Abstract
Clean and green renewable energy is of paramount importance in the world today. Ab initio calculations using density functional theory demonstrate that superlattice structures can result into lowering lattice thermal conductivity and have improved electronic properties, which result in higher electrical conductivity. It is possible to achieve improved thermoelectricity-generating properties of materials with new superlattices and have large effective mass, as well as density of states at the Fermi level composed of 2D/2D AlN/C4H6N6Ni2. However, higher electrical conductivity requires high-mobility charge carriers, narrow-gap semiconductors and lower electron scattering. Thus, band structure, projected density of state, density of state, as well as spin density of state difference between alpha and beta eigenstates contributions, are used to reveal that heterostructures have advantage over the isolated materials. New superlattice structures would result in improving the charge generation/separation and yield a better thermoelectric material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aluminium nitride (AlN) along with indium nitride and gallium nitride is a well-known wide energy gap semiconductor. However, due to the high energy of the surface optical phonons, AlN has become a prominent substrate for atomic layered composites [1]. The material is known to reduce remote interfacial phonon scattering, thereby improving the devices performance [1] to provide high charge mobility and dielectric constant [2]. The high thermal conductivity of AlN enables effective heat dissipation, which limit self-heating [3,4,5]. Its films can be reliably integrated [6] in large scale, for example 6″ and 8″ Si wafers [7, 8], making it both commercially and technologically significant for layered material production. In addition, de Almeida et al. [9] investigated the likelihood of apprehending two-dimensional (2D) hexagonal AlN by first-principles density functional theory (DFT) calculations [9]. Furthermore, de Almeida et al.’s [9] first-principles calculations revealed stability of hexagonal-shaped AlN sheet. The simulations model system consisted of 96 atoms per supercell of a hexagonal AlN. De Almeida et al. predicted that AlN has an indirect gap of 2.81 eV and a cohesive energy, which is 6% lower than the bulk AlN.
Nickel diamminebis[m-(cyano-C:N)]bis(cyano-C)di (C4H6N6Ni2) clathrate is as a depiction of phonon glass–electron crystal materials; the clathrate structures have been studied for possible applications in thermoelectricity generation [10,11,12,13,14,15]. Type-I clathrate crystal structure is covalently bonded with the lattice composed of elements primarily from groups 13 and 14 of the periodic table. Its structure has cages, where metal atoms can fill in [13, 16]. Clathrate compounds have been found with high figure of merit (ZT) values of 1.35 at 900 K but contain expensive elements such as Ge and Ga in Ba8Ga16Ge30 [17] crystal. About 46-mm-long crystal of the Ba8Ga16Ge30 clathrate has been grown using Czochralski method [17, 18]. Saramat et al. [17] revealed electrical, thermal conductivities, as well as Seebeck coefficient transiting from extrinsic to intrinsic behaviour in the 600–900 K temperature range with ZT equal to 1.63 at 1100 K. Thus, Ba8Ga16Ge30 clathrate is a strong candidate for high temperatures, as well as medium thermoelectric application. Efforts have also been made to include cheap elements such as Si and Al for thermoelectric application [19]. Bobnar et al. [19] developed and structurally studied Ba8AlxSi46−x clathrate-I phase, which revealed aluminium and silicon atoms: 5.7 aluminium atoms and 0.3 silicon atoms occupying the crystallographic site 6c, while 1.2 aluminium atoms and 22.8 Si atoms occupy site 24k. Further, atomic distribution was established using wavelength-dispersive X-ray spectroscopy [19], X-ray single-crystal diffraction [20], as well as NMR [19, 21] experiments. Condron et al. [22] generated Ba8Al14Si31 crystal using an aluminium flux method. Electron microprobe, 27Al MAS NMR and single-crystal neutron diffraction were used to determine the aluminium site occupancies. However, Si-based clathrates show low ZT values. A cross-substitution improves the properties by introducing more scattering centres and changing the electronic band structure, thus bringing value to the basic investigative process of chemical composition, such as structural variations and stability [23,24,25,26,27,28].
