Fan L, Liu P. Novel porous three-dimensional nanofibrous scaffolds for accelerating wound healing. Chem Eng J 2019;369:253–62.
Bhattarai RS, Bachu RD, Boddu SHS, Bhaduri S. Biomedical applications of electrospun nanofibers: drug and nanoparticle delivery. Pharmaceutics 2019;11:5.
Drosou C, Krokida M, Biliaderis CG. Composite pullulan-whey protein nanofibers made by electrospinning: impact of process parameters on fiber morphology and physical properties. Food Hydrocoll 2018;77:726–35.
Yoon J, Yang H-S, Lee B-S, Yu W-R. Recent progress in coaxial electrospinning: new parameters, various structures, and wide applications. Adv Mater 2018;30:1704765.
Rieger KA, Birch NP, Schiffman JD. Designing electrospun nanofiber mats to promote wound healing—a review. J Mater Chem B 2013;1:4531–41.
Abrigo M, McArthur SL, Kingshott P. Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol Biosci 2014;14:772–92.
Ding JX, Zhang J, Li JN, Li D, Xiao CS, Xiao HH, Yang HH, Zhuang XL, Chen XS. Electrospun polymer biomaterials. Prog Polym Sci 2019;90:1–34.
Mazoochi T, Hamadanian M, Ahmadi M, Jabbari V. Investigation on the morphological characteristics of nanofiberous membrane as electrospun in the different processing parameters. Int J Ind Chem 2012;3:2.
Kailasa S, Reddy MSB, Maurya MR, Rani BG, Rao KV, Sadasivuni KK. Electrospun nanofibers: materials, synthesis parameters, and their role in sensing applications. Macromol Mater Eng 2021;306:2100410.
Jarusuwannapoom T, Hongrojjanawiwat W, Jitjaicham S, Wannatong L, Nithitanakul M, Pattamaprom C, Koombhongse P, Rangkupan R, Supaphol P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur Polym J 2005;41:409–21.
Fong H, Chun I, Reneker DH. Beaded nanofibers formed during electrospinning. Polymer 1999;40:4585–92.
Miguel SP, Figueira DR, Simoes D, Ribeiro MP, Coutinho P, Ferreira P, Correia IJ. Electrospun polymeric nanofibres as wound dressings: a review. Colloids Surf B 2018;169:60–71.
Zhang X, Shi XT, Gautrot JE, Peijs T. Nanoengineered electrospun fibers and their biomedical applications: a review. Nanocomposites 2021;7:1–34.
Shahriar SMS, Mondal J, Hasan MN, Revuri V, Lee DY, Lee Y-K. Electrospinning nanofibers for therapeutics delivery. Nanomaterials 2019;9:532.
Guo W, Yang K, Qin X, Luo R, Wang H, Huang R. Polyhydroxyalkanoates in tissue repair and regeneration. Eng Regen 2022;3:24–40.
Xue JJ, Xie JW, Liu WY, Xia YN. Electrospun nanofibers: new concepts, materials, and applications. Acc Chem Res 2017;50:1976–87.
Wang X, Zhao H, Turng L-S, Li Q. Crystalline morphology of electrospun poly(ε-caprolactone) (PCL) nanofibers. Ind Eng Chem Res 2013;52:4939–49.
Park J-Y, Lee I-H. Controlled release of ketoprofen from electrospun porous polylactic acid (PLA) nanofibers. J Polym Res 2011;18:1287–91.
Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008;29:4100–7.
Zhuo H, Hu J, Chen S, Yeung L. Preparation of polyurethane nanofibers by electrospinning. J Appl Polym Sci 2008;109:406–11.
Ding W, Wei S, Zhu J, Chen X, Rutman D, Guo Z. Manipulated electrospun PVA nanofibers with inexpensive salts. Macromol Mater Eng 2010;295:958–65.
Amiri N, Ajami S, Shahroodi A, Jannatabadi N, Amiri Darban S, Fazly Bazzaz BS, Pishavar E, Kalalinia F, Movaffagh J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int J Biol Macromol 2020;162:645–56.
Zhang L, Wang Z, Xiao Y, Liu P, Wang S, Zhao Y, Shen M, Shi X. Electrospun PEGylated PLGA nanofibers for drug encapsulation and release. Mater Sci Eng C 2018;91:255–62.
Valizadeh A, Bakhtiary M, Akbarzadeh A, Salehi R, Frakhani SM, Ebrahimi O, Rahmati-yamchi M, Davaran S. Preparation and characterization of novel electrospun poly(ϵ-caprolactone)-based nanofibrous scaffolds. Artif Cells Nanomed Biotechnol 2016;44:504–9.
