Abstract
Concern regarding the environment is increasing day by day. Industrialized countries are aware of the problem that waste creates and are focusing efforts toward solving it by recycling and reusing different kinds of waste. Posidonia oceanica is a seagrass species endemic to the Mediterranean Sea, which creates tons of fiber waste that accumulates along the shoreline. This work demonstrates the feasibility of obtaining substrates for plants from P. oceanica fibers. We analyze the fiber structure by scanning electron microscopy, and observe the germination rate and the average germination time for lentils seeded on Posidonia substrates in comparison to those seeded on cotton. Two different lengths of Posidonia fiber are studied, as well as the influence of desizing and bleaching. The values for gemination rate and germination time showed comparable or even better results than cotton, allowing us to conclude that Posidonia substrates can be used for agricultural purposes. These results offer a new application for the valorization of waste from the Mediterranean coast by reusing the Posidonia fibers. Moreover, when the fibers are bound together by means of a biopolymer, chitosan, the results show that the germination rate is higher than without chitosan treatment, and the average germination time is reduced by approximately 1 day. Thus, we can conclude that the germination process is faster and more effective.
Similar content being viewed by others
1 Introduction
Posidonia oceanica (PO) is an endemic marine plant from the Mediterranean Sea. The fiber residues of the plants are commonly observed on Mediterranean beaches. PO plays a critical ecological role, as it acts as natural protection for the seabed, thus preventing the advance of erosive processes [1]. PO fruit can be found in the sand on beaches as balls of brown fibers, also known as Neptune balls, as shown in Fig. 1.
These balls can be considered as a renewable resource, as they are made of lignocellulosic fibers. Many studies have been conducted regarding PO composition. Ferrero et al. [2] analyzed its composition and reported that it is composed of 70% cellulose, 8% hemicellulose, 12% inorganic components and around 10% lignin. This means that cellulose is the predominant component, and there is a large carbohydrate fraction, around 90%. Lignocellulosic-based fibers are the most widely used as biodegradable reinforcing elements for composite materials [3]. Other authors [4] have found the same percentage of inorganic components but a different percentage of cellulose (40%). This difference could be due to differences in the hydrolysis method used or the area from which the material was collected.
Local municipalities invest huge sums of money in removing these fibers from the sand or beaches in order to keep them clean, especially during the summer season. The problem is not only the funds invested for removal, but also for management of the tons of this waste product. One possible solution is to use the fibers for various purposes. PO has been studied as a low-cost adsorbent for removing dyes [5] and ammonium [6], as a substrate for papermaking [4], as a starting material for cellulose derivatives [7] and as green composites [2]. Valorization of these fibers is important, as noted by Khiari et al. [3], and they can also be considered as a potential fuel for combustion [8]. Indeed, a wide variety of applications exist. However, to the best of our knowledge, the current study is the first to examine the use of the fibers in agriculture.
Focusing the study on agricultural solutions, and moving to seed germination, it is important to note that the growth of seeds is important for human subsistence. Seeds can be contaminated by external processes such as transport, conditioning, storage and packaging, resulting in germination problems. Mitra et al. [9] conducted studies on plasma treatment to demonstrate the importance of reducing the microbiota attached to the seed surface to improve the rate of germination.
Therefore, the aim of this study was to determine whether the fibers from PO could be used for agricultural purposes. We prepared substrates from PO fibers, and lentil seeds were germinated in order to determine the possibility of reusing the residues as agricultural substrate. The separation of fibers from Neptune balls was conducted manually, producing what we called long fibers, or by mechanical milling treatment, which produced short fibers. We prepared the PO fibers by washing them with water. Some of the fibers were tested without any other treatment, whereas some were tested after a bleaching treatment. All treated fibers (rinsed or bleached) were also studied once they had been compacted as a nonwoven material with chitosan. Different substrates were prepared, and lentils were seeded on them and compared with cotton substrates.
