Abstract
Rare earth elements concentrate (REEs) which has been prepared from Rosetta monazite assays 44, 23, 16.94 and 5.91% for Ce, La, Nd and Pr, respectively. Highly pure individual separation of Pr(III) from this concentrate was achieved using a cationic Dowex 50W-X8 ion exchange resin. An EDTA solution assaying 0.015 mol L−1 was used for the complete REEs elution. In this study, the proposed method was applied for the determination of individual Pr(III) after the separation of Ce(IV) by oxidation at 200 °C and separation of Pr(III) from REEs concentrate via ion exchange. Simple perchlorate method was applied at the achieved optimum conditions for the separate spectrophotometric determination of Pr(III) at λmax 444 nm using the fourth derivative spectrum. A fourth derivative molecular absorption spectrophotometric was used to overcome the overlapped interfering spectra to determine the individual components.
Similar content being viewed by others
1 Introduction
The Egyptian beach sand deposits usually contain about 15% heavy economic minerals (ilmenite, rutile, magnetite, zircon, and monazite). The mean relative frequency of monazite in the beach deposits in some samples collected from Demietta and Rosetta (Egypt) attains 0.3–1.5%. The reserves of heavy economic minerals in million metric tons as about 30 in the top meter and about 615 in the top 20 m. Therefore, the Egyptian black sand beach deposits are the chief thorium ore in Egypt due to the presence of monazite [1].
Chemically the mineral monazite is an important source of rare earth elements, especially the light lanthanides [2]. Monazite-bearing sands usually contain other rare earth (RE) minerals such as xenotime, gadolinite, thorite, uranothorite and samarkite [3]. This mineralogical complexity highlights the importance of mineralogical studies for the efficient extraction of the mineral of interest [4].
Such a complex mixture requires intensive treatment to separate the marketable products. Production of RE oxides cake from Egyptian monazite, after its separation as a by-product during the recovery of more abundant minerals using appropriate upgrading technique was previously achieved [5].
The rarest earth bearing minerals are Bastnaesite, (Ce, La) (CO3) (OH, F); xenotime, YPO4; and monazite, (Ce, La, Nd, Th) PO4·SiO4 [6]. Bastnaesite and monazite are sources of light REEs and account for 95% of REE currently used [7]. A number of methods including co-precipitation, solvent extraction, ion exchange and solid phase extraction have been employed for the separation of lanthanide elements. Ion exchange separation and purification techniques used in analytical procedures differ from those used in preparative applications due to the demands on the intensity and quality of the analytical signal rather than on the product quantity and purity. Ion exchange was used for individual separated on the strongly acidic cation-exchangers.
The successful separation of rare earth elements by ion exchange by employing a citric acid and ammonium citrate as eluent. The citrate process has however not been very successful in the separating bulk of heavy rare earth elements. Besides the citrate, a number of eluting agents and process conditions have been actively evaluated, and they have been reviewed by Gupta and Krishnamurthy [8]. The use of amino poly acetic acids such as NTA and ethylene diamine tetra acetic acid (EDTA) as eluents was investigated but was unsuccessful due to the problem of aqueous insolubility. This and many other limitations of the elution process were solved in the band displacement technique developed by Zachariou [9], using the resin in a special cation state. Firstly using Fe3+ indicated pH dependence wherein acidic solutions H4Y would precipitate while in basic solutions hydrous ferric oxide would clog the resin pores. These limitations were indeed overcome by using Cu(II) instead of Fe(III) [8].
Abdelfattah [10] using the band displacement technique for individual separation of lanthanides from monazite concentrate.
Ce can be separated through changes in its oxidation state. The Ce(III) is oxidized to Ce(IV) by drying Ln-hydroxide in the air at 120–130 °C or by chlorination or electrolysis [11, 12].
Pr(III) is one of light rare earth elements (LREEs); which has a different application in different fields as used in the production of atomic battery [13]. Pr(III) is soft silvery metal used as an alloying agent with Mg for the production of high strength metal alloys used in aircraft engines. It forms the core of carbon arc lights for the motion picture industry and didymium glass to make certain types of welders and glass blowers goggles (with yellow colour). It is added to fiber optic cables as a doping agent where it acts as a signal amplifier [14].