Fu S-Z, Meng X-H, Fan J, Yang L-L, Wen Q-L, Ye S-J, Lin S, Wang B-Q, Chen L-L, Wu J-B, Chen Y, Fan J-M, Li Z. Acceleration of dermal wound healing by using electrospun curcumin-loaded poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) fibrous mats. J Biomed Mater Res Part B 2014;102:533–42.
Sundaramurthi D, Krishnan UM, Sethuraman S. Electrospun nanofibers as scaffolds for skin tissue engineering. Polym Rev 2014;54:348–76.
Ding FY, Deng HB, Du YM, Shi XW, Wang Q. Emerging chitin and chitosan nanofibrous materials for biomedical applications. Nanoscale 2014;6:9477–93.
Castro KC, Campos MGN, Mei LHI. Hyaluronic acid electrospinning: challenges, applications in wound dressings and new perspectives. Int J Biol Macromol 2021;173:251–66.
Yang Y, Chang S, Bai Y, Du Y, Yu D-G. Electrospun triaxial nanofibers with middle blank cellulose acetate layers for accurate dual-stage drug release. Carbohydr Polym 2020;243:116477.
Wang Q, Ju J, Tan Y, Hao L, Ma Y, Wu Y, Zhang H, Xia Y, Sui K. Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydr Polym 2019;205:125–34.
Fonseca LM, Cruxen CEdS, Bruni GP, Fiorentini ÂM, Zavareze EdR, Lim L-T, Dias ARG. Development of antimicrobial and antioxidant electrospun soluble potato starch nanofibers loaded with carvacrol. Int J Biol Macromol 2019;139:1182–90.
Moydeen AM, Padusha MSA, Thamer BM, Ahamed NA, Al-Enizi AM, El-Hamshary H, El-Newehy MH. Single-nozzle core-shell electrospun nanofibers of PVP/dextran as drug delivery system. Fibers Polym. 2019;20:2078–89.
Ramadass SK, Nazir LS, Thangam R, Perumal RK, Manjubala I, Madhan B, Seetharaman S. Type I collagen peptides and nitric oxide releasing electrospun silk fibroin scaffold: a multifunctional approach for the treatment of ischemic chronic wounds. Colloids Surf B 2019;175:636–43.
Khan AuR, Huang K, Jinzhong Z, Zhu T, Morsi Y, Aldalbahi A, El-Newehy M, Yan X, Mo X. Exploration of the antibacterial and wound healing potential of a PLGA/silk fibroin based electrospun membrane loaded with zinc oxide nanoparticles. J Mater Chem B 2021;9:1452–65.
Razzaq A, Khan ZU, Saeed A, Shah KA, Khan NU, Menaa B, Iqbal H, Menaa F. Development of cephradine-loaded gelatin/polyvinyl alcohol electrospun nanofibers for effective diabetic wound healing: in-vitro and in-vivo assessments. Pharmaceutics 2021;13:349.
Antunes BP, Moreira AF, Gaspar VM, Correia IJ. Chitosan/arginine-chitosan polymer blends for assembly of nanofibrous membranes for wound regeneration. Carbohydr Polym 2015;130:104–12.
Okhawilai M, Rangkupan R, Kanokpanont S, Damrongsakkul S. Preparation of Thai silk fibroin/gelatin electrospun fiber mats for controlled release applications. Int J Biol Macromol 2010;46:544–50.
Zhang D, Li L, Shan Y, Xiong J, Hu Z, Zhang Y, Gao J. In vivo study of silk fibroin/gelatin electrospun nanofiber dressing loaded with astragaloside IV on the effect of promoting wound healing and relieving scar. J Drug Delivery Sci Technol 2019;52:272–81.
Yin J, Xu L. Batch preparation of electrospun polycaprolactone/chitosan/aloe vera blended nanofiber membranes for novel wound dressing. Int J Biol Macromol 2020;160:352–63.
Hussein Y, El-Fakharany EM, Kamoun EA, Loutfy SA, Amin R, Taha TH, Salim SA, Amer M. Electrospun PVA/hyaluronic acid/L-arginine nanofibers for wound healing applications: nanofibers optimization and in vitro bioevaluation. Int J Biol Macromol 2020;164:667–76.
Maleki A, He J, Bochani S, Nosrati V, Shahbazi M-A, Guo B. Multifunctional photoactive hydrogels for wound healing acceleration. ACS Nano 2021;15:18895–930.
Yang X, Yang J, Wang L, Ran B, Jia Y, Zhang L, Yang G, Shao H, Jiang X. Pharmaceutical intermediate-modified gold nanoparticles: against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 2017;11:5737–45.
Qiu H, Zhu S, Pang L, Ma J, Liu Y, Du L, Wu Y, Jin Y. ICG-loaded photodynamic chitosan/polyvinyl alcohol composite nanofibers: anti-resistant bacterial effect and improved healing of infected wounds. Int J Pharm 2020;588:119797.
