Abstract
To find out some novel antimicrobial chemotherapeutic agents, fifteen (E)-Substituted-N-(1,3-diphenyl-1H-pyrazol-4-yl)methylene)benzeneamine derivative (1–15) were synthesized. Structural elucidation of the prepared derivatives (1–15) was achieved by FTIR, NMR (1H or 13C), Mass spectroscopy and elemental analysis. After structural analyses the derivatives were subjected for biological assessment to estimate the antimicrobial therapeutic potential as well as the percent viability of the cells by disk diffusion methods and MTT assay. All the experiments were performed in triplicate; Ciprofloxacin and DMSO were used as positive and negative control. The results exhibited that the compounds 3, 4, 7–14 were found to exhibit significant antimicrobial therapeutic effect against all microorganisms, while some compounds of the series like 5, 6, 15, possessed moderate and compound 1 was found less significant potential. The percent viability of the cells were observed in the range 91–96% at lowest concentration 3.125 µM while 70–75% at the highest 100 µM.
Similar content being viewed by others
1 Introduction
The antimicrobial resistance (pathogens developed resistance to the available chemotherapeutic agents) has been a matter of great concern and reported the cause of 700,000 Deaths/Annum globally [1]. The pathogenic resistance has been a threat to the global community that once again become the major cause of the death due to the infectious disease [2]. To get rid off with this issue there is always requirement for the preparation of new chemotherapeutic agents to combat multi-drug resistant (MDR) strains. Pyrazole is one of the important heterocyclic nuclei in heterocyclic chemistry and represented various medicinal properties and a lot of research has been performed targeting this functional nucleus. Pyrazole moiety has been found in some currently used chemotherapeutic agents like Rimonabant, Tapoxalin, Lonazolac, Pyrazofurin, Epirizole, Celecoxib and Fezolamine etc. [3]. The versatile therapeutic application of the pyrazole derivative have been reported such as antimicrobial [4], Antimalarial [5], anticancer [6], anti-inflammatory [7], antidepressant [8], anticonvulsant [9], selective enzyme inhibitory activities [10], antipyretic [11], analgesic [12], fungicidal [13], fungistatic [14], antihyperglycemic [15], antiviral [16], antitumor [16], etc. On the other hand the derivatives with HC=N functionality have been reported to exhibit many therapeutic effects like antibacterial, antifungal, antimicrobial, anticonvulsant, anti-HIV, and antitumor etc. [17,18,19].
2 Results and discussion
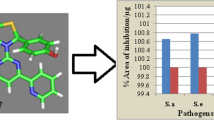
Series of fifteen (E)-Substituted-N-((1,3-diphenyl-1H-pyrazol-4-yl)methylene)benzeneamine derivative (1–15) was aimed for the synthesis, following the three step procedure (Fig. 1). The first step includes the formation of Schiff bases (a1–a5) by the reaction in between phenylhydrazine and substituted ketones. In the second step the Schiff bases undergoes cyclization to yield pyrazole carboxaldehyde (b1–b5) nucleus by using dimethylformamide (DMF) and the desired compounds (1–15) by the condensation reaction between pyrazole carboxaldehyde (b1–b5) and substituted amines in ethanol and drop of acetic acid under reflux. The prepared derivatives (a1–a5), (b1–b5) and (1–15) were then analyzed for structural confirmation by variety of analytical techniques such as FTIR, NMR, Mass Spectroscopy, Elemental analysis. The FTIR bands around 1590–1602 and 3190–3222 cm−1 due to the presence of C=N and NH functional groups confirmed the formation of compounds (a1–a5), the singlet around 1098–11.18 ppm in 1H-NMR spectra due to the NH proton and a strong signal around 166.08–166.90 ppm in 13C-NMR spectra due to C=N carbon further confirmed the formation of compounds (a1–a5). The appearance of bands around 1603–1619 and 1718–1725 cm−1 in FTIR spectra due to the presence of C=N and C=O confirmed the synthesis of compounds (b1–b5). Further confirmation was achieved by 1H-NMR and 13C-NMR spectra, 1H-NMR spectra represented the singlet signals around 8.518–8.539 and 10.032–10.063 ppm due to Pyrazole Proton and HC=O proton, the presence of carbon signals in 13C-NMR spectra around 106.59–188.91 ppm and 186.89–190.20 ppm due to pyrazole carbon and HC=O carbon atom in the structure. The disappearance of the band in the FTIR spectra of the compounds (1–15) 1718–1725 cm−1 and the appearance of ne bands around 1609–1625 cm−1 due the formation of HC=N functional group. Further confirmation of the structures was carried out by the 1H-NMR and 13C-NMR spectra, the 1H-NMR spectra exhibited the disappearance of the singlet around 10.032–10.063 ppm due to the HC=O proton and simultaneous appearance of the singlet around 8.109–8.381 ppm due to the HC=N proton and 13C-NMR spectra portrayed the disappearance of the signal around 186.89–190.20 ppm due to the HC=O carbon, and the appearance of the signal around 151.09–153.67 due to the HC=N carbon atom. Other signals were also observed around 114.24–116.01 ppm, 130.07–130.90 and 150.20–152.89 due to the three carbons of the pyrazole nucleus. Antimicrobial analysis of the compounds 1–15 was performed against the microbes [S. aureus (ATCC-25923), S. epidermidis (ATCC-29887), E. coli (ATCC-25922), P. mirabilis (ATCC-25933) using the disc diffusion protocol in terms of zone of inhibition and minimum inhibitory concentration. The antimicrobial findings revealed that the compounds 3, 4, 7–14 were found to exhibit very good activity against all microorganisms. While some compounds of the series like 5, 6, 15 possessed moderate and compound 1 was found less significant potential. The detailed results for zone of inhibition and MIC reported in Tables 1 and 2. We also calculated the percent area of inhibition/µg for all compounds and the results are represented in Fig. 2. The synthesized compounds were then assessed for MTT assay to observe the percent viability of the cells using Hep G2 cells. The results of MTT assay portrayed that the percent viability of the cells was found to be concentration dependent. All the compounds possessed the percent viability of the cells in the range 91–96% at 3.125 µM and while on increasing concentration up to 100 µM, the percent viability of the cells were found 70–75% at 100 µM. the detailed MTT assay results are represented in Fig. 3.