Pr(III) separation and determination are one of the most difficult problems in inorganic chemistry due to the similarity of their chemical properties derived from their same valence and similar ion radii
El-Dessouky et al. [15], the use of TVEX–PHOR resin for the sorption of Pr(III), Ho(III) and Co (II) from nitrate medium was carried out using batch and column techniques. Various parameters affecting the uptake of these metal ions such as v/m ratio, pH and the metal ion concentration were separately studied. Effect of temperature on the equilibrium distribution values has been studied to evaluate the changes in standard thermodynamic quantities. Experimental results of the investigated metal ions were found to fit to Freundlich isotherm model over the entire studied concentration range. Selectivity sequence of the resin for these metals is Ho > Pr > Co. The recovery of the investigated metals from the loaded resin is performed with 0.1 M sulfuric acid.
Akseli and Kutun [16], sodium trimetaphosphate is a very suitable new eluting agent for the separation of REEs and Th(IV) at room temperature. Sodium trimetaphosphate has the following advantages over α-hydroxyisobutyrate as an eluting agent. Its eluent concentration is smaller than for other eluents. The elution peaks are narrow and nearly symmetrical. The tailing effects are very small and there is no overlapping. Dy and Y are well separated. It is possible to recover Sodium trimetaphosphate. The separation time is shorter than those of other known methods.
In the presence of perchloric acid solution, rare earth elements of Ce, Nd, Sm, Eu, Gd, Dy and Pr at large concentrations have well shaped absorption spectra in the ultraviolet region even without the addition of chromogenic reagent. However, it is difficult to determine each of them individually, because they have serious overlapping peaks. In this study a fourth derivative molecular absorption spectrophotometric was used to resolve the overlapped spectra and to determine the individual components. The proposed method was applied to the determination of Pr(III) individual after separation of cerium by air oxidation at 200 °C and separation of Pr(III) from rare earth elements by ion exchange. This method is convenient for the determination, preparative separation, and purification of rare earth elements.
2 Experimental
2.1 Instruments
A double beam Unicam spectrophotometer (England) equipped with 1 cm cell path length was used for all absorbance measurements. The pH values were measured with NEL pH meter which calibrated using pH standard buffers either 4 and 7 or 7 and 10. An inductively coupled plasma optical emission spectroscopy (ICP-OES) and a XL 30 PHLIPS type environmental scanning electron microscope (ESEM) were used for determining the separated REEs.
2.2 Materials
The monazite used in this study was obtained from the black sand deposited on the Rosetta area on the Mediterranean coast, using appropriate upgrading techniques [3]. The REEs concentrate required for this study was already prepared via alkaline processing of Egyptian Rosetta monazite concentrate. The yielded hydrous cake was completely dissolved in conc. HCl where REEs concentrate precipitate was isolated at pH 9. The precipitate was well washed with distilled H2O and then dried at 200 °C for 6 h to oxidize Ce(III) to Ce(IV). The dried REEs concentrate was ground and then dissolved in 5% HCl with stirring for 4 h where the insoluble Ce(IV) was collected over the filter paper. The REEs filtrate free from Ce was treated with oxalic acid at pH 1.1 followed by calcination to prepare the working REEs concentrate [17]. On the other hand, Pr(III) standard solution (2 g L−1) was prepared by complete dissolution a weighted 0.2415 g of Pr6O11 in hot 1.5 mol L−1 HCl and complete up to 100 mL in a volumetric measuring flask using double distilled water.
2.3 Individual separation of Pr(III)
Individual REEs separation from their concentrate was achieved by applying the ion exchange separation technique using EDTA elution. The experimental set up was all the time composed of two columns of 2.5 cm internal diameters; namely the loading column and the retaining column. The cation exchange resin Dowex 50W-X8 (100–200 mesh size) in the H+ form and Cu(II) form was used to achieve the separation of light rare earth elements. In the first column, the RECl3 solution was adjusted at pH 2.5 using ammonia solution and passed it through the ion exchange column for saturation. The retaining column was then joined to the loading column and the mixed REE bed was eluted by EDTA solution through the retaining column. A 0.015 mol L−1 EDTA solution in its NH4+ form at pH 8.3 for REEs elution at a flow rate of 1 mL/min, where bed volumes (25 mL) of individual REEs were successfully collected. After complete elution of Cu(II)these fractions were then subjected for Pr(III) determination using ICP-OES analysis. The concentrated Pr(III) fraction was treated with 10% oxalic acid solution to precipitate the corresponding Pr-oxalate at pH 1.1 with stirring for 1 h at room temperature. The prepared Pr-oxalate cake was calcinated at 950 °C to prepare Pr6O11 which identified using SEM-EDAX.