Wang W, Ding D, Zhou K, Zhang M, Zhang W, Yan F, Cheng N. Prussian blue and collagen loaded chitosan nanofibers with NIR-controlled NO release and photothermal activities for wound healing. J Mater Sci Technol 2021;93:17–27.
Feng X, Li J, Zhang X, Liu T, Ding J, Chen X. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J Controlled Release 2019;302:19–41.
Liu Y, Zhou S, Gao Y, Zhai Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J Pharm Sci 2019;14:130–43.
Felgueiras HP, Amorim MTP. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surf B 2017;156:133–48.
He J, Liang Y, Shi M, Guo B. Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly(ε-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chem Eng J 2020;385:123464.
Ren Y, Huang L, Wang Y, Mei L, Fan R, He M, Wang C, Tong A, Chen H, Guo G. Stereocomplexed electrospun nanofibers containing poly (lactic acid) modified quaternized chitosan for wound healing. Carbohydr Polym 2020;247:116754.
Ambekar RS, Kandasubramanian B. Advancements in nanofibers for wound dressing: a review. Eur Polym J 2019;117:304–36.
Tao F, Cheng Y, Shi X, Zheng H, Du Y, Xiang W, Deng H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr Polym 2020;230:115658.
Mousavi S-M, Nejad ZM, Hashemi SA, Salari M, Gholami A, Ramakrishna S, Chiang W-H, Lai CW. Bioactive agent-loaded electrospun nanofiber membranes for accelerating healing process: a review. Membranes 2021;11:702.
Zhang X, Lv S, Lu X, Yu H, Huang T, Zhang Q, Zhu M. Synergistic enhancement of coaxial nanofiber-based triboelectric nanogenerator through dielectric and dispersity modulation. Nano Energy 2020;75:104894.
Lan X, Liu Y, Wang Y, Tian F, Miao X, Wang H, Tang Y. Coaxial electrospun PVA/PCL nanofibers with dual release of tea polyphenols and epsilon-poly (L-lysine) as antioxidant and antibacterial wound dressing materials. Int J Pharm 2021;601:120525–120525.
Cheng G, Yin C, Tu H, Jiang S, Wang Q, Zhou X, Xing X, Xie C, Shi X, Du Y, Deng H, Li Z. Controlled co-delivery of growth factors through layer-by-layer assembly of core-shell nanofibers for improving bone regeneration. ACS Nano 2019;13:6372–82.
McClellan P, Landis WJ. Recent applications of coaxial and emulsion electrospinning methods in the field of tissue engineering. BioRes Open Access 2016;5:212–27.
Basar AO, Castro S, Torres-Giner S, Lagaron JM, Turkoglu Sasmazel H. Novel poly(ε-caprolactone)/gelatin wound dressings prepared by emulsion electrospinning with controlled release capacity of Ketoprofen anti-inflammatory drug. Mater Sci Eng C 2017;81:459–68.
Zhao Q, Zhou Y, Wang M. Three-dimensional endothelial cell incorporation within bioactive nanofibrous scaffolds through concurrent emulsion electrospinning and coaxial cell electrospraying. Acta Biomater 2021;123:312–24.
Lan X, Liu Y, Wang Y, Tian F, Miao X, Wang H, Tang Y. Coaxial electrospun PVA/PCL nanofibers with dual release of tea polyphenols and ε-poly (L-lysine) as antioxidant and antibacterial wound dressing materials. Int J Pharm 2021;601:120525.
Huang C-K, Zhang K, Gong Q, Yu D-G, Wang J, Tan X, Quan H. Ethylcellulose-based drug nano depots fabricated using a modified triaxial electrospinning. Int J Biol Macromol 2020;152:68–76.
Han D, Steckl AJ. Triaxial electrospun nanofiber membranes for controlled dual release of functional molecules. ACS Appl Mater Interfaces 2013;5:8241–5.
Samavedi S, Olsen Horton C, Guelcher SA, Goldstein AS, Whittington AR. Fabrication of a model continuously graded co-electrospun mesh for regeneration of the ligament–bone interface. Acta Biomater 2011;7:4131–8.
Al-Baadani MA, Hii Ru Yie K, Al-Bishari AM, Alshobi BA, Zhou Z, Fang K, Dai B, Shen Y, Ma J, Liu J, Shen X. Co-electrospinning polycaprolactone/gelatin membrane as a tunable drug delivery system for bone tissue regeneration. Mater Des 2021;209:109962
Hivechi A, Bahrami SH, Siegel RA, Milan PB, Amoupour M. In vitro and in vivo studies of biaxially electrospun poly(caprolactone)/gelatin nanofibers, reinforced with cellulose nanocrystals, for wound healing applications. Cellulose 2020;27:5179–96.