3 Experimental
3.1 Chemistry
3.1.1 General procedure for the synthesis of compounds a1–a5
To a 100 mL round bottom flask was added an appropriate ketone (10 mmol), phenylhydrazine (10 mmol), few drops of glacial acetic acid and 50 mL absolute ethanol. The reaction mixture was refluxed at 80 °C. Completion of reaction was monitored by TLC, solid precipitate was obtained, filtered, dried and recrystallized from ethanol.
[a 1 ] (2E)-1-phenyl-2-(1-phenylethylidene)hydrazine
Yield: 88%; mp: °C; yellow crystals; Anal. calc. for C14H14N2: C 79.97%, H 6.71%, N 13.32%, found: C 78.20%, H 6.72%, N 13.30%; IR νmax(cm−1): 1595 (C=N), 3012 (CH–Ar), 3220 (NH); 1H NMR (CDCl3)δ(ppm): 1.12 (s, 3H, CH3), 7.28–7.58 (m, Ar–H), 11.08 (s, 1H, NH); 13C NMR (CDCl3)δppm: 14.12 (CH3), 116.10, 118.12, 128.24, 129.02, 130.20, 131.60, 166.90 (C=N); ESI–MS(m/z): [M++1] 210.28.
[a 2 ] (E)-1-phenyl-2-(1-(p-tolylethylidene)hdrazine
Yield: 86%; mp: °C; yellow crystals; Anal. calc. for C15H16N2: C 80.32%, H 7.19%, N 12.49%, found: C 80.28%, H 7.20%, N 12.52%; IR νmax(cm−1): 1590 (C=N), 3018 (CH–Ar), 3206 (NH); 1H NMR (CDCl3)δ(ppm): 1.02 (s, 3H, CH3), 2.20 (s, 3H, CH3), 7.01–7.50 (m, Ar–H), 10.98 (s, 1H, NH); 13C NMR (CDCl3)δppm: 13.90 (CH3), 25.45 (CH3) 116.42, 118.88, 128.22, 129.32, 130.10, 131.24, 166.42 (C=N); ESI–MS(m/z): [M++1] 224.12.
[a 3 ] (E)-2-(1-(4-methoxyphenylethylidene)-1-phenylhdrazine
Yield: 88%; mp: °C; creamy yellow crystals; Anal. calc. for C15H16N2O: C 74.97%, H 6.71%, N 11.66%, found: C 74.88%, H 6.72%, N 11.66%; IR νmax(cm−1): 1596 (C=N), 3005 (CH–Ar), 3190 (NH); 1H NMR (CDCl3)δ(ppm): 1.06 (s, 3H, CH3), 3.80 (OCH3) 6.82–7.52 (m, Ar–H), 11.10 (s, 1H, NH); 13C NMR (CDCl3)δppm: 14.22 (CH3), 56.5 (OCH3) 116.40, 118.32, 128.12, 129.54, 130.25, 131.43, 161.22 (CO), 166.08 (C=N); ESI–MS(m/z): [M++1] 240.10.
[a 4 ] (E)-2-[1-(4-chlorophenyl)ethylidene]-1-phenylhdrazine
Yield: 84%; mp: °C; white crystals; Anal. calc. for C14H13ClN2: C 68.71%, H 5.35%, N 11.45%, found: C 68.72%, H 5.38%, N 11.52%; IR νmax(cm−1): 1602 (C=N), 3010 (CH–Ar), 3222 (NH); 1H NMR (CDCl3)δ(ppm): 1.06 (s, 3H, CH3), 6.46–7.62 (m, Ar–H), 11.18 (s, 1H, NH); 13C NMR (CDCl3)δppm: 14.52 (CH3), 116.11, 118.02, 128.23, 129.65, 130.09, 131.22, 166.66 (C=N); ESI–MS(m/z): [M++1] 244.10.