Defects play a significant role in influencing the physical properties, as well as the applicability of materials [29,30,31]. Clathrate type-I structure has been observed for compounds containing defects, such as Rb8Sn442 and Ba8Ge433 [32, 33]. In these compounds, the defects can order in the crystal structure and give a superstructure belonging to Ia\(\bar{3}\)d space group. At elevated temperatures, the defects randomise and a structural phase transition into the Pm\(\bar{3}\)n structure is observed. From the experimental optical spectra study, schematic energy band for SrTiO3 preformed by Mishra et al. [34] revealed that the detection of oxygen defect is important from a technological point of view, as these vacancies are known to improve the photo-catalytic activities and magneto-optical properties. The idea of disorder and tunnelling states in the Sr and Eu systems but not in the Ba systems, and the corroborative nature of the difference Fourier nuclear density and lattice thermal conductivity has been established in the literature [35,36,37]. Chakoumakos et al. [38] revealed that Sr (2) in the large cage should have displacement parameters of one order of magnitude larger than that of the framework atoms and Sr (1) atom in the small cage. This addressed the guest atom disorder and dynamics in multi-temperature single-crystal and powder neutron diffraction study of Sr8Ga16Ge30. Difference Fourier maps with the Sr (2) atom removed from the reference model showed non-ellipsoidal features in the nuclear density with low density at the centre of the cage. These features in the nuclear density suggest a four-site disorder model. Refinements of single-crystal and the neutron powder diffraction data resulted in similar residual factors for models with four-site disorder on 24k or 24j, and this suggests that the disorder could be more complex than just four sites [39]. The metal atoms in cages, however, introduce low lattice thermal conductivity in clathrates [40,41,42]; the doped material results in high thermoelectric performance [43,44,45] as determined by \({\text{ZT}} = S^{2} \sigma T/(k_{e} + k_{ph} )\). Where S, T and σ represent thermal power or Seebeck coefficient, the absolute temperature and electrical conductivity. Nevertheless, because of the inversion relationships between these parameters [46], it is challenging to improve thermoelectric performance by decreasing k or increasing S and σ. In this study, we investigate the traditional Clathrate C4H6N6Ni2 thermoelectric material with 2D AlN to form AlN/C4H6N6Ni2 heterostructures. Using ab initio methods, we study the geometry, electronic properties and optical properties to demonstrate that heterostructures can result in lowering lattice thermal conductivity, and can have improved electronic properties and reduced energy gap of composites, thus resulting in electrical conductivity increase. The study reveals the importance of heterostructures in electronic analysis, and demonstrates that improved electronic properties would lead to improving the charge generation and result in a better thermoelectric material.
2 Computational details
The ab initio calculations were made using the Cambridge Sequential Total Energy Package [47] program and employing Ultrasoft pseudopotentials as implemented in Biovia Materials Studio 2019 [48]. In this study, geometry optimisation, energy, electronic structure optical properties and charge generation and separation are computed. Additional functionals have been used to correct the underestimation of density functional theory. These include the generalised gradient approximation functional [49] with Perdue–Burke–Ernzerhof [50] to account for electron correlation effects. Geometry optimisation was done on ultra-fine quality with energy convergence tolerance of 5.0 × 10−6 eV/atom. The electronic cut-off energy was set at 400 eV with medium SCF tolerance. Discrete Fourier-transform algorithm [51] grid density was set at 42 × 48 × 160. The convergence window was set mixing at three electronic densities with charge mixing amplitude of 0.5 having a charge mixing cut-off. The Monkhorst–Pack k-points to define the accuracy of the Brillouin zone sampling were set at 8 × 8 × 1 for energy, and Monkhorst–park grid 16 × 16 × 1 k-point defined density of state (DOS) giving the actual spacing of (0.00704 × 0.000704 × 0.05604) 1/Å. Spin mixing amplitude was set at 2.0 having a cut-off of 1.51/Å. To prevent the heterostructure layers from collapsing on each other, a vacuum slab was set at 20 Å and this allowed geometric relaxation.
3 Results and discussion
3.1 Computed models geometry
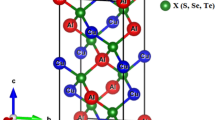
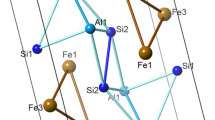
Many physical properties of crystals are associated with their point groups. Some of these properties are piezoelectricity, pyroelectricity, external morphology, ferroelectricity, optical activity or shape of the crystal, indices of refraction, as well as etch figures. A point group is a group of symmetry operations that leave at least one point of an object or pattern unmoved. In 2D, the elements of the point group include the identity and may include one or more mirrors and/or one or more n-fold rotations. The symmetry operations are represented by matrices. C4H6N6Ni2 is a point group 4 with an order of four and four symmetry operations (1, 2, 4+ and 4−). Figure 1a shows an example of an abstract motif of n(C4H6N6Ni2) with 3D tetragonal lattice length parameters of 7.235 (Å), 8.310 (Å) along (a = b, c), with a 83 P4/M spacegroup. The primitive cells angle symmetry constraints of α = β = γ = 90°, respectively, with the symmetry point group 4 and a projection of clathrate. (C6D6)2n, catena-(bis(hexadeuterobenzene) tetrakis(m2-cyano)-tetrakis(ammonio)-di-nickel clathrate) are also members having the same symmetry. In this study, we generated models of AlN with lattice parameters of a = 0.311, b = 0.9385 and c = 0.498 nm, with a Fm3m space group. The primitive cells atomistic position was set at 90°, 90°, 120° for the α, β and γ, respectively [37], and built unit cells, which were magnified into supercells (Fig. 1b). Surfaces for reaction were cut along (101) (Fig. 1c) for C4H6N6Ni2 and layered heterostructures (Fig. 1d–f).