Mohamady Hussein MA, Guler E, Rayaman E, Cam ME, Sahin A, Grinholc M, Sezgin Mansuroglu D, Sahin YM, Gunduz O, Muhammed M, El-Sherbiny IM, Megahed M. Dual-drug delivery of Ag-chitosan nanoparticles and phenytoin via core-shell PVA/PCL electrospun nanofibers. Carbohydr Polym 2021;270:118373.
Ashraf SS, Parivar K, Hayati Roodbari N, Mashayekhan S, Amini N. Fabrication and characterization of biaxially electrospun collagen/alginate nanofibers, improved with Rhodotorula mucilaginosa sp. GUMS16 produced exopolysaccharides for wound healing applications. Int J Biol Macromol 2021;196:194–203.
Rashtchian M, Hivechi A, Bahrami SH, Milan PB, Simorgh S. Fabricating alginate/poly(caprolactone) nanofibers with enhanced bio-mechanical properties via cellulose nanocrystal incorporation. Carbohydr Polym 2020;233:115873.
Nagiah N, Murdock CJ, Bhattacharjee M, Nair L, Laurencin CT. Development of tripolymeric triaxial electrospun fibrous matrices for dual drug delivery applications. Sci Rep 2020;10:609.
Zhao Y, Qiu Y, Wang H, Chen Y, Jin S, Chen S. Preparation of nanofibers with renewable polymers and their application in wound dressing. Int J Polym Sci 2016;2016:4672839.
Yang Y, Chang S, Bai Y, Du Y, Yu D-G. Electrospun triaxial nanofibers with middle blank cellulose acetate layers for accurate dual-stage drug release. Carbohyd Polym 2020;243:116477.
Sagitha P, Reshmi CR, Sundaran SP, Sujith A. Recent advances in post-modification strategies of polymeric electrospun membranes. Eur Polym J 2018;105:227–49.
Yang C, Yan Z, Lian Y, Wang J, Zhang K. Graphene oxide coated shell-core structured chitosan/PLLA nanofibrous scaffolds for wound dressing. J Biomater Sci Polym Ed 2020;31:622–41.
Tanha S, Rafiee-Tehrani M, Abdollahi M, Vakilian S, Esmaili Z, Naraghi ZS, Seyedjafari E, Javar HA. G-CSF loaded nanofiber/nanoparticle composite coated with collagen promotes wound healing in vivo. J Biomed Mater Res Part A 2017;105:2830–42.
Huang R, Li W, Lv X, Lei Z, Bian Y, Deng H, Wang H, Li J, Li X. Biomimetic LBL structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing. Biomaterials 2015;53:58–75.
Li L, Wang X, Li D, Qin J, Zhang M, Wang K, Zhao J, Zhang L. LBL deposition of chitosan/heparin bilayers for improving biological ability and reducing infection of nanofibers. Int J Biol Macromol 2020;154:999–1006.
Wang Z, Hu W, You W, Huang G, Tian W, Huselstein C, Wu C-L, Xiao Y, Chen Y, Wang X. Antibacterial and angiogenic wound dressings for chronic persistent skin injury. Chem Eng J 2021;404:126525.
Sofi HS, Ashraf R, Khan AH, Beigh MA, Majeed S, Sheikh FA. Reconstructing nanofibers from natural polymers using surface functionalization approaches for applications in tissue engineering, drug delivery and biosensing devices. Mater Sci Eng C 2019;94:1102–24.
Chen P, Liu L, Pan J, Mei J, Li C, Zheng Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater Sci Eng C 2019;97:325–35.
Li J, He J, Wei F, Huang Y, Chen X. Progress in collagen electrospinning nanofibers. Polym Bull 2015;5:43–49
Esbah Tabaei PS, Asadian M, Ghobeira R, Cools P, Thukkaram M, Derakhshandeh PG, Abednatanzi S, Van Der Voort P, Verbeken K, Vercruysse C, Declercq H, Morent R, De Geyter N. Combinatorial effects of coral addition and plasma treatment on the properties of chitosan/polyethylene oxide nanofibers intended for bone tissue engineering. Carbohydr Polym 2021;253:117211
Ojah N, Saikia D, Gogoi D, Baishya P, Ahmed GA, Ramteke A, Choudhury AJ. Surface modification of core-shell silk/PVA nanofibers by oxygen dielectric barrier discharge plasma: studies of physico-chemical properties and drug release behavior. Appl Surf Sci 2019;475:219–29.
Permyakova ES, Polčak J, Slukin PV, Ignatov SG, Gloushankova NA, Zajíčková L, Shtansky DV, Manakhov A. Antibacterial biocompatible PCL nanofibers modified by COOH-anhydride plasma polymers and gentamicin immobilization. Mater Des 2018;153:60–70.
Albright V, Xu M, Palanisamy A, Cheng J, Stack M, Zhang B, Jayaraman A, Sukhishvili SA, Wang H. Micelle-coated, hierarchically structured nanofibers with dual-release capability for accelerated wound healing and infection control. Adv Funct Mater 2018;7:1800132.
