Abstract
It is well known that the hydrolysable tannin (tannin acid) and condensed tannins in a plant can influence the availability of soil nitrogen (N) through the formation of tannin–organic N complexes. However, the lack of research on the effect of tannin acid–organic N complexes on soil N hinders our understanding of the subsequent role of these complexes. In this line, an incubation experiment was carried out with the addition of tannin acid-arginine and tannin-bovine serum albumin (BSA) complexes, where tannin acid was represented as the hydrolysable tannin. For the necessary comparisons, tannin acid, arginine, or BSA was added to the soil samples. The results showed that tannic acid addition quickly decreased protein contents at the beginning of incubation and decreased soluble organic N (SON) after 3 days to produce the inorganic N in the soil as compared to control. Tannin-arginine complexes increased NH4+–N compared with control and this increased degree was found lower than arginine treatment. Moreover, the addition of tannin–BSA complexes did not significantly increase soil NH4+–N as BSA alone treatment did. NO3−–N and N2O emission increased in each treatment compared with control indicating nitrification was not limited. The increase of NH4+–N was mainly attributed to the decrease of SON after 3 days of incubation. These results suggested that tannin acid quickly formed the complexes with protein to impact soil N. While, the effects of tannin-arginine and tannin–BSA complexes on soil N transformation were quite dissimilar. Thus, this study highlighted the subsequent role of tannin–organic N complexes under the influence of tannin. Tannin and tannin–organic N complexes naturally coexist in the ecosystem to impact soil N dynamics and may interact with each other as well.
Similar content being viewed by others
1 Introduction
Plant tannins, also known as plant polyphenols, are kind of polyphenolic compounds that widely exist in plants [1, 2]. It is reviewed that tannins are the fourth most abundantly occurring biochemical substance produced by vascular plant tissues after cellulose, hemicellulose and lignin, while leaves and bark may contain up to 40% tannin by dry weight [3]. Therefore, these plant materials, fallen on the soil surface or released from roots could be considered to play an important role in soil carbon (C) or nitrogen (N) cycling, and sustainable ecosystem functioning [4,5,6,7].
The dynamic effects of tannins on C and N in the soil leaching layer have been studied [4]. It was found that tannins had a much higher ability to inhibit ammonification rate than cellulose. It was also found that tannin acid reduced the contents of NO3−–N and NH4+–N in soils [8]. In addition, condensed tannins decreased C and N mineralization and enzyme activity, however, increased the ratio of fungi to bacteria [5]. The presence of roots significantly increased the stable SOM–N due to the formation of complexes with root-derived condensed tannins in boreal forest soils [7]. Therefore, tannins may alter the quantity and forms of N availability for plants or microbes, and inhibit microorganism’s activity [5, 9]. It may reduce soil enzyme activities, such as acid phosphatase, chitinase, and beta-glucosidase [10,11,12]. In contrast, tannins also act as an unstable C source [4] to induce soil N cycling. It was reported that the complexes with tannic acid (hydrolysable tannin) seemed to be more easily degraded than the ones with condensed tannins [13, 14]. Furthermore, the effects of complexes on soil were dependent on soil types, such as N-poor and N-rich soil [15] and different forest types, such as boreal pine forest soil [7] and silver birch forest soil [15].
Tannins can produce insoluble compounds by reacting with proteins, alkaloids, polysaccharides in plant tissues, litters and soils [3] and also do compound reactions with metal ions to produce various complexes [16]. It was reported that arginine in amino acids, insulin in the polypeptide, protein, N base, chitin and chitosan in amino sugars could be precipitated with tannins [17]. Furthermore, the reaction ratio of tannin to organic N and pH would largely affect the amount and proportion of tannin and organic N, involved in the reaction [18]. Therefore, the formation of tannin–organic N complexes is highly important for the mechanisms of soil organic N retention [4, 17, 18] and could induce a shift from mineral- to organic-dominated N cycling [7]. Besides the effect of tannin on soil, it would be of great interest to understand the combination of tannin and different organic N complexes and its effect on soil N cycling. So, it was found that the addition of condensed tannins decreased the rates of C mineralization (only in N-poor soil) and net nitrification, but organic N-condensed tannin complexes increased net mineralization [15]. However, the effects of organic N-tannins acid complexes on soil N had not been studied [17]. Furthermore, the interaction of bovine serum albumin (BSA) and tannic acid, and its influence was studied for the food industry, human or animal nutrition and health [19], but not in the soil.
