Abstract
The aim of this study was to check the ethanol yield of locally available lignocellulosic biomass (Eragrostis airoides Nees) by enzymatic saccharification and microbial fermentation. Two types of physico-chemical pretreatments were studied (dilute acid and alkaline). Physico-chemical pretreatment with dilute acid showed to be superior over the alkaline treatment with respect to the rate of enzyme hydrolysis and ethanol productivity. In the pretreatment conditions, the 5% acid + autoclave yielded the highest concentrations of total reducing sugar (25.46 g/L) and enzymatic hydrolysis (9.13 g/L). Fermentation of the E. airoides hydrolyzates by Saccharomyces cerevisiae produced a fermentation yield of 17.56 g/L ethanol. Therefore, E. airoides could be utilized to produce bioethanol and it can be a potential candidate for future bioethanol production as suggested by the analysis.
Similar content being viewed by others
1 Introduction
The use of fossil fuels and its adverse effects resulting in the global warming by emitting greenhouse gases became a matter of concern to the human nation [1]. Several countries have already started working on progressive alternative renewable energy production in order to replace the conventional fossil fuels, especially in the transport sector. In this context, bioethanol from lignocellulosic biomass (LCB) is considered to be one of the important promising alternative liquid fuels [2]. The use of lignocellulosic biomass as feedstock for bioethanol production [3] has certain advantages over the fossil fuels. LCB has a high potential for ethanol production, they are abundant renewable resources, has a variety of source, do not compete with the first generation of biofuel, and could integrate as Integrated Biomass Utilization System (IBUS) [4].
The traditional production of bioethanol involves fermentation of sugars derived from sugarcane or starch followed by ethanol recovery [5]. Biofuels produced from these processes is termed as “first generation of biofuels”. The first generation of biofuel has certain disadvantages like; it competes with the food crops. The second generation of biofuel (based on non-food crops) has a greater potential of sustainability as compared to former. LCB falls under the second generation of biofuel and showed potential conversion to bioethanol. Structurally, LCB is composed of cellulose, hemicellulose and lignin [6, 7] with a trace amount of inorganic materials. A Cellulose portion of the LCB is the predominant natural biopolymer with great abundance, sustainability supply and relatively low cost [8]. LCB is particularly attractive as feedstock and could be utilized for production of ethanol in three-step process: firstly, it is pretreated with dilute acid to rupture the polymeric structure thereby increasing the enzyme susceptibility; secondly, the cellulose is converted to glucose sugars by enzymatic hydrolysis; and thirdly, conversion of the resulting hexose sugars into ethanol by fermentation.
The pretreatment technology is one of the main steps involved in the conversion of biomass to bioethanol production [9, 10]. It helps in loosened the cellulose fibres from the matrix of lignin, thereby allowing the enzyme to hydrolyze. The treatment also provides more enzyme susceptibility to the biomass. Various types of pretreatment (physical, chemical and/or biological or combination of all) have been developed in other to yield more ethanol cost-effectively. However, not every kind of LCB can be pretreated in the same way in the same conditions [11]. The pretreatment methods, conditions, will be different according to the type of biomass used. For example, steam explosion pretreatment of agro-residues like corn stover and rice straw had been tested and found that an additional acid hydrolysis step was needed for producing high sugars from the biomass, thus need to put more research into determine the best possible pretreatment strategy that can satisfy the features of good pretreatment [11].
There are mainly two approaches to saccharification; acid based and enzyme based. The enzyme -based technology has certain advantages over the acid based treatment due to higher conversion efficiency, lack of inhibitory compounds, no need of recycling the acid, non-corrosive, non-toxic to the environment etc. [12]. For example, several strains of fungal have been developed since then to enhance the cellulase production due to increase cost of the fermentation process. One such commercially available enzyme used in the production of cellulase is Trichoderma reesei [13].
In India, the ethanol is mainly produced from sugarcane and molasses in large quantity [14]. India stands at fifth position in total ethanol production in the world. It contribute around 3% whereas, USA, Brazil, EU and China contributes 53%, 21%, 6%, 7% respectively. The present context of 5% blending will increase to 20% by 2017 according to National Biofuel Policy 2008, Govt. of India. It can be mentioned that the total demand for ethanol in India might rise to 5.7 billion liters by 2017 [15]. To fulfil this gap between demand and supply, India needs to look upon the use of non-food crops, lignocellulose biomass and other waste materials for bioethanol production.
India is the world’s second largest producer of sugar but contributes relatively low ethanol [16]. It can be noted that India is home to an extraordinary variety of climate regions, having tropical, temperate and alpine regions. This indicates that India has a high potential of producing a variety of lignocellulosic biomass in terms of biodiversity and availability. India has great advantages of being as three biodiversity hotspot (The Western Ghats, The Eastern Himalayas and Indo-Burma), allow an easy adaptation of different species and exploration of new raw material as energy crops such as grasses. North-East India is one of the national important regions with Indo-Burma biodiversity hotspot. The region has eight states with varieties of flora and fauna.