Song DW, Kim SH, Kim HH, Lee KH, Ki CS, Park YH. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: implications for wound healing. Acta Biomater 2016;39:146–55.
Ahn S, Ardoña HAM, Lind JU, Eweje F, Kim SL, Gonzalez GM, Liu Q, Zimmerman JF, Pyrgiotakis G, Zhang Z, Beltran-Huarac J, Carpinone P, Moudgil BM, Demokritou P, Parker KK. Mussel-inspired 3D fiber scaffolds for heart-on-a-chip toxicity studies of engineered nanomaterials. Anal Bioanal Chem 2018;410:6141–54.
Chen Y, Qiu Y, Wang Q, Li D, Hussain T, Ke H, Wei Q. Mussel-inspired sandwich-like nanofibers/hydrogel composite with super adhesive, sustained drug release and anti-infection capacity. Chem Eng J 2020;399:125668.
Leung CM, Dhand C, Dwivedi N, Xiao A, Ong ST, Chalasani MLS, Sriram H, Balakrishnan Y, Dolatshahi-Pirouz A, Orive G, Beuerman RW, Ramakrishna S, Verma NK, Lakshminarayanan R. Combating microbial contamination with robust polymeric nanofibers: elemental effect on the mussel-inspired cross-linking of electrospun gelatin. ACS Appl Bio Mater 2019;2:807–23.
Kim SE, Tiwari AP. Mussel-inspired polydopamine-enabled in situ-synthesized silver nanoparticle-anchored porous polyacrylonitrile nanofibers for wound-healing applications. Int J Polym Mater Polym Biomater 2020;71:1–10.
Chen X, Wang X, Wang S, Zhang X, Yu J, Wang C. Mussel-inspired polydopamine-assisted bromelain immobilization onto electrospun fibrous membrane for potential application as wound dressing. Mater Sci Eng C 2020;110:110624.
Wang Y, Chen Z, Luo G, He W, Xu K, Xu R, Lei Q, Tan J, Wu J, Xing M. In-situ-generated vasoactive intestinal peptide loaded microspheres in mussel-inspired polycaprolactone nanosheets creating spatiotemporal releasing microenvironment to promote wound healing and angiogenesis. ACS Appl Mater Interfaces 2016;8:7411–21.
Liang Y, Li M, Huang Y, Guo B. An integrated strategy for rapid hemostasis during tumor resection and prevention of postoperative tumor recurrence of hepatocellular carcinoma by antibacterial shape memory cryogel. Small 2021;17:2101356.
Liu MH, Duan XP, Li YM, Yang DP, Long YZ. Electrospun nanofibers for wound healing. Mater Sci Eng C 2017;76:1413–23.
Tavakoli S, Kharaziha M, Nemati S, Kalateh A. Nanocomposite hydrogel based on carrageenan-coated starch/cellulose nanofibers as a hemorrhage control material. Carbohydr Polym 2021;251:117013.
Gu BK, Park SJ, Kim MS, Kang CM, Kim JI, Kim CH. Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydr Polym 2013;97:65–73.
Gu BK, Park SJ, Kim MS, Lee YJ, Kim J-I, Kim C-H. Gelatin blending and sonication of chitosan nanofiber mats produce synergistic effects on hemostatic functions. Int J Biol Macromol 2016;82:89–96.
Li Z, Milionis A, Zheng Y, Yee M, Codispoti L, Tan F, Poulikakos D, Yap CH. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat Commun 2019;10:5562.
Li Y, Niu F, Zhao X, Yap CH, Li Z. Nonwetting nanostructured hemostatic material for bleeding control with minimal adhesion. Adv Mater Interfaces 2021;8:2101412.
Xie X, Li D, Chen Y, Shen Y, Yu F, Wang W, Yuan Z, Morsi Y, Wu J, Mo X. Conjugate electrospun 3D gelatin nanofiber Sponge for rapid hemostasis. Adv Funct Mater 2021;10:2100918.
Zhang K, Bai X, Yuan Z, Cao X, Jiao X, Li Y, Qin Y, Wen Y, Zhang X. Layered nanofiber sponge with an improved capacity for promoting blood coagulation and wound healing. Biomaterials 2019;204:70–9.
Park J-Y, Kyung K-H, Tsukada K, Kim S-H, Shiratori S. Biodegradable polycaprolactone nanofibres with β-chitosan and calcium carbonate produce a hemostatic effect. Polymer 2017;123:194–202.
Atashgahi M, Ghaemi B, Valizadeh A, Moshiri A, Nekoofar MH, Amani A. Epinephrine-entrapped chitosan nanoparticles covered by gelatin nanofibers: a bi-layer nano-biomaterial for rapid hemostasis. Int J Pharm 2021;608:121074.