2 Experimental
2.1 Materials
PO balls were collected from Oliva and Denia beaches, both located on the Spanish Mediterranean Coast at the Comunidad Valenciana. Lentils were purchased from the agricultural cooperative in Cocentaina and in the Comunidad Valenciana. They were classified as ecological agriculture. Leophen RA was used as a wetting agent and was supplied by BASF. NaOH, sodium silicate and H2O2 were supplied by Panreac. Chitosan of medium molecular weight was supplied by Sigma Aldrich, and acetic acid to solubilize chitosan was supplied by Panreac.
2.2 Preparation of Posidonia fiber
PO balls were subjected to mechanical or hand milling to separate the PO fibers from the ball. After the milling process, fibers were washed with distilled water in order to remove sand, vegetables and other waste caught on the ball. Clean fibers were dried in an oven at 60 °C for 4 h and kept at room temperature.
2.3 Desizing and bleaching of Posidonia fiber
Washed fibers were treated with NaOH (8 g/L, 1:40 w/v) and Leophen RA (wetting agent; 1 g/L, 1:40) solution at 90–100 °C for 1 h. All fibers were then washed with distilled water and dried at 60 °C for 4 h.
Bleaching was applied at a 1:40 w/v ratio. The liquor bath composition was as follows:
-
25 g/L H2O2
-
1 g/L NaOH
-
0.5 g/L Leophen RA (wetting agent)
-
1 g/L Sodium silicate (Na2O 8%, SiO2 27%) (stabilizer)
The bath was heated to 100 °C and the temperature maintained for 1 h. All fibers were then washed with distilled water and dried at 60 °C for 4 h.
2.4 Germination process
There are standard procedures commonly used to enhance the germination of dormant seeds [10], which are based on washing and chilling. Other types of seed treatments to improve plant growth and productivity have been described by Taylor et al. [11]. However, this study is not based on germination efficiency or yield; consequently, in our test we used commercial lentil seeds, and they were only washed.
In order to prepare the substrate, 5 g of fiber was placed on a glass vessel, and four lentils were distributed with approximately equidistant spacing (see Fig. 2). The lentils were then covered with an additional 5 g of the same fiber. Five vessels were prepared for the same kind of fiber, resulting in a total of 20 seeds. The study was conducted with four different substrates:
-
Posidonia oceanica washed (PO washed)
-
Posidonia oceanica washed + desized (PO desized)
-
Posidonia oceanica washed + desized + bleached (PO bleached)
-
Bleached cotton fibers (cotton)
Germinated seeds were counted at the same time every day. When the roots reached 10 mm in length, the seed was considered germinated [12]. When germination was complete, the germination rate was calculated according to Eq. (1).
The average germination time was calculated according to the following formula [13], where f is the number of seeds germinated on the census day, and x indicates the number of days counted
In order to ensure that the fibers were adequately moist to induce the germination process, 5 g of water was sprayed on the upper fibers every morning for 7 days; on the eighth day, germination was evaluated. Samples were kept in the laboratory at room temperature (20–23 °C).
2.5 Scanning electron microscopy
Every sample was observed by means of scanning electron microscopy (SEM) with a field-emission scanning electron microscope (FESEM, ZEISS ULTRA 55). Each sample was placed on a surface and covered with a layer of gold in order to make them conductive using a sputter coater (EMITECH SC7620, Quorum Technologies Ltd., East Sussex, UK). The samples were analyzed with the appropriate magnification and with an acceleration voltage of 10 kV.
2.6 Cohesion treatment
Once the fibers had been separated from the Neptune ball, they were tested without any cohesive treatment. However, considering the possibility for developing industrial products, if the fibers were bound together into a fixed shape, they could be sold, for example, as flowerpots. This is achieved using a binding biopolymer. In this study, we used chitosan, a biopolymer from the shells of shrimp and other crustaceans. We prepared 5 g/L of chitosan, which was dissolved by magnetic stirring for 24 h at room temperature with 3 g/L of acetic acid.