[a 5x ] (E)-2-[1-(4-bromophenyl)ethylidene]-1-phenylhdrazine
Yield: 92%; mp: °C; yellow crystals; Anal. calc. for C14H13BrN2: C 58.15%, H 4.53%, N 9.69%, found: C 58.20%, H 4.50%, N 9.72%; IR νmax(cm−1): 1600 (C=N), 3014 (CH–Ar), 3202 (NH); 1H NMR (CDCl3)δ(ppm): 1.16 (s, 3H, CH3), 6.52–7.56 (m, Ar–H), 11.12 (s, 1H, NH); 13C NMR (CDCl3)δppm: 13.8 (CH3), 116.06, 118.32, 128.12, 129.07, 130.63, 131.63, 166.48 (C=N); ESI–MS(m/z): [M++1] 288.05.
3.1.2 General procedure for the synthesis of compound b1–b5
Phosphorous oxychloride (25 mmol) was added to DMF (100 mL) at 0 °C and stirred for 30 min. Compound a1–a5 (10 mmol) was added slowly to this mixture and stirred for 5 h. The crude reaction mixture was then quenched into water (1 L) and stirred for an additional 1 h and extracted with ethyl acetate. The organic layer was separated, washed with water, dried and evaporated under reduced pressure. The crude was recrystallized from ethanol.
[b 1 ] 1,3-diphenyl-1H-pyrazole-4-carbaldehyde
Yield: 95%; mp: 218–220 °C; yellowish crystals; Anal. calc. for C16H12N2O: C 77.40%, H 4.87%, N 11.28%, found: C 77.92%, H 5.10%, N 11.18%; IR νmax(cm−1): 1603 (C=N), 1719 (C=O), 3022 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 7.260–7.647 (m, Ar–H), 7.792–7.944 (m, Ar–H), 8.532 (s, 1H, CH, pyrazole ring), 10.062 (s, 1H, HC=O); 13C-NMR (CDCl3)δppm: 107.19 (1C-pyrazole ring), 120.57, 127.03, 127.68, 229.31, 129.92, 135.93 (1C-pyrazole ring), 139.75, 151.03 (1C pyrazole ring), 189.20 (HC=O); ESI–MS(m/z): [M++1] 248.11.
[b 2x ] 1-phenyl-3-p-tolyl-1H-pyrazole-4-carbaldehyde
Yield: 95%; mp:198–200 °C; white crystals; Anal. calc. for C17H14N2O: C 77.84%, H 5.38%, N 10.68%; found: C 77.79%, H 5.42%, N 10.65%; IR νmax(cm−1): 1619 (C=N), 1723 (C=O), 3022 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.388 (s, 3H, CH3), 7.259–7.413 (m, Ar–H), 7.485–7.537 (m, Ar–H), 7.702 (d, 1H, Ar–H), 7.781(d, 1H, Ar–H), 8.539 (s, 1H, CH, pyrazole ring), 10.051 (s, 1H, HC=O); 13C-NMR (CDCl3)δppm: 21.39 (CH3), 107.44 (1C-pyrazole ring), 120.35, 127.64, 127.81, 229.74, 129.76, 135.89 (1C-pyrazole ring), 139.70, 151.23 (1C-pyrazole ring), 188.91 (HC=O); ESI–MS(m/z): [M++1] 262.13.
[b 3 ] 3-(4-methoxyphenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde
Yield: 95%; mp: 210–212 °C; creamy white crystals; Anal. calc. for C187H14N2O2: C 73.37%, H 5.07%, N 10.07%; found: C 73.39%, H 5.11%, N 10.04%; IR νmax (cm−1): 1611 (C=N), 1729 (C=O), 3032 (CH–Ar); 1H-NMR (CDCl3) δ (ppm): 3.875 (s, 3H, OCH3)7.014–7.532 (m, Ar–H), 7.777–7.807 (m, Ar–H), 8.518 (s, 1H, CH, pyrazole ring), 10.032 (s, 1H, HC=O); 13C-NMR (CDCl3) δ ppm: 56.71 (OCH3), 107.58 (1C-pyrazole ring), 120.75, 127.22, 127.91, 229.53, 129.76, 135.85 (1C-pyrazole ring), 139.25, 151.19 (1C-pyrazole ring), 190.20 (HC=O); ESI–MS(m/z): [M++1] 278.35.
[b 4 ] 3-(4-chlorophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde
Yield: 95%; mp: 220–222 °C; white crystals; Anal. calc. for C16H11ClN2O: C 67.97%, H 3.92%, N 9.91%; found: C 68.02%, H 3.88%, N 9.88%; IR νmax(cm−1): 1609 (C=N), 1719 (C=O), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 7.261–7.549 (m, Ar–H), 7.773–7.848 (m, Ar–H), 8.537 (s, 1H, CH, pyrazole ring), 10.063 (s, 1H, HC=O); 13C-NMR (CDCl3)δppm: 106.59 (1C-pyrazole ring), 120.87, 127.59, 127.69, 229.78, 129.38, 135.56 (1C-pyrazole ring), 139.68, 151.11 (1C-pyrazole ring), 190.11 (HC=O); ESI–MS(m/z): [M++1]282.70.