Cheng H-H, Xiong J, Xie Z-N, Zhu Y-T, Liu Y-M, Wu Z-Y, Yu J, Guo Z-X. Thrombin-loaded poly(butylene succinate)-based electrospun membranes for rapid hemostatic application. Macromol Mater Eng 2018;303:1700395.
Li W, Yu Q, Yao H, Zhu Y, Topham PD, Yue K, Ren L, Wang L. Superhydrophobic hierarchical fiber/bead composite membranes for efficient treatment of burns. Acta Biomater 2019;92:60–70.
Yu Q, Han Y, Tian T, Zhou Q, Yi Z, Chang J, Wu C. Chinese sesame stick-inspired nano-fibrous scaffolds for tumor therapy and skin tissue reconstruction. Biomaterials 2019;194:25–35.
Jeckson TA, Neo YP, Sisinthy SP, Gorain B. Delivery of therapeutics from layer-by-layer electrospun nanofiber matrix for wound healing: an update. J Pharm Sci 2021;110:635–53.
Chanda A, Adhikari J, Ghosh A, Chowdhury SR, Thomas S, Datta P, Saha P. Electrospun chitosan/polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int J Biol Macromol 2018;116:774–85.
Hassiba AJ, El Zowalaty ME, Webster TJ, Abdullah AM, Nasrallah GK, Khalil KA, Luyt AS, Elzatahry AA. Synthesis, characterization, and antimicrobial properties of novel double layer nanocomposite electrospun fibers for wound dressing applications. Int J Nanomed 2017;12:2205–13.
Zahid S, Khalid H, Ikram F, Iqbal H, Samie M, Shahzadi L, Shah AT, Yar M, Chaudhry AA, Awan SJ, Khan AF, Rehman IU. Bi-layered alpha-tocopherol acetate loaded membranes for potential wound healing and skin regeneration. Mater Sci Eng C 2019;101:438–47.
Chen DW-C, Liao J-Y, Liu S-J, Chan E-C. Novel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: an in vitro and in vivo study. Int J Nanomed 2012;7:763–71.
Ma B, Xie J, Jiang J, Wu J. Sandwich-type fiber scaffolds with square arrayed microwells and nanostructured cues as microskin grafts for skin regeneration. Biomaterials 2014;35:630–41.
Chogan F, Mirmajidi T, Rezayan AH, Sharifi AM, Ghahary A, Nourmohammadi J, Kamali A, Rahaie M. Design, fabrication, and optimization of a dual function three-layer scaffold for controlled release of metformin hydrochloride to alleviate fibrosis and accelerate wound healing. Acta Biomater 2020;113:144–63.
Graça MFP, de Melo-Diogo D, Correia IJ, Moreira AF. Electrospun asymmetric membranes as promising wound dressings: a review. Pharmaceutics 2021;13:183.
Pal P, Dadhich P, Srivas PK, Das B, Maulik D, Dhara S. Bilayered nanofibrous 3D hierarchy as skin rudiment by emulsion electrospinning for burn wound management. Biomater Sci 2017;5:1786–99.
Miguel SP, Ribeiro MP, Coutinho P, Correia IJ. Electrospun polycaprolactone/aloe vera_chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers 2017;9:183.
Wu C, Chen T, Xin Y, Zhang Z, Ren Z, Lei J, Chu B, Wang Y, Tang S. Nanofibrous asymmetric membranes self-organized from chemically heterogeneous electrospun mats for skin tissue engineering. Biomed Mater 2016;11:035019.
Morgado PI, Aguiar-Ricardo A, Correia IJ. Asymmetric membranes as ideal wound dressings: An overview on production methods, structure, properties and performance relationship. J Membr Sci 2015;490:139–151.
Yu D-G, Li J-J, Zhang M, Williams GR. High-quality Janus nanofibers prepared using three-fluid electrospinning. Chem Commun 2017;53:4542–5.
Yang J, Wang K, Yu D-G, Yang Y, Bligh SWA, Williams GR. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater Sci Eng C 2020;111:110805.
Ji X, Li R, Liu G, Jia W, Sun M, Liu Y, Luo Y, Cheng Z. Phase separation-based electrospun Janus nanofibers loaded with Rana chensinensis skin peptides/silver nanoparticles for wound healing. Mater Des 2021;207:109864.
Ji X, Li R, Jia W, Liu G, Luo Y, Cheng Z. Co-axial fibers with janus-structured sheaths by electrospinning release corn peptides for wound healing. ACS Appl Bio Mater 2020;3:6430–8.
Shi L, Liu X, Wang W, Jiang L, Wang S. A self-pumping dressing for draining excessive biofluid around wounds. Adv Mater 2019;31:1804187.
Dai B, Li K, Shi L, Wan X, Liu X, Zhang F, Jiang L, Wang S. Bioinspired Janus textile with conical micropores for human body moisture and thermal management. Adv Mater 2019;31:1904113.