For cohesion of the fibers, 5 g of PO was placed on a glass vessel and was sprayed with 10 mL of chitosan solution. The glass vessel with the fibers and chitosan solution was then dried in an oven at 150 °C for 10 min. Once it was cooled, we could observe that the fibers were bound, and the structure was compacted and easy to manipulate. Another sample with the same kind of fibers (washed, desized, bleached, or cotton) was prepared to cover the seeds and conduct the germination test.
3 Results and conclusions
Fibers were obtained by opening or separating the fibers from the Neptune balls by hand or by mechanical milling. During this process, a solid residue was clearly observed, consisting basically of sand. PO fibers were examined by SEM, and it could be clearly observed (Fig. 3a) that the fibers consisted of a series of thin, hollow microfibers covered by a thin layer that kept them compactly adhered. Moreover, it was noted that some particles still remained on the fiber surface after it had been washed with water (Fig. 3b).
The desizing treatment applied to the fibers directly affected the coating keeping the microfibrils bound together and compact (Fig. 3c). When the bleaching process was applied to the fibers, it was clearly observed that the coating surface was practically dissolved, and only small particles from the original coating remained on the microfiber surface, as shown in Fig. 4.
Figure 5 illustrates the ability of lentil seeds to germinate on PO fiber. The lentil shoot clearly appears among the brown PO fibers.
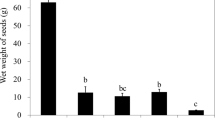
Regarding the germination process, Table 1 shows the results for the different samples tested. Comparisons between cotton and PO show that seeds on cotton germinated later (6.88 days) than those on PO (5.72–5.24 days). This longer germination time demonstrates the feasibility of using PO as a substrate. It also appears to be more effective than other substrates such as cotton fibers that have traditionally been used for such experiments. This behavior can be attributed to the water retention by both the cellulose and the inner part of the fibrils, which allowed more moisture to be retained by the PO versus cotton fibers.
Among PO samples, the germination time was around 5 days. The germination time was slightly higher when the PO fiber was only washed or desized (5.72 days) in comparison to bleaching (100%). It seems that the chemical treatment on the fiber improves the germination process and allows the seeds to germinate faster. This could be due to the fact that the chemical treatment damages the coating that keeps the microfibers compacted. When the coating was practically dissolved (PO bleached), the average germination time (5.24 days) was lower than when the PO was desized and the coating was partially damaged (5.54 days) (Table 1). Germination values for PO once the coating has been removed were the highest (100%), which could be because the fiber retains more water and the seeds can germinate faster. The coating of the fiber limits water penetration. Once the coating has been removed, it is easier for water to reach the cellulose in the fiber, and moisture is retained there.
For the Neptune balls that were mechanically treated to separate the fiber from the ball, the length was measured, and they were found to be considerably shorter (2–5 mm) than the original fiber (6–15 mm). To determine whether length had an influence, the same test was conducted with long fibers manually separated from the Neptune balls. Table 2 shows the results for the long fibers. Apparently, the longer fiber allowed the seeds to germinate slightly faster than short fiber. This can be attributed to the interfibrillar space: the longer the fiber, the greater the interfibrillar space, and consequently, more water can be retained on the substrate.
Despite PO presenting some level of salinity, Tables 1 and 2 both show no difference between the washed and desized fibers; however, the influence of salinity on plant growth should be investigated in further studies. It is possible that salinity has not influenced the results, as spraying water washes it out from the fibers. However, further tests will be conducted to demonstrate this. Once it was demonstrated that PO fibers are suitable for hosting seed growth, a biopolymer was used to give cohesion to the fibers and simulate a flowerpot.
As described in the experimental section, chitosan was the selected biopolymer, and Table 3 shows the results from the germination test.
Chitosan is known for its antimicrobial effects. The results from Table 3 show that the chitosan treatment led to an increase in the rate of germination and a reduction in germination time, which can be attributed to its antibacterial properties. This effect was also demonstrated by Mitra et al. [9], who noted the importance of reducing the microbiota on the seed surface. Thus, it seems that the germination process is enhanced by the presence of chitosan.