In terrestrial ecosystems, plant litter often deposits on the organic horizon with mineral soil underneath. Tannin is released from litter to impact the soil, and thereafter it coexists with tannin–organic N complexes. Besides, tannin influences soil N by the formation of tannin–organic N complexes, the effect of the complexes is needed to be investigated to know the relationship between litter and soil. So, the objective of this study was to investigate the impact of tannin–organic N complexes on soil N in a subtropical forest. In this context, an incubation experiment was conducted to investigate the effects of tannic acid, amino acid, protein and their complexes on the soil after determining soil N types and soluble organic C with different incubation intervals.
2 Materials and methods
2.1 Soil collection
The soil samples were collected from Jianou Wanmulin Nature Reserve (27° 03 ′N, 118° 09 ′E) in the Northern part of Fujian Province, the Southeast of Mount Wuyi and Northwest of the Mount Jiufeng Range. We have been carried out experiments in 2009, so soil sampling and related procedures were approved and permitted by “Jianou Wanmulin Nature Reserve”. The climate of this region is characterized as a typical subtropical monsoon with a mean annual temperature of 19.4 °C, mean annual precipitation of 1731.4 mm, mean annual evaporation of 1466 mm and mean annual relative humidity of 81%. The frost-free period of the whole year is usually present for 277 days. The soil samples were taken from the Cunninghamia lanceolata plantation which was developed by artificial afforestation after clear-cutting in 1969. It was in the Northwest slope direction with a slope of 27° and a forest density of 1117 trees per hectare, with single tree species and a simple stand structure. Three sampling sites were randomly selected to collect surface soil (0–15 cm), removed stones and litter, fully mixed the soil, and brought it back to the laboratory. Passed it through 2 mm sieve and soil was stored in a refrigerator at 4 °C for following incubation experiment. The soil had a pH 5.7 and water-holding capacity (WHC) (g kg−1) 638.2, total C 23.8 g kg−1, total N 1.8 g kg−1. Other basic physical and chemical properties of the soil are as follows: C/N 13.0, NH4+–N 20.6 mg kg−1, NO3−–N 11.1 mg kg−1, soluble organic N (SON) 17.7 mg kg−1 and soil bulk density 1.20 g cm−3.
2.2 Experimental design
To estimate the effects of tannin–organic N complexes on dynamics of soil N in fir planted red soil, an indoor incubation experiment was carried out after preparing complexes by reacting tannic acid with arginine or bovine serum albumin (BSA). The tannin and organic N ratio of 2:1 were used to prepare the complexes for the tannin–BSA complexes and tannin–arginine complexes as well at pH 4.7 [18].
Six treatments including: CK (control), T (adding tannic acid, 6.25 mg), A (adding arginine, 4.37 mg), TA (adding tannin-arginine complexes, 10.6 mg), B (adding BSA, 4.97 mg), TB (adding tannin-BSA complexes, 11.2 mg) were formulated. Fresh soil samples equivalent to 30 g dry soil were considered and the matter in six treatments was added in 300 ml plastic bottles, respectively. Thereafter, the incubation was carried out with sealed bottles at 25 °C and the soil moisture was adjusted to 60% WHC with distilled water. Soil samples were taken on days 0, 3 and 14, respectively, to determine the contents of NH4+–N, NO3−–N, TSN, SOC, soil protein and tannin. Before incubation, three culture bottles were randomly selected and the air above the soil was collected on day 0 as the control for first sampling. Then, the bottle was sealed for incubation. During incubation, 20 ml gas was collected from the bottles with a 50 ml injector on 3, 7, and 14 days and injected into an aluminum film gas bag (50 mL, China Dalian Delin) for nitrous oxide (N2O) concentration determination.
The basic physical and chemical properties of different treatments are shown in Table 1. The C and N contents of the complexes formed by tannin and organic N were different from those of tannin and organic N alone. The difference in the properties caused by the change of C/N ratio affected the transformation of soil N.
2.3 Chemical analysis
The fresh soil sub-samples equal to 6 g of dry weight were directly extracted with 30 ml of 0.5 mol L−1 K2SO4 solution. The NH4+, NO3− and TSN levels in soil extracts were determined by using continuous flow analysis (SKALAR San++, the Netherlands). Total soil C and N were determined by C and N element analyzer (Elemantar Vario MAX CN, Germany). The concentration of N2O in gas samples was measured by gas chromatography (GCv2014, Shimadzu, Japan). The content of tannin and protein was detected with an ultraviolet band [17, 20].