Li L, Mai Y, Wang Y, Chen S. Stretchable unidirectional liquid-transporting membrane with antibacterial and biocompatible features based on chitosan derivative and composite nanofibers. Carbohydr Polym 2022;276:118703.
Luo Z, Jiang L, Xu C, Kai D, Fan X, You M, Hui CM, Wu C, Wu Y-L, Li Z. Engineered Janus amphipathic polymeric fiber films with unidirectional drainage and anti-adhesion abilities to accelerate wound healing. Chem Eng J 2021;421:127725.
Reddy VJ, Radhakrishnan S, Ravichandran R, Mukherjee S, Balamurugan R, Sundarrajan S, Ramakrishna S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen 2013;21:1–16.
Wang Y, Gong J, Yao Y. Extracellular nanofiber-orchestrated cytoskeletal reorganization and mediated directional migration of cancer cells. Nanoscale 2020;12:3183–93.
Xi Y, Dong H, Sun K, Liu H, Liu R, Qin Y, Hu Z, Zhao Y, Nie F, Wang S. Scab-inspired cytophilic membrane of anisotropic nanofibers for rapid wound healing. ACS Appl Mater Interfaces 2013;5:4821–6.
Chen S, Wang H, Su Y, John JV, McCarthy A, Wong SL, Xie J. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater 2020;108:153–67.
Du J, Yao Y, Wang M, Su R, Li X, Yu J, Ding B. Programmable building of radially gradient nanofibrous patches enables deployment, bursting bearing capability, and stem cell recruitment. Adv Funct Mater 2021;32:2109833.
Jiang Y, Han Y, Wang J, Lv F, Yi Z, Ke Q, Xu H. Space-oriented nanofibrous scaffold with silicon-doped amorphous calcium phosphate nanocoating for diabetic wound healing. ACS Appl Bio Mater 2019;2:787–95.
Zhu Z, Liu Y, Xue Y, Cheng X, Zhao W, Wang J, He R, Wan Q, Pei X. Tazarotene released from aligned electrospun membrane facilitates cutaneous wound healing by promoting angiogenesis. ACS Appl Mater Interfaces 2019;11:36141–53.
Liao HT, Lai Y-T, Kuo C-Y, Chen J-P. A bioactive multi-functional heparin-grafted aligned poly(lactide-co-glycolide)/curcumin nanofiber membrane to accelerate diabetic wound healing. Mater Sci Eng C 2021;120:111689.
Kim JH, Jang J, Jeong YH, Ko TJ, Cho D-W. Fabrication of a nanofibrous mat with a human skin pattern. Langmuir 2015;31:424–31.
Sun L, Gao W, Fu X, Shi M, Xie W, Zhang W, Zhao F, Chen X. Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the basketweave pattern of collagen fibrils in native skin. Biomater Sci 2018;6:340–9.
Chen H, Baptista DF, Criscenti G, Crispim J, Fernandes H, van Blitterswijk C, Truckenmueller R, Moroni L. From fiber curls to mesh waves: a platform for the fabrication of hierarchically structured nanofibers mimicking natural tissue formation. Nanoscale 2019;11:14312–21.
Memic A, Abudula T, Mohammed HS, Joshi Navare K, Colombani T, Bencherif SA. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl Bio Mater. 2019;2:952–969.
Liu L, Xu W, Ding Y, Agarwal S, Greiner A, Duan G. A review of smart electrospun fibers toward textiles. Compos Commun 2020;22:100506.
Puiggalí-Jou A, Cejudo A, del Valle LJ, Alemán C. Smart drug delivery from electrospun fibers through electroresponsive polymeric nanoparticles. ACS Appl Bio Mater 2018;1:1594–605.
Nudelman R, Alhmoud H, Delalat B, Fleicher S, Fine E, Guliakhmedova T, Elnathan R, Nyska A, Voelcker NH, Gozin M, Richter S. Jellyfish-based smart wound dressing devices containing in situ synthesized antibacterial nanoparticles. Adv Funct Mater 2019;29:1902783.
Politi S, Carotenuto F, Rinaldi A, Di Nardo P, Manzari V, Albertini MC, Araneo R, Ramakrishna S, Teodori L. Smart ECM-based electrospun biomaterials for skeletal muscle regeneration. Nanomaterials 2020;10:1781.
Li Y-F, Slemming-Adamsen P, Wang J, Song J, Wang X, Yu Y, Dong M, Chen C, Besenbacher F, Chen M. Light responsive hybrid nanofibres for on-demand therapeutic drug and cell delivery. J Tissue Eng Regener Med 2017;11:2411–20.
Bazbouz MB, Tronci G. Two-layer electrospun system enabling wound exudate management and visual infection response. Sensors 2019;19:991.