2.4 The determination of Pr(III) using perchloric acid
Working sample contains more than 100 µg of Pr(III), was prepared by dissolving REEs concentrate in a minimum quantity of perchloric acid and diluting to volume with water in a 10 mL measuring flask to give a final (1.5 mol L−1) perchloric acid, then records the fourth-derivative spectrum against a water blank using 1 cm cell at λmax = 444 nm using fourth derivative spectrum.
2.5 Application
To investigate the utility of the suggested method for the determination of Pr(III) using the perchloric acid method, two standard samples and two REEs concentrate samples were used.
3 Results and discussion
3.1 Composition of monazite rare earth oxides concentrate
ICP-OES analysis and ESEM analysis of RE2O3 is listed in Table 1 and shown in Fig. 1. The obtained results actually revealed the presence of high concentrations of La, Nd, Ce together with considerable concentrations of Pr, Eu, Sm, Gd, Dy and Y oxides. This RE2O3 concentrate is the raw material for the present study which concerns with selective separation and determination of Pr element.
3.2 Selective separation of Ce(IV)
Separation of Ce, the major element in the prepared RE-concentrate cake was carried out by thermal oxidation. Where the RE-concentrate cake was dried at 200 °C for 2 h with continuous oxidation by air to oxidize all Ce(III) to Ce(IV). The present Ce(IV) was easily separated from RE-concentrate cake by dissolving in 5% HCl acid, where Ce(IV) insoluble and left behind via filtration. The Ce(IV) product was carefully washed and ignited at 750–800 °C for 1 h to yield the corresponding CeO2, XRD pattern Fig. 2. On the other hand, the dissolved REEs free from Ce was precipitated using oxalic acid at pH 1.1 and then ignited to prepare the corresponding RE2O3 cake. This cake was already identified using EDAX analysis technique, Fig. 3 and chemically characterized using ICP-OES analysis technique as given in Table 2. Table 2 revealed actually that not less than 98.7% of Ce was already separated from the prepared RE2O3 concentrate. Accordingly, pre-concentration of the other RE2O3 concentrate such as La2O3, Nd2O3, Pr6O11, Y2O3 and Sm2O3 which are already increased to achieve 43.83, 28.04.12.04, 6.2 and 04.86%, respectively. This concentrate 2.5 g was re-dissolved in HCl solution to prepare the chloride pregnant solution required for the ion exchange separation of Pr(III).
3.3 Ion exchange separation of Pr(III) from RE2O3 concentrate free from Ce
The chloride solution of RE2O3 concentrate free from Ce was allowed to pass through the resin packed in a column in its hydrogen form. After saturation the elution process with EDTA molar-to-molar (metal-EDTA complexes) was applied. However a volume of 600 mL of the eluent was collected in constant volumes of 25 mL and analyzed. It was observed that the first 300 mL are concentrated with Nd(III) without any appearance of Pr(III) which appeared in a volume of 325 mL as listed in Table 3. The second stage was the separation of Pr(III) by elution of the retaining column. The function of the retaining ion is to cause the redeposit ion of the rare earth elements on the resin bed and transport the chelating agent off the column in soluble form to obtain a better separation and resolutions of individual rare earths.The following reaction represents rare earth elements adsorption and elution through Cu(II) as shown in Eqs. (1) and (2).
From the obtained results, Table 3 and Fig. 4, the first three eluate fractions (1–3) are concentrated with both Nd(III) and Pr(III) ion species. Where the following four eluate sample fractions (4–7) are concentrated only with highly pure Pr(III) ion species (99.99% purity). The last eluate fractions were collected and subjected to Pr separation by precipitation with oxalic acid as Pr2(C2O4) cake which was carefully washed and ignited to prepare pure Pr2O3 as identified in EDAX chart Fig. 5. At the end of the elution, the last three eluate sample fractions (8–10) represent low concentrations of Pr(III) ion species compared to La(III).
3.4 Determination of Pr(III)
The determination of individual rare earth elements in their mixtures is still very difficult because they are in closed characters. There are two methods for determination REEs by either selective or separation. Instrumental analysis of REEs by Spectrophotometric is one of the simplest and accurate. With respect to Pr(III) there are two basic methods used for spectrophotometric analysis. The first uses the absorption spectrum of the colored ions where the second is based upon the absorption spectrum developed by the formation of colored complexes.
3.4.1 Absorption spectra of praseodymium perchlorate
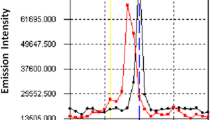
The conventional spectrophotometry for praseodymium perchlorate against water blank was scanned in the UV–VIS spectrum region between (420–600 nm), Fig. 6.