The present work focused on the physico-chemical compositional analysis and cellulosic bioethanol production from grass sp. Eragrostis airoides by enzymatic hydrolysis and microbial fermentation collected from North-East India. To the best of our knowledge, this feedstock has not been reported elsewhere on bioethanol production. The physico-chemical characterization of Eragrostis airoides grass was earlier reported by [17]. The plant biomass was selected based on the fact that, this biomass could be utilized as one of the best potential candidate for bioethanol production [17]. The brief information about the plant species is given in the discussion section (Table 4 of ESM). This biomass T. reesei was used as cellulase for sugar released from the biomass. The solid residue after pretreatment was utilized for enzymatic hydrolysis and then fermentation was carried out by using Saccharomyces cerevisiae. Our overall idea was to produce the bioethanol from LCB which are not explored or attempted earlier for bioethanol production. We aim to explore such biomasses which are growing abundantly in hilly terrain areas and this could be future substrate for bioethanol production.
1.1 Novelty of this research article
We have thoroughly search the literature and found that there is huge gap in producing biofuel using different sources of feedstock. The feedstock such as lignocellulosic biomass which comes under second generation of biofuel will be future raw materials for biofuel production. In addition, the future biofuel production will be more localized to the domestic and resources available in the native villages. In this regards, it is very essential to study the energy content of different raw materials so that it can be utilized as feedstock for biofuel generation. We have explored a potential cellulosic grass biomass called as Eragrostis airoides Nees grass for better yield of bioethanol and we got the results which showing higher rate than other cellulosic biomass.
2 Materials and methods
2.1 Plant material
Eragrostis airoides Nees was collected from Assam, a gateway of North-East India. This grass was collected during the total inflorescence period and handful amount of biomass was handled carefully. Some of the twig containing inflorescence was separated out. The remaining biomass was dried in air. The dried sample was made a fine powder to a particle size less than 3 mm. The ground material was used as feedstock for bioethanol production.
2.2 Herbarium preparation and species identification
The twig containing inflorescence was washed thoroughly, pressed, dried, poisoned and pasted on the two sheets of the herbarium [17]. One copy of the herbarium specimen was submitted to Herbarium Unit of Botany Department of Gauhati University, Assam India for species taxonomical identification. The voucher number obtained from the herbarium unit was recorded properly. Another copy of the same herbarium specimen was maintained at Indian Institute of Technology Guwahati (IITG), Assam, India for future references and documentation.
2.3 Proximate analysis
The physico-chemical parameters of the collected sample were analyzed based on the proximate composition of the biomass as reported earlier [17]. Briefly, proximate analysis includes moisture, volatile, ash, fixed carbon, total solid, Thermogravimetric analysis (TGA) and X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR). The fiber analysis was performed using the fibra plus automatic system (Pelican). The detailed biomass composition is given in the discussion section [17].
2.4 Microorganisms and maintenance
The yeast strain S. cerevisiae (MTCC 170) to be used for fermentation process was procured from IMTECH, Chandigarh India. The microorganism was grown at 30 °C maintained at 4 °C on MGYP agar plates containing (g/L) glucose, 10; yeast extract, 3; malt extract, 3; peptone, 5; agar 20 (pH 5.0). The cellulase from Trichoderma reesei ATCC 26921 was procured from sigma and used for the enzymatic hydrolysis.
2.5 Pretreatments
2.5.1 Physico-chemical pretreatments
The biomass was treated with different concentration of chemicals (acids and alkali). The concentration of the chemicals was examined properly. In all experiment 10% (w/v) powdered biomass in aqueous solution was taken. After the treatment, the biomass was filtered using a muslin cloth and washed repeatedly with distilled water until the neutral pH of the washed water was achieved. The biomass residue was dried overnight in an oven dry at 60 °C. This solid residue was used for further enzymatic hydrolysis. The filtrate was analysed for total reducing sugar released during pretreatment. In this study, the physical and chemical methods of pretreatment were applied simultaneously. The autoclave pressure was released swiftly after the pretreatment.
2.5.1.1 Acid + autoclaving
The biomass was subjected to different concentration of dilute sulphuric acid (1%, 3%, 5%,7% w/v), at 121 °C, 15 psi for 20 min in a 250 ml Erlenmeyer flask.
2.5.1.2 Alkali + autoclaving
The biomass was subjected to different concentration of sodium hydroxide (1%, 3%, 5%, 7% w/v) at 121 °C for 20 min in a 250 ml Erlenmeyer flask.
2.5.2 Total reducing sugars (TRS)
The total reducing sugars produced from pretreatment and hydrolysis of cellulose was measured by the dinitrosalicylic acid (DNS) method [18]. The sugar containing biomass sample (after pretreatment and hydrolysis) were centrifuged at 11,000×g for 10 min. The supernatant was collected and analysed for sugar content using UV- visible spectrophotometer by running the standard glucose solution. The readings were taken at 550 nm.