4 Conclusions
This study showed substantial differences in the behavior of lentil seeds depending on fiber length. The lentils seeded on PO substrates germinated more rapidly than those on cotton, demonstrating the potential for agricultural use of PO fiber and the possible valorization of PO fiber.
However, some differences were observed when the PO fiber had been treated. Desizing affected the shell covering on the fiber surface, and bleaching removed it entirely. When the covering was altered or removed, a greater number of lentil seeds were able to germinate and at a faster rate. This is probably due to the characteristic structure of the fibers, as it has been demonstrated that the presence of a hollow inner region enables greater retention of moisture on the substrate.
For valorization of the PO fibers, chitosan, a biopolymer, was used as a binding agent. The process resulted in a nonwoven PO bound chemically by chitosan. Regarding the germination process, we were able to observe the antibacterial properties conferred by chitosan when the fibers were treated, which improved both the germination rate and the germination time. Consequently, we can confirm the effectiveness of this polymer used as a binding agent.
To sum up, we can conclude that PO fibers can be valorized if they are used as a substrate for seeds, at least for lentils. Further research is needed on different seeds and with other binding agents for the design of flowerpots or planters.
References
Ferrero B, Fombuena V, Fenollar O, Boronat T, Balart R (2015) Development of natural fiber-reinforced plastics (NFRP) based on biobased polyethylene and waste fibers from Posidonia oceanica seaweed. Polym Compos 36(8):1378–1385
Ferrero B, Boronat T, Moriana R, Fenollar O, Balart R (2013) Green composites based on wheat gluten matrix and Posidonia oceanica waste fibers as reinforcements. Polym Compos 34(10):1663–1669
Khiari R, Marrakchi Z, Belgacem MN, Mauret E, Mhenni F (2011) New lignocellulosic fibres-reinforced composite materials: a step forward in the valorisation of the Posidonia oceanica balls. Compos Sci Technol 71(16):1867–1872
Khiari R, Mhenni MF, Belgacem MN, Mauret E (2010) Chemical composition and pulping of date palm rachis and Posidonia oceanica—a comparison with other wood and non-wood fibre sources. Bioresour Technol 101:775–780
Ncibi MC, Mahjoub B, Seffen M (2007) Kinetic and equilibrium studies of methylene blue biosorption by Posidonia oceanica (L.) fibres. J Hazard Mater 139(2):280–285
Wahab MA, Jellali S, Jedidi N (2010) Effect of temperature and pH on the biosorption of ammonium onto Posidonia oceanica fibers: equilibrium, and kinetic modeling studies. Bioresour Technol 101(22):8606–8615
Aguir C, Mhenni MF (2006) Experimental study on carboxymethylation of cellulose extracted from Posidonia oceanica. J Appl Polym Sci 98:1808–1816
Plis A, Lasek J, Skawińska A, Kopczyński M (2014) Thermo-chemical properties of biomass from Posidonia oceanica. Chem Pap 68(7):879–889
Mitra A, Li YF, Klämpfl TG, Shimizu T, Jeon J, Morfill GE, Zimmermann JL (2014) Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioprocess Technol 7(3):645–653
International Seed Testing Association (1985) International rules for seed testing 1985. Seed Sci Technol 13(2):299–513
Taylor AG, Harman GE (1990) Concepts and technologies of selected seed treatments. Annu Rev Phytopathol 28(1):321–339
Goertz SH, ve Coons JM (1989) Germination response of tepary and navy beans to sodium chloride and temperature. HortScience 24(6):923–925
Kaya MD, Kaya G, Kolsarıcı Ö (2005) The effects of NaCl concentrations on germination and excretion of some Brassica species. J Agric Sci 11(4):448–452
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marilés, BA., Jaime, GP., Eva, BB. et al. Fibers of the seagrass Posidonia oceanica as substrate for germination of lentil seeds. SN Appl. Sci. 1, 1414 (2019). https://doi.org/10.1007/s42452-019-1420-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1420-5