[b 5 ] 3-(4-bromophenyl)-1-phenyl-1-H-pyrazole-4-carbaldehyde
Yield: 95%; mp: 202–204 °C; white crystals; Anal. calc. for C16H11BrN2O: C 58.74%, H 3.39%, N 8.56%; found: C 59.07%, H 3.42%, N 8.80%; IR νmax(cm−1): 1611 (C=N), 1725 (C=O), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 7.260–7.645 (m, Ar–H), 7.755–7.796 (m, Ar–H), 8.533 (s, 1H, CH, pyrazole ring), 10.036 (s, 1H, HC=O); 13C-NMR (CDCl3)δppm: 109.21 (1C-pyrazole ring), 120.75, 127.54, 127.75, 229.63, 129.68, 135.76 (1C-pyrazole ring), 139.57, 151.53 (1C-pyrazole ring), 186.89 (HC=O); ESI–MS(m/z): [M++1] 326.03.
3.1.3 General procedure for the synthesis of compound 1-15
To an appropriate aldehyde substituted aromatic amines was added in ethanol. Few drops of glacial acetic acid was also added and refluxed at 80 °C. Precipitate was obtained, filtered, dried and recrystallized from ethanol [19].
(E)-N-((1,3-diphenyl-1H-pyrazol-4-yl)methylene)benzeneamine
Yield: 95%; mp: 228–230 °C; white crystals; Anal. calc. for C22H17N3: C 81.71%, H 5.30%, N 12.99%; found: C 81.62%, H 5.32%, N 13.01%; IR νmax(cm−1): 1611 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 6.683–6.978 (m, 5H, Ar–H), 7.082–7.222 (m, 5H, Ar–H), 7.368–7.498 (m, 5H, Ar–H), 8.215 (s, 1H, HC=N), 8.314 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3) δppm: 114.90 (1C-pyrazole ring), 119.79, 120.82, 127.43, 129.5571, 129.58, 130.70 (1C-pyrazole ring), 136.81, 139.35, 150.59 (1C-pyrazoline ring), 153.67 (HC=N); ESI–MS(m/z): [M++1] 323.15.
(E)-3-methyl-N-((1,3-diphenyl-1H-pyrazol-4-yl)methylene)benzeneamine
Yield: 95%; mp: 236–238 °C; white crystals; Anal. calc. for C23H19N3: C 81.87%, H 5.68%, N 12.45%; found: C 81.91%, H 5.62%, N 12.47%; IR νmax(cm−1): 1619 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.359 (s, 3H, CH3), 6.711–6.957 (m, 5H, Ar–H), 7.097–7.215 (m, 5H, Ar–H), 7.310 (d, 1H, Ar–H), 7.385–7.483 (m, 3H, Ar–H), 8.224 (HC=N), 8.561 (s, 1H, Ar–H), 8.612 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 22.01 (CH3), 114.46 (1C-pyrazole ring), 119.89, 120.77, 127.64, 129.55, 129.76, 130.90 (1C-pyrazole ring), 136.73, 139.60, 150.90 (1C-pyrazoline ring), 152.80 (HC=N); ESI–MS(m/z): [M++1] 337.14.
(E)-4-methyl-N-((1,3-diphenyl-1H-pyrazol-4-yl)methylene)benzeneamine
Yield: 95%; mp: 232–234 °C; yellow crystals; Anal. calc. for C23H19N3: C 81.87%, H 5.68%, N 12.45%; found: C 81.80%, H 5.71%, N 12.43%; IR νmax(cm−1): 1616 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.367 (s, 3H, CH3), 6.790–6.981 (m, 5H, Ar–H), 7.093–7.195 (m, 5H, Ar–H), 7.219–7.343 (m, 2H, Ar–H), 7.431–7.453 (m, 2H, Ar–H), 8.381 (HC=N), 8.502 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 20.99 (CH3), 114.67 (1C-pyrazole ring), 119.45, 120.77, 127.82, 129.65, 129.48, 130.57 (1C-pyrazole ring), 136.74, 139.81, 150.68 (1C-pyrazoline ring), 152.91 (HC=N); ESI–MS(m/z): [M++1] 337.15.
(E)-N-((1-phenyl-3-p-tolyl-1H-pyrazol-4-yl)methylene)benzeneamine
Yield: 95%; mp: 222–224 °C; yellow crystals; Anal. calc. for C23H19N3: C 81.87%, H 5.68%, N 12.45%; found: C 81.90%, H 5.70%, N 12.40%; IR νmax(cm−1): 1624 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.377 (s, 3H, CH3), 6.676–7.028 (m, 5H, Ar–H), 7.115–7.267 (m, 2H, Ar–H), 7.301–7.397 (m, 2H, Ar–H), 7.413–7.533 (m, 5H, Ar–H), 8.110 (s, 1H, HC=N), 8.473 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 21.32 (CH3), 115.17 (1C-pyrazole ring), 118.59, 119.44, 120.22, 120.88, 123.11, 126.02, 127.48, 127.96, 129.28, 129.64, 130.37 (1C-pyrazole ring), 131.18, 131.96, 139.38, 152.04 (1C-pyrazole ring), 152.87 (HC=N); ESI–MS(m/z): [M++1] 337.18.