Soil soluble organic N (SON) (mg N kg−1) is calculated as follows:
where NH4+ is ammonium N (mg N kg−1), and NO3− is nitrate N (mg N kg−1).
Soil soluble organic C (SOC) (mg kg−1) was calculated with the following formula:
where V0 represents the volume of FeSO4 (ml) consumed by titrating blank samples, V1 is the volume of FeSO4 (ml) consumed by titrating samples, c is the concentration of FeSO4 solution (mol L−1), ts is the dilution factor and m is the mass of dried soil (g). The millimole mass fraction of C (mg mmol−1) was 3 and the coefficient of conversion to kg was 1000. While, N2O emission flux (F) (μg kg−1 d−1) in 0–3, 3–7 and 7–14 days are defined as follows:
where M is the molar mass of N2O, V1 is the air volume of 1 mol under the standard conditions, V2 is the volume of incubation bottle (ml), m is the quality of dry soil (kg), dc/dt is the change of N2O concentration per unit time (μg d−1), T is incubation temperature (°C). When F is positive, it indicates that the soil emits N2O to the atmosphere and when it is negative, it indicates that soil absorbs N2O.
Accumulative N2O Emissions (CE) (μg kg−1) is expressed as:
where Fi indicates the N2O emission flux (μg kg−1 d−1) t i times; Fi+1 indicates N2O emission flux at i + 1 times; ti+1 − ti represents the interval between two adjacent measurement dates; and n is the total number of measurements in the cumulative emission observation period.
2.4 Statistical analysis
The least significant difference (LSD) test in one-way ANOVA was applied to access differences among treatments at a 5% significance level. Repeated measures analysis of variance (ANOVA) was used to evaluate the effects of treatments, time and their interaction on soil N, SOC, tannin and protein, which were all significant (p < 0.05) except for the effect of time on NH4+–N, so the results were not provided. All statistical analyses were made using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
3 Results
3.1 NH4 +–N, NO3 −–N and SON in soil with additions of tannins or complexes
Tannin acid addition did not significantly decrease soil inorganic N (Table 2). In contrast, soil NH4+–N was significantly (p < 0.05) increased by 21.5–45.8% in treatment A, by 14.1% and 15.5% in TA treatment on days 3 and 14, and by 11.9% in treatment B only on day 14 compared with CK. NO3−–N in A treatment was significantly (p < 0.01) higher than in CK by 7.5% and 5.3% on days 3 and 14, respectively, and by 8.4% on day 3 for TA treatment. In addition, NO3−–N was significantly (p < 0.05) higher by 4.7% and 5.2% in treatment B, and by 6.5% and 11.0% in TB treatment on days 0 and 3 than in CK, respectively.
The content of soluble organic N (SON) in CK treatment increased slowly with incubation, but SON in A and TA treatments decreased, especially for treatment A with a rapid decline before day 3 (Table 2). Treatments T, B and TB decreased SON from day 3 compared to CK. The SON content in treatment A was significantly higher by 100.4% on day 0, but it was significantly lowered by 27.3–78.3% and 65.8% in T, A, B and TB treatments than in CK on days 3 and 14. The content of SON in TA treatment was significantly (p < 0.05) lower by 30.1–59.3% than in CK.
3.2 The emission of N2O from the soil with different additions
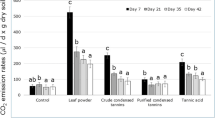
The N2O emission rate in CK treatment decreased significantly with incubation time (Table 3), nonetheless, in T, A and TA treatments, it declined firstly and then increased. The N2O emission rate in B and TB treatments showed a gradual upward trend with incubation time. The N2O emission rate was the highest in A treatment, which was significantly (p < 0.01) higher than in other treatments. The greatly cumulative N2O emission in CK mainly occurred in 0–3 days, accounting for 51.9% and in 7–14 days of incubation A, B and TB treatments accounting for 53.5–56.4% of the total emission. While, the cumulative N2O emission mainly was from 0–3 days to 7–14 days for treatment T accounting for 35.5% and 49.6% and TA treatment accounting for 35.9% and 42.8% of the total emission, respectively.