3.2 Electronic properties
3.2.1 Band structure
The electronic band energy structures were generated in order to find out the influence 2D AlN had on the formation of heterostructures. Since lowering heat conductivity requires scattering of acoustic phonons, this involves anisotropic thermal vibrations, inclusion of heavy atoms with complex structure, filling lattice cages with atoms for disorder, superlattice structures (heterostructures), as well as defects/grain boundary scattering. C4H6N6Ni2 had a zero band gap, and this can be ascribed to the high aromaticity of C4H6N6Ni2 structure, while AlN model had a direct band gap energy of 6.013 eV near the G point. This was much higher than the theoretically determined value by de Almeida et al. [9] (2.81 eV) and an experimental energy gap of 5.0 eV by Mattila et al. [52]. However, Li et al. [53] revealed a direct band gap of 6.12 eV near the G point of AlN from ab initio calculations. The experimental optical reflectance, as well as absorption/transmission cathode luminescence bandgap measurements, reported a 6.03 eV by Silveira et al. [54]. Furthermore, their study ascribes exciton at 6.03 eV and a shoulder intense line at 6.01 eV ascribed to the neutral donor-bound-exciton recombination. It was observed from our plot (Fig. 2) that the number of energy bands increased and induction of energy gap was observed in our heterostructures, i.e. C4H6N6Ni2 surface (2c), C4H6N6Ni2–AlN layer (0.015 eV) (2d), C4H6N6Ni2–AlN–AlN layer (0.060) (2e) and C4H6N6Ni2–AlN–AlN–AlN layer (0.022 eV) (2f). An energy gap is important in that the thermoelectric materials should not be a perfect conductor of heat. Temperature is dependent on band gap, and the optical band gap of semiconductors tends to decrease with the increase in temperature as found out by Mishra et al. [55]. However, the material requires being a good electrical conductor and the small energy gap created is enough to allow the electrons to move from valence band (VB) to conduction band (CB) with minimum energy.
3.2.2 Density of state (DOS)
Increasing Seebeck (α) can be archived by bandgap engineering, large DOS at E = 0 and large effective mass. To understand the materials nature at the Fermi level, the DOS was calculated (Fig. 3a–e), i.e. C4H6N6Ni2, AlN, C4H6N6Ni2–AlN layer, C4H6N6Ni2–AlN–AlN layer and C4H6N6Ni2–AlN–AlN–AlN layer. As shown in Fig. 3a, C4H6N6Ni2 material had increased degeneracy of band extrema. Furthermore, there was a clear luck of energy gap observed in C4H6N6Ni2. An intense decrease in the electron density of state at the Fermi level, i.e. zero energy AlN (Fig. 3b), was seen. The increase in extrema of heterostructures and high intensity in band structure can be ascribed to improve thermoelectric ZT as the Seebeck for the material is derived from the additions of contributions from each extremum [56, 57].
3.2.3 Projected density of state (PDOS)
Individual element constituting our heterostructures was computed for their contribution to the interface projected as a plot of density of state (PDOS) (Fig. 4). Ni, C, N, H and Al projected density of state (E = 0 eV is set as the Fermi level). Ni has an atomic number of 28 with [Ar] 3d8 4s2 or [Ar] 3d9 4s1 electronic configuration; it has 2, 8, 16, 2 or 2, 8, 17, 1 electron per shell. The 3d8 or 3d9 contribution was dominant in the VB, as well as conduction band past the Fermi level (Fig. 4a). However, the s orbital showed dominance past 3 eV into the conduction band. Carbon (C) has an electron configuration of [He] 2s2 2p2 (Fig. 4b) with 2, 4 electrons per shell, and its 2p2 in p orbital occupies the VB and CB with its highest electron density in the VB. N has an atomic number of 7 with [He] 2s2 2p3 electronic configuration, and it has 2, 5 electrons per shell.
The 2p3 contribution dominated in the VB, as well as in the conduction band past the Fermi level (Fig. 4c). However, it had the greatest contribution to the VB. Hydrogen has a symbol of H and atomic number 1. It has 1 electron per shell with electronic configuration of 1s1. It had an equal band contribution at the Fermi level. However, aluminium (Al) had the dominance in the conduction band with the [Ne] 3s2 3p1 electronic configuration, where the 3p1 orbital had the highest contribution at the Fermi level.
It was further observed that the p orbital contribution dominated the heterostructures generated from the individual elements C4H6N6Ni2, AlN, C4H6N6Ni2-AlN, C4H6N6Ni2–AlN–AlN and C4H6N6Ni2–AlN–AlN–AlN layer partial density of state, respectively (Fig. 5), in both the valence and conduction bands. The influence of adding the different Al layers was observed by the increase in the DOS (electrons/eV), with Fig. 6e being the densest.