2.6 Enzyme hydrolysis
The pretreated biomass was used as a carbohydrate substrate for enzymatic hydrolysis. The commercially available cellulase from Trichoderma reesei ATCC 26921 was procured from Sigma and used in enzymatic saccharification. The pretreated biomass (10 gm) was dissolved in a 100 ml of sodium acetate buffer at pH 4.7 and it was autoclave at 121 °C, 15 psi, for 20 min. The enzyme was loaded to the sample after it was cool down. The mixture was incubated in a shaker water bath at 50 °C, 75 rpm for 4 days. The reaction time was calculated as 1, 2, 3 and 4 days. The reaction was stopped by placing the sample flask in boiling water for 5 min. The mixture was filtered and glucose concentration was measured.
2.6.1 Enzyme hydrolysis rate (ν)
The enzyme hydrolysis rate was calculated on the enzyme hydrolysis time. The ν was plotted as the concentration of glucose released per hydrolysis time.
where ν, enzyme hydrolysis rate (mg/ml glucose per hour); Glut, concentration of glucose at time t (mg/ml); Gluo, initial glucose concentration at time, 0 h (mg/ml); t, hydrolysis time (h) and to, 0 h.
2.7 Analytical methods
The presence of sugars (glucose, xylose and arabinose) released during the pretreatment technology in the filtered hydrolysates was detected by HPLC with capable of detecting refraction index using the reported methods [19].
2.8 Fermentation
Fermentation was carried out with the fungal S. cerevisiae. A growth medium (containing (g/L) glucose, 10; yeast extract, 3; malt extract, 3; peptone, 5; (pH 5.0) was prepared and fresh colonies from agar plate were selected and used to inoculate 50 ml of the growth medium in 250 ml Erlenmeyer flasks. The culture was grown in shaker bath; harvested and centrifuged at 11,000×g. Cells were re-suspended in 2 ml of deionized water.
The 1 L flask containing enzyme hydrolysed pretreated biomass sample, 5 g yeast extract, and S. cerevisiae was inoculated. The pH was maintained at 6.0 and fermentation was carried out at 35 °C, 75 rpm, 72 h. After 72 h, the supernatants were collected and analysed for ethanol estimation.
2.8.1 Ethanol determination
The quantitative monitoring of the ethanol production in the fermentation process was carried out by using Gas chromatography (Shimadzu GC-14A Gas Chromatograph with a Restek RTX-5 capillary column and Fisher 1-butanol A383-1 as the internal standard). The column temperature was initially adjusted at 100 °C for 2 min; and further increased to 180 °C for 1 min, 220 °C with a rate of 5 °C/min for 5 min. The carrier gas (N2) flow rate was maintained at 1 ml/min. Four standards (10, 20, 30 and 40 ml of ethanol) are prepared in 50 ml volumetric flask. The percentage of ethanol in volume was counted as volume ethanol/total volume multiply by 100. Approximately 1 µL of standards and unknowns are injected into the column [20].
All the experiment’s results are taken after three consecutive reading.
3 Results
3.1 Plant material
The photograph of the plant material collected from Northeast India (Fig. 1A of ESM), the grounded plant biomass material is given in powder form (Fig. 1B of ESM), herbarium specimen (Fig. 2 of ESM), taxonomical position of the plant species (Table 1 of ESM), and morphological characters of the plants (Table 4 of ESM) can be found from Data in support of Cellulosic bioethanol production from Eragrostis airoides Nees grass collected from Northeast India (as supplementary file).
3.2 Fiber analysis
The cellulose, hemicellulose, lignin, NDF and ADF of the biomass sample are presented in Fig. 1. The cellulose, hemicellulose and lignin content were found to be 43.17%, 30.89%, 10.94% respectively.
Composition of Eragrostis airoides (Adapted from [17])
3.3 Physico-chemical pretreatment
Chemical and physical pretreatment methods were applied simultaneously. Dilute acid (H2SO4) and alkali (sodium hydroxide) of different concentration was treated with biomass and autoclaved were performed. The total fermentable sugar (TFS) was calculated based on the sugar released during pretreatment and enzymatic hydrolysis. The maximum TFS (60.67 g/100 g of biomass) was found in pretreatment condition with 5% acid + autoclave treatment (Table 1). The minimum TFS (44.90 g/100 g of biomass) was seen in 1% alkali + autoclaving.
3.4 Sugar assay
The individual sugars (glucose, xylose and arabinose) released during the pretreatment technology was determined by HPLC. The maximum glucose concentration (g/L) released in pretreatment condition 5% acid + autoclave was found to be 1.62 g/L (Fig. 2). The minimum was found in condition with 1% alkali + autoclave 1.12 g/L. The pentose sugars like xylose and arabinose were also released during pretreatment technology and their values are quite high as compared to glucose. The possible reasons are discussed in the discussion section later.