(E)-3-methyl-N-((1-phenyl-3-p-tolyl-1H-pyrazol-4-yl)methylene)benzeneamine
Yield: 95%; mp: 228–230 °C; brown crystals; Anal. calc. for C24H21N3: C 82.02%, H 6.02%, N 11.96%; found: C 82.10%, H 6.00%, N 11.92%; IR νmax(cm−1): 1627 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.298 (s, 3H, CH3), 2.318 (s, 3H, CH3), 6.666–6.937 (m, 5H, Ar–H), 7.010–7.107 (m, 2H, Ar–H), 7.204–7.389 (m, 2H, Ar–H), 7.414 (d, 1H, Ar–H), 7.473–7.597 (m, 3H, Ar–H), 8.205 (s, 1H, HC=N), 8.309 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 21.02 (CH3), 21.92 (CH3), 114.29 (1C-pyrazole ring), 118.61, 119.53, 120.29, 120.73, 123.54, 126.74, 127.78, 127.76, 129.49, 129.92, 130.44 (1C-pyrazole ring), 131.35, 131.76, 139.74, 152.54 (1C-pyrazole ring), 153.32 (HC=N); ESI–MS(m/z): [M++1] 351.19.
(E)-4-methyl-N-((1-phenyl-3-p-tolyl-1H-pyrazol-4-yl)methylene)benzeneamine
Yield: 95%; mp: 240–242 °C; white crystals; Anal. calc. for C24H21N3: C 82.02%, H 6.02%, N 11.96%; found: C 82.05%, H 5.99%, N 12.01%; IR νmax(cm−1): 16120 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.328 (s, 3H, CH3), 2.359 (s, 3H, CH3), 6.676–6.793 (m, 5H, Ar–H), 6.877–7.195 (m, 2H, Ar–H), 7.217–7.292 (m, 2H, Ar–H), 7.325–7.453 (m, 2H, Ar–H), 8.490–7.527 (m, 2H, Ar–H), 8.188 (s, 1H, HC=N), 8.389 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 21.59 (CH3), 22.10 (CH3), 116.01 (1C-pyrazole ring), 118.33, 119.21, 120.54, 120.38, 123.74, 126.57, 127.89, 127.83, 129.65, 129.09, 130.23 (1C-pyrazole ring), 131.58, 131.67, 139.78, 152.68 (1C-pyrazole ring), 152.99 (HC=N); ESI–MS(m/z): [M++1] 351.18.
(E)-N-((3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)benzeneamine
Yield: 95%; mp: 236–238 °C; white crystals; Anal. calc. for C23H19N3O: C 78.16%, H 5.42%, N 11.89%; found: C 78.10%, H 5.39%, N 11.91%; IR νmax(cm−1): 1621 (C=N), 3025 (CH–Ar);1H-NMR (CDCl3)δ(ppm): 3.781 (s, 3H, OCH3), 6.685–7.022 (m, 5H, Ar–H), 7.105–7.277 (m, 2H, Ar–H), 7.313–7.398 (m, 2H, Ar–H), 7.459–7.593 (m, 5H, Ar–H), 8.221 (s, 1H, HC=N), 8.517 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 21.67 (CH3), 54.19 (OCH3), 114.39 (1C-pyrazole ring), 119.27, 120.71, 127.41, 129.12, 129.75, 130.21 (1C-pyrazole ring), 136.58, 139.36, 150.43 (1C-pyrazoline ring), 152.45 (HC=N); ESI–MS(m/z): [M++1] 353.14.
(E)-N-((3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-3-methyl benzeneamine
Yield: 95%; mp: 214–216 °C; white crystals; Anal. calc. for C24H21N3O: C 78.45%, H 5.76%, N 11.44%; found: C 78.50%, H 5.73%, N 11.41%; IR νmax(cm−1): 1618 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.319 (s, 3H, CH3), 3.758 (s, 3H, OCH3), 6.670–6.885 (m, 5H, Ar–H), 6.935–7.177 (m, 2H, Ar–H), 7.213–7.318 (m, 2H, Ar–H), 7.364 (d, 1H, Ar–H), 7.392–7.533 (m, 3H, Ar–H), 8.127 (s, 1H, HC=N), 8.314 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 21.92 (CH3), 55.30 (OCH3), 114.51 (1C-pyrazole ring), 119.61, 120.56, 127.53, 129.90, 129.74, 130.22 (1C-pyrazole ring), 136.52, 139.43, 150.51 (1C-pyrazoline ring), 152.33 (HC=N); ESI–MS(m/z): [M++1] 367.15.
(E)-N-((3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-4-methyl benzeneamine
Yield: 95%; mp: 222–224 °C; white crystals; Anal. calc. for C24H21N3O: C 78.45%, H 5.76%, N 11.44%; found: C 78.51%, H 5.78%, N 11.40%; IR νmax(cm−1): 1614 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.354 (s, 3H, CH3), 3.764 (s, 3H, OCH3), 6.719–6.888 (m, 5H, Ar–H), 6.877–7.012 (m, 2H, Ar–H), 7.089–7.198 (m, 2H, Ar–H), 7.225–7.350 (m, 2H, Ar–H), 7.385–7.473 (m, 2H, Ar–H), 8.139 (s, 1H, HC=N), 8.320 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 21.00 (CH3), 55.38 (OCH3), 114.24 (1C-pyrazole ring), 119.35, 120.80, 127.33, 129.55, 129.85, 130.09 (1C-pyrazole ring), 136.37, 139.87,150.39 (1C-pyrazoline ring), 152.43 (HC=N); ESI–MS(m/z): [M++1] 367.18.