To explore the electron contribution and the orbitals near the Fermi level (Ef) at the interfaces of C4H6N6Ni2–AlN, C4H6N6Ni2–AlN–AlN and C4H6N6Ni2–AlN–AlN–AlN heterostructures, the PDOS of bulk C4H6N6Ni2, AlN and their heterostructures were plotted (Fig. 5). The absence of bandgap in C4H6N6Ni2 is observed (Fig. 5a) with 4s2 or 4s1 electrons dominating both the VB and CB before and past Ef mainly from Ni (Fig. 5a). At the Fermi level, AlN VB is stuck at the E = 0 eV of VB states with a clear separation (band gap) purely dominated by p orbital (Fig. 5b). N contributes 2p3 at VB, while the CB is made up of 3p1 from Al; this signifies upward band bending and p-type feature. The PDOS analysis of heterostructures from C4H6N6Ni2 and AlN shows that the VBM is largely contributed by 2p3, 4s2 or 4s1 states, respectively, while 3p1 makes the greatest contribution in CB. The electron transition from the VB to CB, i.e. C4H6N6Ni2 states to AlN states of in the composite reveal the orbitals involved and their influence in thermal electron generation or light absorption [15]. Therefore, from Fig. 5c–e, the VB of composites is dominated by p orbital states, as well as the CBM in layer. There is a distinct increase in the density of state of the layer build up, resulting in an increase with both VB and CB, respectively. Under heat (infrared) or visible light, it is possible for the electrons in the C4H6N6Ni2 states to be easily transferred into the AlN states.
3.3 Spin density of state
The C4H6N6Ni2–AlN monolayer and Ni, N, C, Al, and H spin density of state of starting materials were calculated and analysed using DFT against individual components and plotted in the state graph density. In the spin-polarised calculation, individual states contributions from alpha and beta were summarised. The spin-up contributions formed only self-states alpha, and only self-states spin-down made beta. The results and the difference in beta and alpha contributions were shown in a butterfly plot (Fig. 6). Ni, N, C, Al, and H had equal spin-down and spin-up energies. Despite the fact that all the elements had equal alpha and beta spins as observed (Fig. 6a), the composite shows slight shift at the Fermi level and this can be ascribed to the doping effect as seen in the emergence of a bandgap in the composites in the band structures.
3.4 The optical properties
New material gives a wealth of information about the low-energy excitations that govern the physics of material. Some of these excitations indicate the part of the solar spectrum, where they might be expected to appear. A number of quantities, often called optical constants, describe the response of materials to light/heat. These optical constants, such as the dielectric constant (\(\epsilon\)), the electrical conductivity (\(\sigma\)), the susceptibility (\(\chi\)) and the refractive index (n) among others, are neither constant nor independent but are functions of the pressure, external magnetic field, frequency, as well as temperature. By knowing two of these, one that describes the absorption in the solid and one that describes dispersion, all of the others may be calculated using Maxwell’s [58, 59] equations for macroscopic media as expressed using Gaussian units [60].
The value of reflectivity as a function of an incident angle is calculated using Fresnel’s formulas [61] from absorption index of sample material and refractive index. Our calculated reflectivity against wavelength plot reveals that at low wavelengths, i.e. below 200 nm (Fig. 7a), AlN is the highest. Single-layered heterostructure resulted in a drop in the reflectance, and subsequent addition of the layers show improvement in reflectivity with the C4H6N6Ni2–AlN–AlN–AlN layer having the highest reflectivity from 240 nm into the redshift.
Absorption is the process of converting radiant energy into internal energy of the medium. This occurs by two: mainly by Rayleigh scattering [62] and Compton scattering [63]. Thermoelectric effect occurs when photon/heat energy is consumed to release an electron from atom nucleus [64]. This effect arises from the fact that the potential energy barrier for electrons is finite at the surface of the material. AlN (Fig. 7b) has the least absorption, which mainly occurs in the ultraviolent region of the solar spectrum. Improvement was observed when the layered heterostructures were tested with the three layers being the highest at all wavelengths tested, i.e. 200 to 2500 nm.
The calculated optical conductivity (Fig. 7c) showed C4H6N6Ni2 exhibits a high conductivity in the high-frequency region of the solar spectrum; thus, as the wavelength increases the conductivity decreased in a uniform manner. However, a significant improvement in the conductivity with increase in layers was noted particularly at higher wavelengths with C4H6N6Ni2–AlN–AlN–AlN layer dominating. Thermoelectric materials require high electrical conductivity. Increasing electrical conductivity (σ) entails having a material with high charge carrier mobility, narrow energy gap semiconductors. To lower electron scattering, our calculated electrical conductivity (Table 1) using the Boltzmann constant relation [65] is as follows:
where \(\sigma_{0}\) is a constant, \(E_{\text{a}}\) is the activation energy and k is Boltzmann constant. This show a gradual decrease in conductivity from the thermoelectric material C4H6N6Ni2 surface (101) with single layer to double layers; however, the three-layer system indicated an increase in conductivity and this can be ascribed to a reduction in electron affinity and ionisation energy as observed.