Tannin addition significantly (p < 0.05) increased the N2O emission rate by 122.3% from day 7 to 14 compared to CK. It significantly increased by 80.5–548.3% in treatment A, and by 55.3–250.0% in TA treatment through the incubation compared to CK. B and TB treatments significantly decreased the N2O emission rate by 53.5% and 57.2% from 0 to 3 days, but increased it by 43.9–201.2% after day 3 compared to CK.
3.3 Soil soluble organic C (SOC), tannin and protein in different treatments
At the commencement of incubation, SOC in CK treatment was significantly higher than that of all other treatments and SOC in CK, T, A and TB decreased with incubation time (Table 4). SOC in T, A and TA treatments on day 0 were significantly lower by 32.7%, 53.4% and 46.5% than in CK treatment, respectively. However, SOC in T and TA treatments were significantly greater by 21.3% and 31.3% than in CK treatment on day 3, respectively. On the fourteenth day, SOC in T, A and TA treatments were significantly upper by 116.0%, 35.7% and 30.0% than in CK treatment, respectively. B and TB treatments significantly decreased SOC by 24.5–48.7% through the incubation compared to CK.
Tannin contents in CK treatment increased and had the maximum values at the end (Table 4). In T treatment it was found significantly (p < 0.01) higher than the other treatments. Soil tannin was significantly higher by 41.8% and 35.3% in A, by 34.2% and 21.2% in TA treatment on days 0 and 3 compared to CK, respectively. Further, soil tannin was significantly higher by 28.5% in B only on day 0, by 20.4% in TB treatment only on day 14 compared to CK.
The protein contents in CK, TA, B and TB treatments decreased in the start and then increased thereafter with time (Table 4). While, in T and A treatments it increased with time. The highest protein content was found at each stage of incubation for TB treatment. However, the lowest protein contents were attributed to treatment A overall. T and A treatments decreased the protein contents quickly by 60.9% and 59.5% on day 0, respectively. B and TB treatments enlarged the protein contents by 32.9% and 47.9% on day 3, respectively. On the fourteenth day, the A treatment reduced the protein contents by 40.7%, but TB treatment increased it by 35.9%.
3.4 Relationship between indicators of all treatments
Overall, we observed a linear negative correlation between SOC and NO3−–N (Fig. 1a), which was more pronounced in CK (p < 0.01), T (p < 0.01) and A (p < 0.001) treatments with R2 > 0.63, and weaker in both B and TB with R2 around 0.4 (p > 0.05). In addition, SON and NH4+–N also had a linear negative correlation except in A treatment (Fig. 1b), which was recorded stronger in A (p < 0.001) than in CK, TA and TB treatments (p > 0.05). Tannin and SOC exhibited negative deep functional relations (Fig. 1c), which was robust in CK (p < 0.01), TA (p < 0.05), B (p < 0.001) and TB (p < 0.001) treatments than in both T and A (p > 0.05).
At the beginning of incubation, overall a linear negative correlation was observed between protein and tannin (Fig. 2a), in which there were linear positive correlation in T (p < 0.001), TA (p < 0.05) and TB (p = 0.064) treatments. At the beginning of incubation, the linear negative correlation between protein and SON (Fig. 2b) was computed dominating in A treatment (p < 0.01) and opposed in B (p > 0.05) and TB (p > 0.05) with incubation. However, the linear negative correlation between tannin and SON was found at 3 days after incubation (Fig. 2c), which dominated in both T and A treatment (p > 0.05) and suppressed in CK (p < 0.05) with incubation. Moreover, the positive deep functional relation between SOC and SON was also found at 3 days of incubation (Fig. 2d), which dominated in A treatment (p > 0.01) and opposed in CK (p > 0.05) with incubation duration.