3.5 Enzymatic hydrolysis
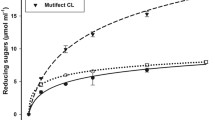
The enzymatic saccharification was carried out in different pretreatment conditions by using T. reesei. The saccharification yield was determined and found that pretreatment condition in 5% acid + autoclaving shows maximum yield (Fig. 2). In all pretreatment conditions, the acid pretreated biomass showed higher saccharification than those treated with alkali (Fig. 3).
Furthermore, the rate of enzyme hydrolysis in acid treatment and alkali treatment were calculated and presented in Figs. 4 and 5. The higher rate of enzyme hydrolysis was obtained for the pretreatment condition 5% acid + autoclaving. As the time passed, the rate of enzyme hydrolysis decreases gradually (Table 2).
3.6 Ethanol production
The ethanol production from fermentation was checked. It can be seen that the maximum ethanol yield (17.56 g/L) was obtained in pretreatment condition with 5% Acid + Autoclave pretreatment (Fig. 6). As the ethanol concentration increases, the level of the sugar in the system reduces simultaneously (Fig. 7).
4 Discussion
The bioconversion of LCB into fermentable sugars for the production of bioethanol is a vibrant research area where enormous efforts are going on in terms of process simplicity and efficiency for higher yield. Although extensive studies have been carried out to meet the future challenges, there is no self-sufficient process or single step protocol for all the biomass to convert into bioethanol. The pretreatment [21] methods and the hydrolysis process are the two crucial stages in the whole conversion process. These two stages mainly determined the overall efficiency process.
The production of bioethanol from lignocellulosic feedstock such as grasses seems very attractive and sustainable. As we know that, using such biomass will drastically help in reducing the greenhouse gas emission. That is why it is very important to utilize LCB for ethanol production in larger scale cost-effectively and in an environmentally sustainable way.
In India, the second generation of bioethanol is mainly confined to few plant species such as Lantana, Saccharum spontaneum, Eichornia, Parthenium sp. [14]. It is highly needed to focus on an exploration of new raw material that could be a potential candidate to be used as feedstock for bioethanol production. It can be noted that the production and utilization of feedstock for bioethanol should be derived from inedible parts of food crops or lignocellulosic biomass, in order to overcome the competition between food and fuel [22].
Basically, various species of lignocellulosic biomass native to North-East India was collected to find out the best potential candidate to be used as feedstock for bioethanol production. Eragrastis airoides was found to be one of the most dominating, widely distributed, and easily available species to this region. The voucher number was assigned to the herbarium specimen. This voucher number was used as the reference for the future study of the particular specimen. The documentation of the specimen was important to discuss the fact that this specimen will provide future references of the sample and local areas where it grows. The collection and identification of new raw material in bioethanol production is equally important. North-East India was selected as the site of collection for LCB. Various plant species are available to this region. The hilly terrains and fallow lands of these regions are lying unused due to difficulty in the cultivation of food crops. However, the grass species could easily grow in such areas. The grasses such as Saccharum spontaneum, Phragmites karka have been used in the production of bioethanol. Our attempt is to explore new feedstock material easily available to this region for biofuel generation. Our selected biomass Eragrostis airoides is profoundly found in this region. The documentation in the form of herbarium specimen of such biomass was highly required in other to know the phenological changes occur due to global warming [23]. The documentation of the specimen provides the ecological habitat of the sample. That is the main reason why we have made herbarium specimen and submitted to the herbarium unit, which is well recognised by Botanical Survey of India (BSI). The systematic position of the plant sample gives details about the Class, Order, Family, Genus and Species it belongs to can be found from supplementary table.
The physico-chemical characterization of Eragrostis airoides was earlier reported by [17]. Briefly, the moisture content was found to be 8.275%. The combustion yield of biomass is affected by the moisture content. High moisture content decreases the combustion yield of biomass [24]. Volatile matter (VM) is considered an important factor for determining fuel efficacy. The VM was found to be 86.840%. The fixed carbon content influenced the biological conversion process of the fuel [25]. Usually, the woody biomass has higher fixed carbon content than lignocellulosic biomass. The fixed carbon content of Eragrostis airoides was found to be 1.225%. The ash content of biomass affects the combustion rate. The ash content was found to be 3.660%. The thermal profile indicates the thermal stability of the sample at a different range of temperature. Thermogravimetric analysis (TGA) is a technique which determined the weight loss kinetic of the biomass sample during the decomposition. The Eragrostis airoides went through three distinct stages ranging from 100 to 200 °C, 200 to 500 °C, and 500 to 900 °C. The cellulose and hemicellulose component degraded at 200–500 °C and lignin being high thermal stability degraded beyond 500 °C. The X-ray Diffraction determined the crystallinity of the biomass sample. The decrease in the degree of crystallinity is an indication of a decrease in the degree of polymerization [26,27,28]. The crystalline index of the sample was found to be 26.663. The elemental composition of Eragrostis airoides is carbon (41.024%), hydrogen (6.723%), Nitrogen (1.138%), and oxygen (51.115%) respectively.