(E)-N-((3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)benzeneamine
Yield: 95%; mp: 234–236 °C; white crystals; Anal. calc. for C22H16ClN3: C 73.84%, H 4.51%, N 11.74%; found: C 73.81%, H 4.53%, N 11.77%; IR νmax(cm−1): 1622 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 6.728–6.880 (m, 5H, Ar–H), 6.920–7.131 (m, 2H, Ar–H), 7.159–7.278 (m, 2H, Ar–H), 7.301–7.419 (m, 5H, Ar–H), 8.126 (s,1H, HC=N), 8.330 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 115.15 (1C-pyrazole ring), 118.59, 119.44, 120.22, 120.88, 123.11, 126.02, 127.48, 127.96, 129.28, 129.64, 130.27 (1C-pyrazole ring), 131.18, 131.96, 139.38, 152.04 (1C-pyrazole ring), 152.85 (HC=N); ESI–MS(m/z): [M++1] 357.12.
(E)-N-((3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-3-methyl benzeneamine
Yield: 95%; mp: 220–222 °C; yellow crystals; Anal. calc. for C23H18ClN3: C 74.29%, H 4.88%, N 11.30%; found: C 74.20%, H 5.01%, N 11.25%; IR νmax(cm−1): 1609 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.336 (s, 3H, CH3), 6.663–6.829 (m, 5H, Ar–H), 6.952–7.067 (m, 2H, Ar–H), 7.119–7.209 (m, 2H, Ar–H), 7.333 (d, 1H, Ar–H), 7.421–7.505 (m, 3H, Ar–H), 8.109 (s,1H, HC=N), 8.382 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 22.00 (CH3), 115.23 (1C-pyrazole ring), 118.18, 119.62, 120.53, 120.74, 123.56, 126.68, 127.34, 127.76, 129.74, 129.17, 130.47 (1C-pyrazole ring), 131.35, 131.71, 139.52, 152.45 (1C-pyrazole ring), 152.58 (HC=N); ESI–MS(m/z): [M++1] 371.15.
(E)-N-((3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-4-methyl benzeneamine
Yield: 95%; mp: 225–227 °C; white crystals; Anal. calc. for C23H18ClN3: C 74.29%, H 4.88%, N 11.30%; found: C 74.32%, H 5.02%, N 11.26%; IR νmax(cm−1): 1610 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.322 (s, 3H, CH3), 6.735–7.110 (m, 5H, Ar–H), 7.172–7.237 (m, 2H, Ar–H), 7.257–7.318 (m, 2H, Ar–H), 7.352–7.418 (m, 2H, Ar–H), 7.452–7.594 (m, 2H, Ar–H), 8.135 (s, 1H, HC=N), 8.310 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 22.13 (CH3), 115.85 (1C-pyrazole ring), 118.78, 119.54, 120.35, 120.77, 123.76, 126.98, 127.88, 127.65, 129.278, 129.86, 130.54 (1C-pyrazole ring), 131.57, 131.56, 139.53, 152.84 (1C-pyrazole ring), 152.61 (HC=N); ESI–MS(m/z): [M++1] 371.14.
(E)-N-((3-(4-bromophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)benzeneamine
Yield: 95%; mp: 234–236 °C; yellow crystals; Anal. calc. for C22H16BrN3: C 65.68%, H 4.01%, N 10.45%; found: C 65.70%, H 3.98%, N 10.50%; IR νmax(cm−1): 1623 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 6.683–6.788 (m, 5H, Ar–H), 6.935–7.055 (m, 2H, Ar–H), 7.118–7.217 (m, 2H, Ar–H), 7.293–7.411 (m, 5H, Ar–H), 8.177 (s, 1H, HC=N), 8.319 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 115.18 (1C-pyrazole ring), 118.59, 119.44, 120.22, 123.11, 126.02, 127.42, 129.64, 130.39 (1C-pyrazole ring), 131.96, 139.38, 152.04 (1C-pyrazole ring), 152.82 (HC=N); ESI–MS(m/z): [M++1] 401.07.
(E)-N-((3-(4-bromophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-3-methyl benzeneamine
Yield: 95%; mp: 224–226 °C; yellow crystals; Anal. calc. for C23H18BrN3: C 66.36%, H 4.36%, N 10.09%; found: C 66.51%, H 4.30%, N 10.10%; IR νmax(cm−1): 1625 (C=N), 3025 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.290 (s, 3H, CH3), 6.717–6.914 (m, 5H, Ar–H), 6.987–7.094 (m, 2H, Ar–H), 7.145–7.256 (m, 2H, Ar–H), 7.332 (d, 1H, Ar–H), 7.377–7.455 (m, 3H, Ar–H), 8.201 (s, 1H, HC=N), 8.355 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 21.40 (CH3), 114.31 (1C-pyrazole ring), 119.82, 120.77, 127.67, 128.28, 129.37, 129.37, 130.73 (1C-pyrazole ring), 135.43, 150.51 (1C-pyrazole ring), 151.09 (HC=N); ESI–MS(m/z): [M++1]415.08.