Our study of the permittivity of a material as function of frequency (dielectric function) (Fig. 7d) is derived from Maxwell’s equations, which state that a dielectric-metallic interface can support surface plasmon polaritons. Unique properties of interface waves arise and are the result of dielectric materials optical frequency-dependent dispersion characteristics. At higher wavelength above 1250 nm, C4H6N6Ni2–AlN–AlN–AlN layer showed a high dielectric function. However, the absence of negative permittivity means that the electric displacement vector and the electric field vector point in the same direction. The high wavelengths are appropriate for thermoelectric application indicating improvement in the generated composites having probable infrared wavelength of red heat.
3.5 Mechanism of charge transfer
To understand the charge transfer mechanism between the layers within heterostructures generated, electron density difference is necessary [66]. This will explain the observation on what happens when two surfaces interact. The interfacial interactions are expected to change the charge distribution between surfaces, which result in charge transfer across the heterostructures.
In order to explore the charge transfer process in the C4H6N6Ni2–AlN and C4H6N6Ni2–AlN–AlN heterostructures, three-dimensional charge density difference (Fig. 8) is plotted with the yellow iso-surface representing electron accumulation, while the green iso-surface represents the electron depletion with an iso-surface value of 0.003 Å−3. Figure 8 reveals that the charge densities are redistributed by forming hole- and electron-rich regions within the bulk C4H6N6Ni2, C4H6N6Ni2 surface (101) and AlN sheet interface. This is attributed to the large separation between the AlN sheet and C4H6N6Ni2 surface (101). In addition, a high charge depletion occurred on the bulk C4H6N6Ni2 accumulating on C4H6N6Ni2 surface (101), resulting in hole-rich C4H6N6Ni2 sites. Thus, holes accumulated in the C4H6N6Ni2 bulk region, while electrons accumulated in the region close to the surfaces; therefore, electrons are expected to be excited from the C4H6N6Ni2 to the surface (101) and crossover to AlN. C4H6N6Ni2 acts as electron donor and conduction medium to transport electrons to the AlN surface.
The C4H6N6Ni2–AlN (Fig. 8a) and C4H6N6Ni2–AlN–AlN layer (Fig. 8b) differences in electron density show the Mulliken population analysis [67, 68], and the three-dimensional charge density difference [66] confirms the transfer of interlayer charge between the C4H6N6Ni2–AlN monolayers as previously observed in PDOS analysis. The charge density difference in the two-layer system (Fig. 8b) shows a strong charge relocation at the AlN interface region, which can lead to substantial quantities of charge generation in C4H6N6Ni2 and subsequent transfer increase in the layers, resulting in more charge accumulation in the AlN. This study, therefore, reveals that the interaction at the interface causes not only strong charge distribution fluctuation in each component, but also significant interfacial charge transfer and separation of heterostructures, which determine the mechanism of the enhanced thermos-catalytic performance of the heterostructures.
3.6 Electron affinity and ionisation energy
C4H6N6Ni2, AlN and their heterostructures were calculated for their electron-transporting properties (Fig. 9). The ionisation energy was obtained from work function + EVBM [69], where EVBM = − Work function + 0.5 energy gap [70] and Electron affinity = ionisation potential-band gap [71]. Energies of the band edges and vacuum level were used in calculations of electron affinity and ionisation energy of the individual monolayers as well as their corresponding heterostructures.
The calculated ionisation energy and electron affinity of AlN, C4H6N6Ni2, C4H6N6Ni2–AlN layer, C4H6N6Ni2–AlN–AlN layer and C4H6N6Ni2–AlN–AlN–AlN layer along the x-axis are shown in Fig. 9. AlN had the least ionisation energy of − 1.135 eV, which was followed by C4H6N6Ni2 with 2.814 eV. Generating a single layer resulted in a further increase in the ionisation energy to a maximum value of 4.102 eV in the two layers. However, after generating two layers, the three layers of AlN resulted in a reduction in the ionisation energy, where a similar trend was observed with the least being − 7.145 eV and the maximum electron affinity was 4.042 eV. The reduction in the three-layer system can be ascribed to the shielding effect and the distance from the thermoelectric material. Thus, it will be easy to move an electron from the thermoelectric material into AlN in the composite generated.
4 Conclusion
Using first-principles calculations, we demonstrate that it is possible to generate layered C4H6N6Ni2–AlN heterostructures with increased electrical conductivity and reduced thermal conductivity because of superlative structures. The new heterostructures resulted in improved properties, such as large effective mass, new energy gaps and large density of states at the Fermi level. Increasing electrical conductivity requires high-mobility charge carriers, narrow-gap semiconductors and lower electron scattering. Optical and electronic properties revealed improvement in the electron density of state, conductivity and dielectric function. The benefit arising from the improved electronic properties would lead to improving the charge generation and separation, thus resulting in a better thermoelectric material.