The heating value is an important parameter defining the effectiveness of any biofuel [29, 30]. The heating value was calculated and found to be 15.273%. The FTIR analysis shows the presence of various functional groups which links with the cellulose, hemicellulose and lignin component. The peaks at 480–815 cm−1 indicate the presence of sugars like arabinan, galactan and B-D fructose. The compositional analysis was conducted in other to know the cellulose, hemicellulose and lignin content in the biomass. The cellulose and hemicellulose component can be hydrolyzed to monomers. These monomers can be further used in fermentation to produce ethanol. The Eragrostis airoides content cellulose (43.17%), hemicellulose (30.89%) and lignin (10.94%). By taking all these parameters into account, the biomass was further processed for pretreatment, enzymatic hydrolysis and fermentation.
The pre-treatment of lignocellulosic biomass is a crucial step before hydrolysis. The objective of pre-treatment is to decrease the crystalline nature of cellulose thereby enhancing the enzymatic saccharification. The efficient enzymatic conversion of cellulosic biomass into fermentable sugars depends heavily on the effectiveness of the pretreatment condition. During the pretreatment, the impeding layers of carbohydrate are disrupted and the cellulose portion becomes easily accessible to enzymes [31]. Numerous pretreatment strategies have been developed to enhance the enzymatic hydrolysis to yield more fermentable sugars [32,33,34,35,36]. However, only a small number of pretreatment methods have been reported as being potentially cost-effective. We have adopted physico-chemical pretreatment of dilute acid and alkali treatment with autoclaving. The different concentration of acid and alkali was prepared (1%, 3%. 5% and 7% respectively). The parameter for autoclaving was kept constant for all pretreatment condition. The most effective pretreatment condition was observed in 5% acid + autoclaving. It was found that the maximum glucose (1.62 g/L) concentration was released in 5% acid + autoclaving condition.
From the HPLC analysis, it was found that pentose sugars like xylose and arabinose were released during the pretreatment methods (Fig. 2). This implies that the pretreatment condition adopted also degrading the hemicellulose (pentose sugars) present in the biomass. It was also seen that the TRS yield in enzymatic hydrolysis did not show much rise. This may be due to the consequences of some cellulose undergoing hydrolysis during acid + autoclave pretreatment which limits the TRS released in enzymatic hydrolysis (Table 2).
The enzymatic hydrolysis of the grass biomass was carried out by cellulase enzyme (T. reesei). Unlike chemical hydrolysis, enzymatic hydrolysis is highly specific to the substrate, pH and a temperature. The hydrolysis was conducted with mild conditions at a pH of 4.7 and temperature of 50 °C. The enzymatic hydrolysis using T. reesei was considered as because the hydrolysis does not corrode the apparatus in spite of taking several days over the chemical hydrolysis, the enzymes are more specific to the substrate and highly dependent on the reaction environment such as pH. Moreover, when the enzyme gets saturated, it affects the process unless they are removed subsequently [37]. However, the use of enzymes in large-scale industry is limited and the process of hydrolysis took around 4 days.
It is well known that the acid pretreatment helps in improving the enzymatic hydrolysis of the cellulosic component, however, it has no effect on lignin composition [38]. Results on the enzymatic hydrolysis of the pretreated grass (Eragrostis airoides) using the enzyme T. reesei shows that the best hydrolysis was obtained with the acid + autoclaving. The 5% acid + autoclave pretreatment is more superior to other conditions as it produces saccharification yield of 91.3 mg/g and TRS of 40.16 g/L (Table 1). The TRS yield depends on the composition and molecular structure of the biomass as well as the condition applied for pretreatment and enzymatic hydrolysis (Cardona et al. 2014). The TRS yield obtained from another type of LCB could not be compared with the TRS obtained in these studies. For instance, a TRS yield of 36.15 mg gdb−1 was reported on Kans grass with 1% biomass loading [39]. A TRS yield of 118.1 mg/g was obtained on rice straw pretreated with 4% NaOH [40]. For the Switchgrass, a yield of 810 mg/g was achieved on pretreatment condition with 2% NaOH [41].
Enzymatic hydrolysis is a crucial step in the conversion of cellulosic biomass to biofuels. There are several steps involved in the process. The cellulose portion present in the biomass is separated out from the lignin mixture. Then, the cellulose is broken down into respective smaller monomers (sugars) and then these molecules are utilized further by S. cerevisiae for fermentation process [42].
It was seen that the rate of enzyme hydrolysis decreases as the time passed (Figs. 4, 5 and Table 2). The rate of the enzyme hydrolysis was maximum in the first day as compared to the 2nd and 3rd day. This may be due to the formation of inhibitory substances in the hydrolysate as well as due to the alteration of cellulose crystallinity by the pretreatment condition which affecting in the rate of enzyme hydrolysis [11].