Zhang H, Niu Q, Wang N, Nie J, Ma G. Thermo-sensitive drug controlled release PLA core/PNIPAM shell fibers fabricated using a combination of electrospinning and UV photo-polymerization. Eur Polym J 2015;71:440–50.
Slemming-Adamsen P, Song J, Dong M, Besenbacher F, Chen M. In situ cross-linked PNIPAM/gelatin nanofibers for thermo-responsive drug release. Macromol Mater Eng 2015;300:1226–31.
A. Tamayol, A. Hassani Najafabadi, P. Mostafalu, A.K. Yetisen, M. Commotto, M. Aldhahri, M.S. Abdel-wahab, Z.I. Najafabadi, S. Latifi, M. Akbari, N. Annabi, S.H. Yun, A. Memic, M.R. Dokmeci, A. Khademhosseini. Biodegradable elastic nanofibrous platforms with integrated flexible heaters for on-demand drug delivery. Sci Rep. 2017;7:9220.
Zhu Y, Zhang J, Song J, Yang J, Du Z, Zhao W, Guo H, Wen C, Li Q, Sui X, Zhang L. A multifunctional pro-healing Zwitterionic hydrogel for simultaneous optical monitoring of pH and glucose in diabetic wound treatment. Adv Funct Mater 2020;30:1905493.
Aydogdu MO, Altun E, Crabbe-Mann M, Brako F, Koc F, Ozen G, Kuruca SE, Edirisinghe U, Luo CJ, Gunduz O, Edirisinghe M. Cellular interactions with bacterial cellulose: polycaprolactone nanofibrous scaffolds produced by a portable electrohydrodynamic gun for point-of-need wound dressing. Int Wound J 2018;15:789–97.
Keirouz A, Chung M, Kwon J, Fortunato G, Radacsi N. 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: a review. WIREs Nanomed Nanobiotechnol 2020;12:e1626.
Jiang K, Long Y-Z, Chen Z-J, Liu S-L, Huang Y-Y, Jiang X, Huang Z-Q. Airflow-directed in situ electrospinning of a medical glue of cyanoacrylate for rapid hemostasis in liver resection. Nanoscale 2014;6:7792–8.
Luo W-L, Qiu X, Zhang J, Hu P-Y, Liu X-F, Liu J-J, Yu M, Ramakrishna S, Long Y-Z. In situ accurate deposition of electrospun medical glue fibers on kidney with auxiliary electrode method for fast hemostasis. Mater Sci Eng C 2019;101:380–6.
Gao Y, Xiang H-F, Wang X-X, Yan K, Liu Q, Li X, Liu R-Q, Yu M, Long Y-Z. A portable solution blow spinning device for minimally invasive surgery hemostasis. Chem Eng J 2020;387:124052.
Zhang J, Zhao Y-T, Hu P-Y, Liu J-J, Liu X-F, Hu M, Cui Z, Wang N, Niu Z, Xiang H-F, Long Y-Z. Laparoscopic electrospinning for in situ hemostasis in minimally invasive operation. Chem Eng J 2020;395:125089.
Zhang J, Liu C-L, Liu J-J, Bai X-H, Cao Z-K, Yang J, Yu M, Ramakrishna S, Long Y-Z. Eluting mode of photodynamic nanofibers without photosensitizer leakage for one-stop treatment of outdoor hemostasis and sterilizing superbacteria. Nanoscale 2021;13:6105–6116.
Dong W-H, Liu J-X, Mou X-J, Liu G-S, Huang X-W, Yan X, Ning X, Russell SJ, Long Y-Z. Performance of polyvinyl pyrrolidone-isatis root antibacterial wound dressings produced in situ by handheld electrospinner. Colloids Surf B 2020;188:110766.
Dong R-H, Jia Y-X, Qin C-C, Zhan L, Yan X, Cui L, Zhou Y, Jiang X, Long Y-Z. In situ deposition of a personalized nanofibrous dressing via a handy electrospinning device for skin wound care. Nanoscale 2016;8:3482–8.
Liu X-F, Zhang J, Liu J-J, Zhou Q-H, Liu Z, Hu P-Y, Yuan Z, Ramakrishna S, Yang D-P, Long Y-Z. Bifunctional CuS composite nanofibers via in situ electrospinning for outdoor rapid hemostasis and simultaneous ablating superbug. Chem Eng J 2020;401:126096.
Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (grant number: 51973172), Natural Science Foundation of Shaanxi Province (No. 2020JC-03 and 2019TD-020), the State Key Laboratory for Mechanical Behavior of Materials, the World-Class Universities (Disciplines) and Characteristic Development Guidance Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Y., Du, Y., Zhang, J. et al. Structural and Functional Design of Electrospun Nanofibers for Hemostasis and Wound Healing. Adv. Fiber Mater. 4, 1027–1057 (2022). https://doi.org/10.1007/s42765-022-00178-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-022-00178-z