(E)-N-((3-(4-bromophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-4-methyl benzeneamine
Yield: 95%; mp: 230–232 °C; white crystals; Anal. calc. for C23H18BrN3: C 66.36%, H 4.36%, N 10.09%; found: C 66.40%, H 4.30%, N 10.12%; IR νmax(cm−1): 1616 (C=N), 3027 (CH–Ar); 1H-NMR (CDCl3)δ(ppm): 2.329 (s, 3H, CH3), 6.671–6.840 (m, 5H, Ar–H), 6.932–7.083 (m, 2H, Ar–H), 7.153–7.228 (m, 2H, Ar–H), 7.295–7.372 (m, 2H, Ar–H), 7.399–7.512 (m, 2H, Ar–H), 8.172 (s, 1H, HC=N), 8.359 (s, 1H, CH, pyrazole ring); 13C-NMR (CDCl3)δppm: 20.99 (CH3), 115.02 (1C-pyrazole ring), 119.42, 120.75, 127.39, 128.97, 129.60, 129.85, 130.07 (1C-pyrazole ring), 135.03, 150.02 (1C-pyrazole ring),151.71 (HC=N); ESI–MS(m/z): [M++1] 415.09.
3.2 Antimicrobial Screening
Screening of the antimicrobial therapeutic effect of the synthesized compounds was carried out employing the disc diffusion method. The stock solution of the test compounds was prepared by dissolving one mg of each compound (1–15) to the 100 mL of dimethysulphoxide (DMSO). The microbes [S. aureus (ATCC-25923), S. epidermidis (ATCC-29887), E. coli (ATCC-25922), P. mirabilis (ATCC-25933) were sub-cultured in nutrient agar media, following by the McFarland Protocol and transferred to the agar plate. Now the 5 mm paper discs were prepared and dipped to the test solution and placed to the agar plate to observe the zone of inhibition (mm) followed by incubation. Minimum inhibitory concentration (MIC) was also estimated by macro dilution to understand the smallest concentration of the test compounds to inhibit the growth of microorganism. For estimation of MIC, serial dilutions were made for all the test compounds to the final concentration 400, 200, 100, 50, 12.5, 6.25 and 3.125 µg/ml. Ciprofloxacin and DMSO were used as positive and negative control in the study [20].
3.3 MTT assay
The enzyme succinate dehydrogenase reduces 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) in the mitochondria of the living cells and produces formazon crystal that can be measured spectrophotometrically. To estimate the percent viability of the cells MTT is widely used. Human hepatocellular carcinoma cell line (HepG2) was used to assess the percent viability of cells, Ciprofloxacin was used as a reference drug in the experiment. All the prepared compounds and the Reference drug tested on the sub-confluent population of HepG2 cells with a concentration range 3.125–100 µM, and percent viability of cells was observed on 48 h incubation and reported in Fig. 3.
4 Conclusion
A series of fifteen pyrazole derivatives (1–15), structurally elucidated, was screened for antimicrobial therapeutic effect followed by percent viability of the cells. The antimicrobial therapeutic potential was measured in terms of zone of inhibition and MIC. The antimicrobial screening findings portrayed that the potential in three states—significant, moderate and less significant. The compounds 3, 4, 7–14 were possessed significant antimicrobial therapeutic effect against all microorganisms, the compounds 5, 6, 15, possessed moderate, while the compound 1 was found less significant. The percent viability of the cells at concentrations 3.125 µM and 100 µM were observed in the range 91–96% and 70–75% respectively.
Change history
22 May 2019
In the initial online publication, there was a typo in the third author’s name. The name is correctly shown here. The original article has been corrected.
References
Babu KR, Eeshwaraiah B, Aravind D, Harshadas MM, Rallabaldi MR, Apurba B, Rakeshwar B (2008) Synthesis of quinoline analogs: search for antimalarial agents. Monatsh Chem 139:179–181. https://doi.org/10.1007/s00706-007-0772-5
Kulandaivelu U, Padmini VG, Suneetha K, Shireesha B, Vidyasagar JV, Rao TR, Jayaveera KN, Basu A, Jayaprakash V (2011) Synthesis, antimicrobial and anticancer activity of new thiosemicarbazone derivatives. Arch Pharm 344:84–90. https://doi.org/10.1002/ardp.201000201
Naim MJ, Alam O, Nawaz F, Alam MJ, Alam P (2016) Current status of pyrazole and its biological activities. J Pharm Bioallied Sci 8:2–17. https://doi.org/10.4103/0975-7406.171694
Chandrakantha B, Isloor AM, Shetty P, Shetty P, Isloor S, Malladi S, Fun HK (2012) Synthesis, characterization and antimicrobial activity of novel ethyl 1-(N-substituted)-5-phenyl-1H-pyrazole-4-carboxylate derivatives. Med Chem Res 21:2702–2708. https://doi.org/10.1007/s00044-011-9796-9