Saccharomyces cerevisiae is one of the most effective ethanol-producing microorganisms which utilized the hexose sugars including glucose, mannose and galactose for ethanol production [43,44,45]. The main component of lignocellulosic hydrolysate is glucose (a hexose sugar). In the enzymatic hydrolysis, the cellulose portion was converted to glucose. Saccharomyces cerevisiae with high ethanol productivity then used up these glucose molecules to ethanol. It is reported that S. cerevisiae is high tolerance to inhibitory compounds present in the hydrolysate of lignocellulosic biomass [46, 47]. However, this strain is unable to utilize the pentose sugars for the production of ethanol through fermentation. Some yeast strains have been developed to ferment xylose into ethanol but the rate and ethanol yield is considerably low as compared to their glucose fermentation [45, 48].
S. cerevisiae is commonly used in industrial ethanol production from sugar or starch-based raw materials. For example, in the fermentation of sugar from Saccharum spontaneum with S. cerevisiae could yield 19.45 g/L ethanol (Table 3). The highest ethanol yield (20 g/L) was obtained in Reed canary grass by yeast strain VTT-B-03339 [49]. However, our study on E. airoides using the S. cerevisiae could produce ethanol yield of 17.56 g/L.
The overall mass balance diagram was produced based on the composition analysis after each step, including physicochemical pretreatment, enzymatic hydrolysis and fermentation (Fig. 8). As compared with pretreatment, the saccharification decreased total sugar by twofold. The fermentation of hydrolysates using S. cerevisiae resulted in their effective conversion to 17.56 g/L of ethanol.
5 Conclusion
Our results show clearly and consistently that the used of feedstock Eragrostis airoides could efficiently produce bioethanol more than the feedstock such as elephant grass and Parthenium hysterophorus. This biomass offers better candidate than other biomass in terms of biofuel production, energy content, calorific value and cellulosic component which are one of the major criteria for selection of using as feedstock for biofuel production. The more beautiful conclusive of using this feedstock is that, the biomass is usually grows in fallows areas of this region without proper maintenance and this could be one of the most concerned factors in regards to food crops versus fuel crops. Taking into account the high energy productivity and cellulosic component of the feedstock, the biomass was considered as substrate for enzymes for bioethanol production. To the best of our knowledge, the grass Eragrostis airoides is a viable feedstock for a source of bioethanol production. This feedstock can be used to produce ethanol as this raw material is abundantly found in North-East India.
Abbreviations
- TRS:
-
Total reducing sugar
- LCB:
-
Lignocellulosic biomass
- IBUS:
-
Integrated Biomass Utilization System
- TGA:
-
Thermogravimetric analysis
- XRD:
-
X-ray diffraction
- FTIR:
-
Fourier transform infrared spectroscopy
- DNS:
-
Dinitrosalicylic acid
- ν :
-
Enzyme hydrolysis rate
- Glut:
-
Glucose concentration at time t
- Gluo:
-
Initial glucose concentration at time
- S. cerevisiae :
-
Saccharomyceae cerevisiae
- GC:
-
Gas chromatography
- NDF:
-
Neutral detergent fiber
- ADF:
-
Acid detergent fiber
- TFS:
-
Total fermentable sugar
- P:
-
Pretreatment
- EH:
-
Enzymatic hydrolysis
- SHF:
-
Separate hydrolysis and fermentation
- SSF:
-
Simultaneous saccharification and fermentation
References
Mehvish I, Umar A, Muhammad I, Zile H, Saba J, Muhammad N, Quratulain S (2018) Production of bioethanol from sugarcane bagasse using yeast strains: a kinetic study. Energy Sources Part A Recov Util Environ Effects 40(3):364–372. https://doi.org/10.1080/15567036.2017.1422056
Venkatesh B (2014) Current challenges in commercially producing biofuels from lignocellulosic biomass. Int Sch Res Not. https://doi.org/10.1155/2014/463074
Ambreen G, Muhammad I, Muhammad N, Quratulain S, Ikram ul H (2018) Kallar grass (Leptochloa fusca L. Kunth) as a feedstock for ethanol fermentation with the aid of response surface methodology. Environ Prog Sustain Energy 37(1):569–576. https://doi.org/10.1002/ep.12701
Larsen J, Petersen MØ, Thirup L, Li HW, Iversen FK (2011) The IBUS process lignocellulosic bioethanol close to a commercial reality. Chem Eng Technol 31:765–0772
Khanal SK (2008) Anaerobic biotechnology for bioenergy production. Principles and application. Willey and Blackwell, New York, pp 161–186