References
Gun OhJ, Ki Hong S, Kim C-K, Hoon Bong J, Shin J, Choi S-Y, Jin Cho B (2014) High performance graphene field effect transistors on an aluminum nitride substrate with high surface phonon energy. Appl Phys Lett 104(19):193112
Ma N, Jena D (2014) Charge scattering and mobility in atomically thin semiconductors. Phys Rev X 4(1):011043
Perebeinos V, Avouris P (2010) Inelastic scattering and current saturation in graphene. Phys Rev B 81(19):195442
Freitag M, Steiner M, Martin Y, Perebeinos V, Chen Z, Tsang JC, Avouris P (2009) Energy dissipation in graphene field-effect transistors. Nano Lett 9(5):1883–1888
Bae M, Islam S, Dorgan V, Pop E (2011) Scaling of high-field transport and localized heating in graphene transistors. ACS Nano 5(10):7936–7944
Chandrasekar H, Mohan N, Bardhan A, Bhat K, Bhat N, Ravishankar N, Raghavan S (2013) An early in situ stress signature of the AlN-Si pre-growth interface for successful integration of nitrides with (111) Si. Appl Phys Lett 103(21):211902
Li J, Lin J, Jiang H (2006) Growth of III-nitride photonic structures on large area silicon substrates. Appl Phys Lett 88(17):171909
Ng GI, Arulkumaran S, Vicknesh S, Wang H, Ang K, Kumar CM, Ranjan K, Lo G-Q, Tripathy S, Boon CC (2012) GaN-on-silicon integration technology. In: 2012 IEEE international symposium on radio-frequency integration technology (RFIT). IEEE, pp 159–161
de Almeida E, de Brito Mota F, de Castilho C, Kakanakova-Georgieva A, Gueorguiev GK (2012) Defects in hexagonal-AlN sheets by first-principles calculations. Eur Phys J B 85(1):1–9
Nolas G, Cohn J, Slack G, Schujman S (1998) Semiconducting Ge clathrates: promising candidates for thermoelectric applications. Appl Phys Lett 73(2):178–180
Ikeda M, Euchner H, Yan X, Tomeš P, Prokofiev A, Prochaska L, Lientschnig G, Svagera R, Hartmann S, Gati E (2019) Kondo-like phonon scattering in thermoelectric clathrates. Nat Commun 10(1):887
Kaur P, Madsen GK, Bera C (2019) Thermoelectric figure of merit and thermal conductivity of type-I clathrate alloy nanowires. MRS Commun 9(1):370–374
Dong Y, Ding X, Yan X, Zhang L, Ju T, Liu C, Rogl P, Paschen S (2019) Structural and thermoelectric properties of Cu substituted Type I clathrates Ba8CuxSi~32−xGa~14. Materials 12(2):237
Grin Y (2019) Inhomogeneity and anisotropy of chemical bonding and thermoelectric properties of materials. J Solid State Chem 274:329–336
Yang J, Wang Y, Yang H, Tang W, Yang J, Chen L, Zhang W (2019) Thermal transport in thermoelectric materials with chemical bond hierarchy. J Phys: Condens Matter 31(18):183002
Rogl P (2006) Formation and crystal chemistry of clathrates. In: Rowe DM (ed) Thermoelectrics handbook: macro to nano. CRC Press, Boca Raton
Saramat A, Svensson G, Palmqvist A, Stiewe C, Mueller E, Platzek D, Williams S, Rowe D, Bryan J, Stucky G (2006) Large thermoelectric figure of merit at high temperature in Czochralski-grown clathrate Ba8Ga16Ge30. J Appl Phys 99(2):023708
Kitamura K, Yamamoto J, Iyi N, Kirnura S, Hayashi T (1992) Stoichiometric LiNbO3 single crystal growth by double crucible Czochralski method using automatic powder supply system. J Cryst Growth 116(3–4):327–332
Bobnar M, Böhme B, Wedel M, Burkhardt U, Ormeci A, Prots Y, Drathen C, Liang Y, Nguyen HD, Baitinger M (2015) Distribution of Al atoms in the clathrate-I phase Ba8AlxSi46−x at x = 6.9. Dalton Trans 44(28):12680–12687
Christensen M, Lock N, Overgaard J, Iversen BB (2006) Crystal structures of thermoelectric n- and p-type Ba8Ga16Ge30 studied by single crystal, multitemperature, neutron diffraction, conventional X-ray diffraction and resonant synchrotron X-ray diffraction. J Am Chem Soc 128(49):15657–15665
Webb GA (1979) C. P. Slichter. Principles of magnetic resonance. Springer series in Solid-State Sciences, Vol. 1, Ed by M. Cardoua, P. Fulde and H. J. Queisser, Springer-Verlag, Berlin (1978). 397 pages. DM 54, US$7.00. Organ Magn Reson 12(3):IV. https://doi.org/10.1002/mrc.1270120316
Condron CL, Martin J, Nolas G, Piccoli PM, Schultz AJ, Kauzlarich SM (2006) Structure and thermoelectric characterization of Ba8Al14Si31. Inorg Chem 45(23):9381–9386
Yan X, Bauer E, Rogl P, Paschen S (2013) Structural and thermoelectric properties of Ba8Cu5SixGe41−x clathrates. Phys Rev B 87(11):115206