Relationship between two indicators of the treatments at different incubation intervals. Such as protein and tannin (a), protein and SON (b) at the beginning of incubation, tannin and SON (c), SOC and SON (d) at 3 days after incubation. Where, black triangle (c) means that data in T treatment are not fit in function
4 Discussion
4.1 Effects of tannin on nitrogen transformation
Tannic acid addition (210 mg tannic acid kg−1 soil, T treatment) quickly decreased protein (Table 4) and exhibited a negative linear correlation between tannin and protein in soil (Fig. 2a). It suggested that tannin addition increased the stability of protein by forming complexes, but the effect of tannin on soil protein did not continue after 3 days. The decline in protein content did not decrease SON at the beginning of incubation (Table 2, Fig. 2b), nonetheless, SON diminished by tannin addition compared with CK after 3 days (Table 2, Fig. 2c) which mineralized to produce the inorganic N in the soil (Fig. 1b). Adamczyk et al. [17] reported that the tannin–organic N complexes were formed after tannins reaction with a variety of organic N such as amino acids, proteins, peptides, polyamines and amino sugar. Therefore, Tannic acid addition did not significantly decrease the contents of NH4+–N and NO3−–N compared with CK in our study. Previous research reported that both low-concentration tannic acid (100 mg tannic acid kg−1 soil) and high-concentration tannic acid (5 g tannic acid kg−1 soil) significantly reduced the soil NH4+–N contents [8]. In this study, the effects of tannin addition on soil N were less than that in the previous research [21] due to reduced addition. Therefore, the tannin content might be one of the factors to influence the results in different past experimentations. Studies had shown that tannin addition reduced the content of inorganic N and converted it to organic N [4, 22], which ultimately reduced the N availability. Additionally, the magnesium, calcium, and manganese affect protein digestibility and the process of complex formation [19]. Thus, the effects of tannin on N might also be dependent on soil types [15] and probably related to metal ion concentrations in different soils, such as the role of iron [23] and fungal necromass [7, 24] in N transformation. There was a strong site-effect reported in differential response of C and N transformations to condensed tannins [15].
Furthermore, as a C source, tannin also participates in N cycling by affecting soil microbial communities and activities [5]. When tannin contents are lower, it can increase soil respiration, promote microbial activity to fix N, and reduce the net N mineralization rate. However, when tannin contents are higher, it inhibits the microbial activity by the toxicity of tannin, reduces soil respiration and the biological fixation of N and slightly promote net N mineralization [21]. It explains a linear negative correlation between SOC and NO3−–N (Fig. 1a) that was found stronger in T treatment. The contrasting effects of low and high concentrations of tannins on soil enzymatic activity in the boreal forest were also observed in another study [25]. So, the increase of SOC with incubation at T treatment compared with CK indicated that the effect of tannic acid on soil C and N might be different and the effect on N was less than condensed tannin. The negative effect of condensed tannin was dominantly focused on when studying the role of litter in soil C and N mineralization, such as of leaves [13] and roots [7]. It was even reported that condensed tannin (0.4 or 4 g kg−1 soil) did not significantly affect net N mineralization compared with the control in birch soil from Finland [15], which might be dependent on reaction time after tannin addition.
4.2 Effects of arginine and tannin-arginine complexes on nitrogen transformation
The results showed that compared with CK treatment, arginine treatment (A treatment) significantly enhanced soil NH4+–N, because amino acid-N, a low molecular weight N compound, was also easily transformed in soil [26] from amino acids to NH4+–N with the decline of SON after 3 days. It was consistent with the relationship between SON and NH4+–N (Fig. 1b). However, arginine addition significantly increased NO3−–N contents after 3 days and thereafter improved slowly which may be related to the weakness of nitrification in subtropical red soil. Arginine addition, in fact, might increase soil NO3−–N before 3 days because the half-life of amino acids was less than 3 days [27, 28] and soil sample was not considered in this duration. Kielland et al. [29] also found that amino acids could rapidly be mineralized into NH4+–N, but the transformation rate of NH4+–N to NO3−–N was very slow.
The addition of arginine significantly promoted the emission of N2O and cumulative emissions (Table 3). These findings are in line with results of an incubation experiment adding amino acids, where, soil mineralization of eleven amino acids (100 mg AA–N g−1 soil) promoted a wide range in the production of N2O (156.0 ± 79.3 ng N2O–N g−1 soil) during 12 days incubations [30]. This may be due to the release of inorganic N from the mineralization of amino acids and further nitrification. On the other hand, arginine addition promoted N2O emission, which may be related to the contents of C-arginine [30] and the promotion of heterotrophic nitrification by added amino acid [31]. It is possible that arginine addition increased NO3−–N after 3 days by heterotrophic nitrification bacteria utilizing SOC (Figs. 1a, 2d). So, net NO3−–N contents were not an indicator of soil N2O emission because of immobilization of NO3−–N by microbes [32]. Another possibility could be that soil N2O emission might occur prior to NO3−–N production in nitrification after arginine addition (Tables 2, 3).