Bekhit AA, Saudi MN, Hassan AMM, Fahmy SM, Ibrahim TM, Ghareeb D, El-Seidy AM, Nasralla SN, Bekhit AEA (2019) Synthesis, in silico experiments and biological evaluation of 1,3,4-trisubstituted pyrazole derivatives as antimalarial agents. Eur J Med Chem 163:353–366. https://doi.org/10.1016/j.ejmech.2018.11.067
Shi JB, Tang WJ, Qi XB, Li R, Liu XH (2015) Novel pyrazole-5-carboxamide and pyrazole–pyrimidine derivatives: synthesis and anticancer activity. Eur J Med Chem 90:889–896. https://doi.org/10.1016/j.ejmech.2014.12.013
Viveka S, Dinesha, Shama P, Gundibasapp-Nagaraja K, Ballav S, Kerkar S (2015) Design and synthesis of some new pyrazolyl-pyrazolines as potential anti-inflammatory, analgesic and antibacterial agents. Eur J Med Chem 101:442–451. https://doi.org/10.1016/j.ejmech.2015.07.002
Abdel-Aziz M, Abuo-Rahma GEA, Hassan A (2009) Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur J Med Chem 44:3480–3487. https://doi.org/10.1016/j.ejmech.2009.01.032
Harit T, Malek F, Bali BE, Khan A, Dalvandi K, Marasini Bishnu P, Noreen S, Malik R, Khan S, Choudhary MI (2012) Synthesis and enzyme inhibitory activities of some new pyrazole-based heterocyclic compounds. Med Chem Res 21:2772. https://doi.org/10.1007/s00044-011-9804-0
Kumar RS, Arif IA, Ahamed A, Idhayadhulla A (2016) Anti-inflammatory and antimicrobial activities of novel pyrazole analogues. Saudi J Biol Sci 23:614–620. https://doi.org/10.1016/j.sjbs.2015.07.005
Malvar DC, Ferreira D, Teixeira R, Castro AD, Raphael C, Ligia Freitas CC, Antonio C, Elson F, Iziara F, Mafra J, Souza G, Vanderlinde F (2013) Antinociceptive, anti-inflammatory and antipyretic effects of 1.5-diphenyl-1H-Pyrazole-3-carbohydrazide, a new heterocyclic pyrazole derivative. Life Sciences. 95:81–88. https://doi.org/10.1016/j.lfs.2013.12.005
Vijesh AM, Isloor AM, Shetty P, Sundershan S, Fun HK (2013) New pyrazole derivatives containing 1,2,4-triazoles and benzoxazoles as potent antimicrobial and analgesic agents. Eur J Med Chem 62:410–415. https://doi.org/10.1016/j.ejmech.2012.12.057
Sridhar R, Perumal PT, Etti S, Shanmugam G, Ponnuswamy MN, Prabavathy VR, Mathivanan N (2004) Design, synthesis and anti-microbial activity of 1H-pyrazole carboxylates. Bioorg Med Chem Lett 14:6035–6040. https://doi.org/10.1016/j.bmcl.2004.09.066
Bebernitz GR, Argentieri G, Battle B, Brennan C, Balkan B, Burkey BF, Eckhardt M, Gao J, Kapa P, Strohschein RJ, Schuster HF, Wilson M, Xu DD (2001) The effect of 1,3-diaryl-[1H]-pyrazole-4-acetamides on glucose utilization in ob/ob mice. J Med Chem 44:2601–2611. https://doi.org/10.1021/jm010032c
El-Sabbagh OI, Baraka MM, Ibrahim SM, Pannecouque C, Andrei G, Snoeck R, Balzarini J, Rashad AA (2009) Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur J Med Chem 44:3746–3753. https://doi.org/10.1016/j.ejmech.2009.03.038
Nitulescu GM, Draghici C, Olaru OT (2013) New potential antitumor pyrazole derivatives: synthesis and cytotoxic evaluation. Int J Mol Sci 14(11):21805–21818. https://doi.org/10.3390/ijms141121805
Kajal A, Bala S, Kamboj S, Sharma N, Saini V (2013) Schiff bases: A versatile pharmacophore. J Catal 1:1–15. https://doi.org/10.1155/2013/893512
Arshad M, Bhat AR, Hoi KK, Choi I, Athar F (2017) Synthesis, characterization and antibacterial screening of some novel 1,2,4-triazine derivatives. Chin Chem Lett 28:1559–1565. https://doi.org/10.1016/j.cclet.2016.12.037
Arshad, M., Bhat, A. R., Pokharel, S., Lee, E. J., Athar, F., Choi, I., European Journal of Medicinal Chemistry. (2014) Synthesis, characterization and anticancer screening of some novel piperonyl–tetrazole derivatives. 71: 229-236. https://doi.org/10.1016/j.ejmech.2013.11.008
Mosmann, T., J. Immunol. Methods. (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. 65: 55. https://doi.org/10.1016/0022-1759(83)90303-4
Acknowledgements
The above research work was supported by the Dean of College of Medicine, Al-Dawadmi, Shaqra University kingdom of Saudi Arabia and University Grant Commission Govt. of India (F40-65(C/M)/1009(SA-III/MANF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article has been revised: A typo in the third author’s name has been corrected.
Rights and permissions
About this article
Cite this article
Arshad, M., Khan, M.S., Nami, S.A.A. et al. (E)-Substituted-N-((1,3-diphenyl-1H-pyrazol-4-yl)methylene)benzeneamine: synthesis, characterization, antibacterial, and MTT assessment. SN Appl. Sci. 1, 548 (2019). https://doi.org/10.1007/s42452-019-0571-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0571-8