Mashallah R, Saeed S (2019) Lignin-chitosan blend for methylene blue removal: adsorption modeling. J Mol Liq 274:778–791
Mashallah R, Ahmad BA, Gavin MW, Saeed S (2018) Quantum chemical calculations and molecular modeling for methylene blue removal from water by a lignin-chitosan blend. Int J Biol Macromol 120:2065–2075
Lynd LR, Weimer PJ, Zyl W Hv, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577
Singh YD (2019) Comprehensive characterization of indigenous lignocellulosic biomass from Northeast India for biofuel production. SN Appl Sci 1(5):458. https://doi.org/10.1007/s42452-019-0453-0
Singh YD, Satapathy KB (2018) Conversion of lignocellulosic biomass to bioethanol: an overview with a focus on pretreatment. Int J Eng Technol 15:17–43
Waleed KEZ, Maha MI, Yasser RAF, Nadia AS, Morsi MM (2011) Acid and enzyme hydrolysis to convert pretreated lignocellulosic materials into glucose for ethanol production. Carbohydr Polym 84:865–871
Harmsen P, Huijgen W, Bermudez L, Bakker R, (2010) Literature review of physical and chemical pretreatment processes for lignocellulosic biomass. Biosynergy report. 1184
Toyama N (1969) Applications of cellulase in Japan, cellulase and their application. In: Gould RF (ed) Advances in chemistry series 95. American chemical Society, Washington DC, pp 356–390
Mohan SSDT, Amol D, Sanjeeva RD, Arjuna VRP, Uttam AG, Rao VY, Rao VB, Subba VRM (2016) Bioethanol production through separate hydrolysis and fermentation of Parthenium hysterophorus biomass. Renew Energy 86:1317–1323
National policy on biofuel. Govt. of India 2016. http://mnre.gov.in/filemanager/UserFiles/biofuel_policy.pdf. Accessed on 3 Jan 2016
Aradhay A (2013) India Biofuels Annual. GAIN. 6. Report No. IN3073
Singh YD, Pinakeswar M, Utpal B (2016) Comprehensive characterization of lignocellulosic biomass through proximate, ultimate and compositional analysis for bioenergy production. Renew Energy 103:490–500. https://doi.org/10.1016/j.renene.2016.11.039
Gail LM (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Saratale GD, Kshirsagar SD, Sampange VT, Saratale RG, Oh SE, Govindwar SP, Oh MK (2014) Cellulolytic enzymes production by utilizing agricultural wastes under solid state fermentation and its application for biohydrogen production. Appl Biochem Biotechnol 174(8):2801–2817
Buckee GK, Mundy AP (1993) Determination of ethanol in beer by gas chromatography (direct injection)—collaborative trial. J Inst Brew 99:381–384
Misbah G, Muhammad I, Muhammad N (2018) Statistical modeling and optimization of pretreatment of Bombax ceiba with KOH through Box–Behnken design of response surface methodology. Energy Sources Part A Recov Util Environ Effects 40(9):1114–1124. https://doi.org/10.1080/15567036.2018.1474291
Sakai S, Tsuchida Y, Okino S, Ichihashi O, Kawaguchi H, Watanabe T, Yukawa H (2007) Effect of lignocellulose-derived inhibitors on growth of and ethanol production by growth-arrested Corynebacterium glutamicum R. Appl Environ Microbiol 73:2349–2353
Abraham J, Rushing M, Richard BP, Primack D, Mukunda S (2006) Photographs and herbarium specimens as tools to document phenological changes in response to global warming. Am J Bot 93:1667–1674. https://doi.org/10.3732/ajb.93.11.1667
García R, Pizarro C, Lavín AG, Bueno JL (2013) Biomass proximate analysis using thermogravimetry. Biores Technol 139:1–4. https://doi.org/10.1016/j.biortech.2013.03.197
Vassilev SV, Baxter D, Andersen LK, Vassileva CG (2010) An overview of the chemical composition of biomass. Fuel 89:913–933
Driscoll M, Stipanovic A, Winter W, Kun C, Manning M, Jesica S, Galloway RA, Cleland MR (2009) Electron beam irradiation of cellulose. Radiat Phys Chem 78:539–542
Gumuskaya E, Usta M, Kirci H (2003) The effects of various pulping conditions on crystalline structure of cellulose in cotton linters. Polym Degrad Stab 81:559–564
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18
Mallick N, Mandal S, Singh AK, Bishai M, Dash A (2012) Green microalga Chlorella vulgaris as a potential feedstock for biodiesel. J Chem Technol Biotechnol 87:137–145. https://doi.org/10.1002/jctb.2694
Myrsini S, Michael K (2019) Chlorella vulgaris as a green biofuel factory: comparison between biodiesel, biogas and combustible biomass production. Biores Technol 273:237–243
Gary B, Elizabeth Y, Kimberly B, John C, Ramachandran KB, Subramanian R (2011) Chemical and physicochemical pretreatment of lignocellulosic biomass: a review. Enzyme Res. https://doi.org/10.4061/2011/787532