Compared with CK treatment, the addition of tannin-arginine complexes (TA treatment) significantly increased NH4+–N which was less than treatment A, but more than treatment T. Their influences on NO3−–N were somewhat different with a small degree among them, suggesting a limited effect on nitrification due to N2O emission which was yet higher as compared with CK. These results indicated that after tannin influencing the soil, tannin-arginine complexes still have a role in impacting SON, but their effect on ammonification was weaker compared with tannin alone. As a substrate, tannin-arginine complexes like tannin acid and arginine significantly decreased SOC and protein on day 0 and SON through the incubation, which suggested that the precipitation of organic N by tannic acid might decrease N leaching and may act as a source of N for plants [17]. As tannin-arginine complexes might be relatively unstable and still could form complexes with proteins (Table 4, Fig. 2a). It was reported that condensed tannins act as vital phenolic compounds, show inhibitory effects on net N mineralization, nitrification and regulate microbial communities [9, 18], thus, inhibiting microbial biomass N [15, 21]. It was concluded that purified tannin always precipitated more organic N complexes than tannic acid [17]. Therefore, condensed tannin–organic N complexes might be of higher stability. Due to condensed tannins released from roots, boreal forest soil even had a great loss of organic matter before 3 years, and the formation of stable soil organic matter-N and increase of dissolved organic N after 3 years [7]. In conclusion, after the differential effects of tannin, the formation of tannin–organic N complexes might still impact soil to some extent.
4.3 Effects of BSA and tannin-BSA complexes on nitrogen transformation
Bovine serum albumin (BSA), as an exogenous protein, has been often involved in research as a protein representative [17, 33]. Compared with CK treatment, the addition of BSA (B treatment) significantly increased NH4+–N only on day 14, which was lower than that in A treatment. Jones and Kielland [33] argued that BSA was converted to NH4+–N in the litter layer only about 10 days in an artificially adding BSA experiment and thereafter converted to NO3−–N at almost 30 days. Since the present experiment only carried out for 14 days, the mineralization of added BSA by microbes was not enough to produce more NH4+–N as results of 4 weeks compared to 2 weeks had been reported in the research [15]. The soil NO3−–N was higher than SON, the increase of NO3−–N and N2O emission coupled with the decline of SON and protein with BSA addition compared with CK treatment suggested that heterotrophic nitrification also played a key important role in soil N transformation as amino acid did. These results showed that protein transformation was slower and needed more time that limited the N mineralization [33, 34], so SON decreased prior to the mineralization of protein (Table 2). However, considering the changing of each N form the decline of SON still mainly contributed to the increase of inorganic N, especially NH4+–N.
The addition of tannin–BSA complexes (TB treatment) had non-significant effect on soil NH4+–N, which was different from the effect of TA treatment. While, TB increased NO3−–N more than B treatment compared with CK, and also enhanced soil N2O emission similar to that of BSA. A previous study reported that the addition of BSA–tannins complexes (BSA–tannic acid) to the soil resulted in a decrease in net N mineralization compared to protein added alone [14]. The findings of Joanisse et al. [34] also showed that BSA alone increased net N mineralization much more than BSA complexed with tannic acid (hydrolysable tannin). Tannin–protein complexes are stable and difficult to be directly utilized [4] and hard to be degraded by enzymes [10], which might enhance the utilization of SON by microbes to increase NO3−–N (Table 2), and increase protein content (Table 4). In addition, purified tannin-protein can be utilized by plant mycorrhizal symbionts [34], allowing the retained organic N to re-enter the N cycle. Therefore, in this experiment the mineralization might be less affected by TB treatment. Furthermore, TB treatment could play a significant role in slowing down the degradation of soil nitrogenous compounds [35], which was different from the role of TA.
Plant tannins entered the soil through litter, root exudation, and produced tannin-protein complexes with proteins. However, there was a different extent of degradation among the complexes with tannic acid (hydrolysable tannin) and complexes with condensed tannins [13, 14]. Moreover, there was diverse net N mineralization or net nitrification with the addition of condensed tannin-Rubisco, -chitin or -BSA [15]. The presence of plant roots in boreal forest soil even enhanced the formation of stable soil organic matter-N after 3 years, attributing to the condensed tannins [7]. Therefore, when we are concerned about the effects of tannin on soil N dynamics, originating from differentially decomposing litters, the role of tannin–organic N complexes should also be paid decent attention because these are used to coexist on or in the soil.