Xu J, Wu J, Heguri S, Mu G, Tanabe Y, Tanigaki K (2012) Low-temperature physical properties of Ba8NixGe46−x (x = 3, 4, 6). J Electron Mater 41(6):1177–1180
Shi X, Yang J, Bai S, Yang J, Wang H, Chi M, Salvador JR, Zhang W, Chen L, Wong-Ng W (2010) On the design of high-efficiency thermoelectric clathrates through a systematic cross-substitution of framework elements. Adv Funct Mater 20(5):755–763
Falmbigl M, Grytsiv A, Rogl P, Heinrich P, Royanian E, Bauer E (2013) Tuning of band gap and thermoelectric properties of type-I clathrate Ba8NixZnyGe46−x−y−zSnz. J Alloys Compd 567:65–72
Sirusi AA, Ross JH, Yan X, Paschen S (2015) NMR study of Ba8Cu5SixGe41−x clathrate semiconductors. Phys Chem Chem Phys 17(26):16991–16996
Ångqvist M, Lindroth DO, Erhart P (2016) Optimization of the thermoelectric power factor: coupling between chemical order and transport properties. Chem Mater 28(19):6877–6885
Jupille J, Thornton G (2015) Defects at oxide surfaces. In: Jacques J, Geoff T (eds) Springer series in surface sciences, vol 58. Springer, Cham
von Oertzen GU, Gerson AR (2007) The effects of O deficiency on the electronic structure of rutile TiO2. J Phys Chem Solids 68(3):324–330
Mishra V, Warshi MK, Sati A, Kumar A, Mishra V, Sagdeo A, Kumar R, Sagdeo PR (2018) Diffuse reflectance spectroscopy: an effective tool to probe the defect states in wide band gap semiconducting materials. Mater Sci Semicond Process 86:151–156
Carrillo-Cabrera W, Budnyk S, Prots Y, Grin Y (2004) Ba8Ge43 revisited: a 2a′ × 2a′ × 2a′ superstructure of the Clathrate-I type with full vacancy ordering. Z Anorg Allg Chem 630(13–14):2267–2276
Kaltzoglou A, Fässler T, Christensen M, Johnsen S, Iversen B, Presniakov I, Sobolev A, Shevelkov A (2008) Effects of the order–disorder phase transition on the physical properties of A8Sn44□2 (A = Rb, Cs). J Mater Chem 18(46):5630–5637
Mishra V, Sati A, Warshi MK, Phatangare AB, Dhole S, Bhoraskar V, Ghosh H, Sagdeo A, Mishra V, Kumar R (2018) Effect of electron irradiation on the optical properties of SrTiO3: an experimental and theoretical investigations. Mater Res Express 5(3):036210
Zerec I, Keppens V, McGuire M, Mandrus D, Sales BC, Thalmeier P (2004) Four-well tunneling states and elastic response of clathrates. Phys Rev Lett 92(18):185502
Suekuni K, Avila M, Umeo K, Takabatake T (2007) Cage-size control of guest vibration and thermal conductivity in Sr8Ga16Si30−xGex. Phys Rev B 75(19):195210
Avila M, Suekuni K, Umeo K, Fukuoka H, Yamanaka S, Takabatake T (2006) Glasslike versus crystalline thermal conductivity in carrier-tuned Ba8Ga16X30 clathrates (X = Ge, Sn). Phys Rev B 74(12):125109
Chakoumakos B, Sales B, Mandrus D, Nolas G (2000) Structural disorder and thermal conductivity of the semiconducting clathrate Sr8Ga16Ge30. J Alloys Compd 296(1–2):80–86
Christensen M, Johnsen S, Iversen BB (2010) Thermoelectric clathrates of type I. Dalton Trans 39(4):978–992
Nolas G, Weakley T, Cohn J, Sharma R (2000) Structural properties and thermal conductivity of crystalline Ge clathrates. Phys Rev B 61(6):3845–3850
Nolas GS, Chakoumakos BC, Mahieu B, Long GJ, Weakley T (2000) Structural characterization and thermal conductivity of type-I tin clathrates. Chem Mater 12(7):1947–1953
Fässler TF (2011) Zintl phases: principles and recent developments, vol 139. Springer, Heidelberg
DiSalvo FJ (1999) Thermoelectric cooling and power generation. Science 285(5428):703–706
Cole T (1983) Thermoelectric energy conversion with solid electrolytes. Science 221(4614):915–920
Wood C (1988) Materials for thermoelectric energy conversion. Rep Prog Phys 51(4):459
Snyder GJ, Toberer ES (2011) Complex thermoelectric materials. In: Dusastre V (ed) Materials for sustainable energy: a collection of peer-reviewed research and review articles from Nature Publishing Group. World Scientific, Singapore, pp 101–110
Segall MD, Philip JDL, Probert MJ, Pickard CJ, Hasnip PJ, Clark SJ, Payne MC (2002) First-principles simulation: ideas, illustrations and the Castep code. J Phys: Condens Matter 14(11):2717–2744
Materials Studio Simulation Environment (2019) Release 2019. Accelrys Software Inc, San Diego, CA
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868