Asma S, Ambreen G, Muhammad I, Muhammad N, Quratulain S (2017) Comparison of different pretreatment methods for efficient conversion of bagasse into ethanol. Biofuels 8(1):135–141. https://doi.org/10.1080/17597269.2016.1215066
Fouzia T, Muhammad I, Hafiz AS, Javed IQ (2017) Statistical optimization for deconstruction of poplar substrate by dilute sulfuric acid for bioethanol production. Green Chem Lett Rev 10(2):69–79. https://doi.org/10.1080/17518253.2017.1293176
Hassan SS, Williams GA, Jaiswal AK (2018) Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour Technol 262:310–318
Zabeda HM, Akter S, Yun J, Zhang G, Awad FN, Qi X, Sahu JN (2019) Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew Sustain Energy Rev 105:105–128
Shen Z, Zhang K, Si M, Liu M, Zhuo S, Liu D et al (2018) Synergy of lignocelluloses pretreatment by sodium carbonate and bacterium to enhance enzymatic hydrolysis of rice straw. Bioresour Technol 249:154–160
Duff SJB, Murray WD (1996) Bioconversion of forest products industry waste cellulosics to fuel ethanol: a Review. Bioresour Technol 55:1–33
Yu Z, Jameel H, Chang H, Park S (2011) The effect of delignification of forest biomass on enzymatic hydrolysis. Bioresour Technol 102(19):9083–9089
Rashmi K, Sanjoy G (2011) Saccharification of Kans grass using enzyme mixture from Trichoderma reesei for bioethanol production. Biores Technol 102:9970–9975
Cheng Y, Zheng Y, Yu CW, Dooley TM, Jenkins BM, Vandergheynst JS (2010) Evaluation of high solids alkaline pretreatment of rice straw. Appl Biochem Biotechnol 162(6):1768–1784
Zheng Y, Pan Z, Zhang R, Wang D, Labavitch J, Jenkins BM (2006) Dilute acid pretreatment and enzymatic hydrolysis of saline biomass for sugar production. ASABE 067003:1–12
Zhang YP, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysism of cellulose: noncomplexed cellulose systems. Biotechnol Bioeng 88:797–823
Cuevas M, Sánchez S, Bravo V, García JF, Baeza J, Parra C, Freer J (2010) Determination of optimal pre-treatment conditions for ethanol production from olive-pruning debris by simultaneous saccharification and fermentation. Fuel 89(10):2891–2896
Hiba AT, Naim N, Carlos D, Ahmed T, Hassan A (2014) Potential of bioethanol production from olive mill solid wastes. Biores Technol 152:24–30
Lin TH, Huang CF, Guo GL, Hwang WS, Huang SL (2012) Pilot-scale ethanol production from rice straw hydrolysates using xylose-fermenting Pichia stipitis. Bioresour Technol 116:314–319
Hahn-Hagerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa- Grauslund MF (2007) Toward industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol 74:937–953
Matsushika A, Inoue H, Kodaki T, Sawayama S (2009) Mini review—ethanol production from Saccharomyces cerevisiae strains: current state and perspectives. Appl Microbiol Biotechnol 84:37–53
Ferreira AD, Mussatto SI, Cadete RM, Rosa CA, Silvio SS (2011) Ethanol production by a new pentose-fermenting yeast strain, scheffersomyces stipitis UFMG-IMH 43.2, isolated from the Brazilian forest. Yeast 28:547–554
Kallioinen A, Uusitalo J, Pahkala K, Kontturi M, Viikari L, Weymarn Nv, Siika-Aho M (2012) Reed canary grass as a feedstock for 2nd generation bioethanol production. Bioresour Technol 123:669–672. https://doi.org/10.1016/j.biortech.2012.07.023
Anuj KC, Lakshmi MN, Chandrasekhar G, Manikyam A, Venkateswar LR (2009) Use of Saccharum spontaneum (wild sugarcane) as biomaterial for cell immobilization and modulated ethanol production by thermotolerant Saccharomyces cerevisiae VS3. Biores Technol 100:2404–2410
Shuchi S, Shyamal S, Mayank A, Arun G, Vijayanand SM (2015) Ultrasound enhanced ethanol production from Parthenium hysterophorus: a mechanistic investigation. Biores Technol 188:287–294. https://doi.org/10.1016/j.biortech.2014.12.038
Cardona E, Rios J, Peña J, Rios L (2014) Effects of the pretreatment method on enzymatic hydrolysis and ethanol fermentability of the cellulosic fraction from elephant grass. Fuel 118:41–47. https://doi.org/10.1016/j.fuel.2013.10.055
Acknowledgements
The author acknowledged the IIT Guwahati, center for energy and CHF Pasighat for providing the facilities and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no confict of interest. Mentioning trade names or commercial products in this paper is solely for the purpose of providing specifc information and does not imply recommendation or endorsement by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, Y.D. Cellulosic bioethanol production from Eragrostis airoides Nees grass collected from Northeast India. SN Appl. Sci. 1, 889 (2019). https://doi.org/10.1007/s42452-019-0952-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0952-z