5 Conclusions
Tannin acid might quickly precipitate the protein by forming the complexes. Tannin-arginine complexes did not affect soil inorganic N as exhibited by tannin alone. It is possible that the tannin-amino acid complexes served as N source to promote soil N transformation and might have a priming effect on soil N contents. Tannin–BSA complexes acted as a stable N source in the soil which was difficult to be directly utilized by microorganisms that played a key role in immobilizing SON. In the process of plant litter decomposition, it was not only tannin contents that affected its decomposition, but also N metabolism, including protein hydrolysis, the interaction between tannin and organic N would also regulate soil N cycling. It is a supplement to the mechanism for clarifying the effect of tannin–organic N complexes on soil N transformation with litter decomposition.
References
Peng K, Jin L, Niu YD, Huang Q, Mcallister TA, Yang HE, Denise H, Xu ZJ, Acharya S, Wang SX, Wang YX (2017) Condensed tannins affect bacterial and fungal microbiomes and mycotoxin production during ensiling and upon aerobic exposure. Appl Environ Microbiol 84:e02274-17
Gripenberg S, Rota J, Kim J, Wright SJ, Garwood NC, Fricke EC, Zalamea PC, Salminen JP (2017) Seed polyphenols in a diverse tropical plant community. J Ecol 106:87–100
Kraus TEC, Dahlgren RA, Zasoski RJ (2003) Tannins in nutrient dynamics of forest ecosystems—a review. Plant Soil 256:41–66
Kraus TEC, Zasoski RJ, Dahlgren RA, Horwatha WR, Preston CM (2004) Carbon and nitrogen dynamics in a forest soil amended with purified tannins from different plant species. Soil Biol Biochem 36:309–321
Ushio M, Balser TC, Kitayama K (2013) Effects of condensed tannins in conifer leaves on the composition and activity of the soil microbial community in a tropical montane forest. Plant Soil 365:157–170
Shimada T, Takahashi A, Shibata M, Yagihashi T (2015) Effects of within-plant variability in seed weight and tannin content on foraging behaviour of seed consumers. Funct Ecol 29:1513–1521
Adamczyk B, Sietiö O-M, StrakováP Prommer J, Wild B, Hagner M, Pihlatie M, Fritze H, Richter A, Heinonsalo J (2019) Plant roots increase both decomposition and stable organic matter formation in boreal forest soil. Nat Commun 10:3982
Ma HL, Gao R, Yin YF, Yang YS (2016) Effects of leaf litter tannin on soil ammonium and nitrate content in two different forest soils of mount Wuyi, China. Toxicol Environ Chem 98:395–409
Smolander A, Kanerva S, Adamczyk B, Kitunen V (2012) Nitrogen transformations in boreal forest soils—does composition of plant secondary compounds give any explanations? Plant Soil 350:1–26
Adamczyk B, Kitunen V, Smolander A (2009) Polyphenol oxidase, tannase and proteolytic activity in relation to tannin concentration in the soil organic horizon under silver birch and Norway spruce. Soil Biol Biochem 41:2085–2093
Adamczyk S, Adamczyk B, Kitunen V, Smolander A (2015) Monoterpenes and higher terpenes may inhibit enzyme activities in boreal forest soil. Soil Biol Biochem 87:59–66
Triebwasser DJ, Tharayil N, Preston CM, Gerard PD (2012) The susceptibility of soil enzymes to inhibition by leaf litter tannins is dependent on the tannin chemistry, enzyme class and vegetation history. New Phytol 196:1122–1132
Zhang QF, Laanbroek HJ (2018) The effects of condensed tannins derived from senescing Rhizophora mangle leaves on carbon, nitrogen and phosphorus mineralization in a Distichlis spicata salt marsh soil. Plant Soil 433:37–53
Mutabaruka R, Hairiah K, Cadisch G (2007) Microbial degradation of hydrolysable and condensed tannin polyphenol-protein complexes in soils from different land-use histories. Soil Biol Biochem 39:1479–1492
Adamczyk B, Kitunen V, Smolander A (2013) Response of soil C and N transformations to condensed tannins and different organic N-condensed tannin complexes. Appl Soil Ecol 64:163–170
Witwicki M, Jerzykiewicz M, Ozarowski A (2015) Understanding natural semiquinone radicals—multifrequency EPR and relativistic DFT studies of the structure of Hg (II) complexes. Chemosphere 119:479–484