Song J-W, Yamashita K, Hirao K (2011) Communication: a new hybrid exchange correlation functional for band-gap calculations using a short-range Gaussian attenuation (Gaussian–Perdue–Burke–Ernzerhof). J Chem Phys 135(7):071103
White J, Bird D (1994) Implementation of gradient-corrected exchange-correlation potentials in Car-Parrinello total-energy calculations. Phys Rev B 50(7):4954–4957
Mattila T, Nieminen RM (1996) Ab initio study of oxygen point defects in GaAs, GaN, and AlN. Phys Rev B 54(23):16676–16682
Li J, Nam K, Nakarmi M, Lin J, Jiang H, Carrier P, Wei S-H (2003) Band structure and fundamental optical transitions in wurtzite AlN. Appl Phys Lett 83(25):5163–5165
Silveira E, Freitas J, Schujman S, Schowalter L (2008) AlN bandgap temperature dependence from its optical properties. J Cryst Growth 310(17):4007–4010
Mishra V, Warshi MK, Sati A, Kumar A, Mishra V, Kumar R, Sagdeo P (2019) Investigation of temperature-dependent optical properties of TiO2 using diffuse reflectance spectroscopy. SN Appl Sci 1(3):241
Hicks L, Dresselhaus M (1993) Effect of quantum-well structures on the thermoelectric figure of merit. Phys Rev B 47(19):12727–12731
Larson P, Mahanti S, Kanatzidis MG (2000) Electronic structure and transport of Bi2Te3 and BaBiTe3. Phys Rev B 61(12):8162–8171
Maxwell JC (1865) VIII. A dynamical theory of the electromagnetic field. Philos Trans R Soc Lond 155:459–512
Maxwell JC (1864) A dynamical theory of the electromagnetic field, vol 13. The Society, London
Potter R (1997) Optical properties. In: Ghosh G, Palik ED (eds) Handbook of optical constants of solids, five-volume set: handbook of thermo-optic coefficients of optical materials with applications. Elsevier, Amsterdam, p 11
Judd DB (1942) Fresnel reflection of diffusely incident light. J Res Natl Bur Stand 29(5):329–332
Clays K, Persoons A (1991) Hyper-Rayleigh scattering in solution. Phys Rev Lett 66(23):2980–2983
Williams B (1977) Compton scattering: the investigation of electron momentum distributions, vol 10. McGraw-Hill International Book Co, New York
Šantić B, Desnica U (1990) Thermoelectric effect spectroscopy of deep levels—application to semi-insulating GaAs. Appl Phys Lett 56(26):2636–2638
Gaur K, Lal H (1985) Electrical transport in light rare-earth vanadates. JMatS 20(9):3167–3176
Shi L, Xu C, Yuan H (2011) A CASTEP study on magnetic properties of C-doped ZnO crystal. Phys B 406(17):3187–3191
Mulliken RS (1955) Electronic population analysis on LCAO–MO molecular wave functions. I. J Chem Phys 23(10):1833–1840
Csizmadia I (1976) Theory and practice of MO calculations on organic molecules. In: Csizmadia I (ed) Progress in theoretical organic chemistry, vol 1. Elsevier Scientific Publishing Company, New York
Yuan K, Chen L, Li F, Chen Y (2014) Nanostructured hybrid ZnO@CdS nanowalls grown in situ for inverted polymer solar cells. J Mater Chem C 2(6):1018–1027
Opoku F, Govender KK, van Sittert CGCE, Govender PP (2018) Insights into the photocatalytic mechanism of mediator-free direct Z-scheme g-C3N4/Bi2MoO6(010) and g-C3N4/Bi2WO6(010) heterostructures: a hybrid density functional theory study. Appl Surf Sci 427:487–498
Louis E, San-Fabián E, Díaz-García MA, Chiappe G, Vergés JA (2017) Are electron affinity and ionization potential intrinsic parameters to predict the electron or hole acceptor character of amorphous molecular materials? J Phys Chem Lett 8(11):2445–2449
Acknowledgements
The authors would like to acknowledge the financial contributions from the Faculty of Science: University of Johannesburg, South Africa: Centre for Nanomaterials Science Research, Department of chemical sciences and the National Research Foundation (TTK14052167682). EMK acknowledges the financial support from URC International Scholarship from the University of Johannesburg. The authors are also grateful to the Centre for High Performance Computing (CHPC) for computational resources provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kiarii, E.M., Govender, K.K. & Govender, P.P. A theoretical study of 2D AlN on 3D C4H6N6Ni2 clathrate thermoelectric material composites. SN Appl. Sci. 1, 1638 (2019). https://doi.org/10.1007/s42452-019-1696-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1696-5