Adamczyk B, Adamczyk S, Smolander A, Kitunen V (2011) Tannic acid and Norway spruce condensed tannins can precipitate various organic nitrogen compounds. Soil Biol Biochem 43:628–637
Adamczyk S, Kiikkilä O, Kitunen V, Smolander A (2013) Potential response of soil processes to diterpenes, triterpenes and tannins: nitrification, growth of microorganisms and precipitation of proteins. Appl Soil Ecol 67:47–52
Kaspchak E, Goedert AC, Igarashi-Mafra L, Mafra MR (2019) Effect of divalent cations on bovine serum albumin (BSA) and tannic acid interaction and its influence on turbidity and in vitro protein digestibility. Int J Biol Macromol 136:486–492
Adamczyk B, Salminen JP, Smolander AA (2012) Precipitation of proteins by tannins: effects of concentration, protein/tannin ratio and pH. Int J Food Sci Technol 47:875–878
Kanerva S, Kitunen V, Kiikkilä O, Loponen J, Smolander A (2006) Response of soil C and N transformations to tannin fractions originating from Scots pine and Norway spruce needles. Soil Biol Biochem 38:1364–1374
Tharayil N, Alpert P, Bhowmik P, Gerard P (2013) Phenolic inputs by invasive species could impart seasonal variations in nitrogen pools in the introduced soils: a case study with Polygonum cuspidatum. Soil Biol Biochem 57:858–867
Heil J, Vereecken H, Brüggemann N (2016) A review of chemical reactions of nitrification intermediates and their role in nitrogen cycling and nitrogen trace gas formation in soil. Eur J Soil Sci 67:23–39
Adamczyk B, Sietiö O-M, Biasi C, Heinonsalo J (2019) Interaction between tannins and fungal necromass stabilizes fungal residues in boreal forest soils. New Phytol 223:16–21
Adamczyk B, Karonen M, Adamczyk S, Engström MT, Laakso T, Saranpää P, Kitunen V, Smolander A, Simon J (2017) Tannins can slow-down but also speed-up soil enzymatic activity in boreal forest. Soil Biol Biochem 107:60–67
Dippold M, Biryukov M, Kuzyakov Y (2014) Sorption affects amino acid pathways in soil: implications from position-specific labeling of alanine. Soil Biol Biochem 72:180–192
Geisseler D, Horwath WR (2014) Investigating amino acid utilization by soil microorganisms using compound specific stable isotope analysis. Soil Biol Biochem 74:100–105
Ma HL, Pei GT, Gao R, Yin YF (2017) Mineralization of amino acids and its signs in nitrogen cycling of forest soil. Acta Ecol Sin 37:60–63
Kielland K, Olson K, Ruess RW, Boone RD (2006) Contribution of winter processes to soil nitrogen flux in taiga forest ecosystems. Biogeochemistry 81:349–360
McLain JET, Martens DA (2005) Nitrous oxide flux from soil amino acid mineralization. Soil Biol Biochem 37:289–299
Zhang JB, Wang J, Zhong WH, Cai ZC (2015) Organic nitrogen stimulates the heterotrophic nitrification rate in an acidic forest soil. Soil Biol Biochem 80:293–295
Ma HL, Yin YF, Gao R, Taqi R, He XH (2019) Response of nitrogen transformation to glucose additions in soils at two subtropical forest types subjected to simulated nitrogen deposition. J Soils Sediments 19:2166–2175
Jones DL, Kielland K (2012) Amino acid, peptide and protein mineralization dynamics in a taiga forest soil. Soil Biol Biochem 55:60–69
Joanisse GD, Bradley RL, Preson CM, Bending GD (2009) Sequestration of soil nitrogen as tannin-protein complexes may improve the competitive ability of sheep laurel (Kalmia angustifolia) relative to black spruce (Picea mariana). New Phytol 181:187–198
Erktan A, Balmot J, Merino-Martín L, Monnier Y, Pailler F, Coq S, Abiven S, Stokes A, Bissonnais YL (2017) Immediate and long-term effect of tannins on the stabilization of soil aggregates. Soil Biol Biochem 105:197–205
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31770659, 31170578 and 31570607) and Natural Science Foundation of Fujian Province, China (2018J01716). Authors sincerely thank Yanyu Lin, Yuanzhen Peng and Liuming Yang for their assistance in soil sampling and analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, C., Qiu, H., Ma, H. et al. Response of the subtropical forest soil N transformations to tannin acid-organic nitrogen complexes. SN Appl. Sci. 2, 1209 (2020). https://doi.org/10.1007/s42452-020-3006-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-3006-7