Figure 6 represents the absorption bands of Pr(III) as perchlorate at wavelengths, λmax 444, 469,482 and 589 nm, while the maximum wavelengths at λmax 444 nm. These bands are due to f → f transitions. At absorption band of λmax 444 nm, it could determine trace amounts of Pr(III).
3.4.2 REEs interfering effect on Pr(III) determination
It was necessary to study the interference effect of some REEs associated with praseodymium. The expected elements associated praseodymium are Ce, Nd, Sm, Eu, Gd and Dy. The interference effect of these elements was studied using synthetic solutions containing 0.6 g L−1 of praseodymium in the presence of a mixture of REEs. The background absorption of La and Y was not giving any indication.
From Table 4 the tolerance limits of Ce(III), Nd(III), Sm(III), Eu(III), Gd(III) and Dy(III) concentrations associated with Pr(III) should not exceed 2, 1, 0.3, 0.2, 0.2, and 0.2 g L−1, respectively. It was clearly observed that the conventional spectrophotometry method was inapplicable for determination of Pr(III) in the presence of other elements due to the interfering effect of REEs. Accordingly, it was found necessary to apply derivative spectrophotometry technique to overcome the effect of interfering elements on the determination of Pr(III). The determination of Pr(III) in lanthanide concentrate using first to fourth derivative orders were studied.
The principal advantages of derivative spectra (DS) were the elimination the influence of matrix interference (without prior separation), DS measurement depends strongly on the relative width of the interfering peak compared to that of the analyte peak. The derivative spectra of praseodymium perchlorate is shown in Fig. 7, the first derivative spectrum is simply the gradient (dA/dλ) of the absorption envelope and fourth derivative (d4A/dλ4) increased sensitivity without any prior extraction. A simple method is proposed for simultaneous determination of Pr, based on a fourth derivative molecular absorption spectrophotometric.
From the Fig. 7, the λmax in the first derivative at 442 nm (absorbance 0.056), the λmax in the second derivative at 444 nm (absorbance − 0.19), the λmax in the third derivative at 446 nm (absorbance 0.56), the λmax in the fourth derivative at 444 nm (absorbance 0.91).
3.4.3 Calibration curve of praseodymium perchlorate
The calibration curve of Pr(III) perchlorate complex, Fig. 8 was done at λmax 444 nm between the absorbance of different concentrations of Pr(III) perchlorate complex ranged from 100 to 2000 mg L−1 and water blank. Evidentially, the results indicated that the detection limit is 100 mg L−1 and the molar absorptivity of zero order and fourth-derivative spectra were found to be 0.1 × 103 M−1 cm−1 and 0.95 × 103 M−1 cm−1 respectively. The linear part of the calibration curve of praseodymium perchlorate represents the concentration range (100–2000) mg L−1 through which Pr(III) could be determined by fourth-derivative spectra with good accuracy.
3.5 Application
To test the utility of the suggested method, two standard and REE concentrate samples were used these samples were already analyzed by using both ICP-OES and the suggested methods as shown in Tables 5, 6 and 7 and Fig. 9 Showed the fourth derivative for two REEs concentrate samples.
The collecting comparison results of the investigated samples using both ICP-OES and perchloric acid suggested method listed in Table 8. The results obtained from Table 8 showed that the determination of Pr(III) gives quite results. Also there is a good accuracy in determination of Pr(III) in the presence of other rare earth elements by using perchloric acid. Through the variation of % error results, it showed be removed Ce(III) from both standard and REEs concentrate samples before applied the suggested perchloric acid method for Pr(III) determination.
4 Conclusion
The proposed method was applied for the determination of individual Pr(III) after the separation of Ce(IV) by oxidation at 200 °C and separation of Pr(III) from REEs concentrate via ion exchange. A fourth derivative molecular absorption spectrophotometric was used to overcome the overlapped interfering spectra to determine the individual components. The determination of Pr(III) gives quite results. Also there is a good accuracy in the determination of Pr(III) in the presence of other rare earth elements by using perchloric acid. Through the variation of % error results, it showed be removed Ce(III) from both standard and REE concentrate samples before applied the suggested perchloric acid method for Pr(III) determination.
Change history
10 May 2019
The article Development of a procedure for spectrophotometric
References
El-Nadi YA, Daoud JA, Aly HF (2005) Modified leaching and extraction of uranium from hydrous oxide cake of Egyptian monazite. Int J Miner Process 76:101–110
Gupta CK, Krishnamurthy N (1992) Extractive metallurgy of rare earths. Int Mater Rev 37:197–248
Mustafa M (2003) Separation of economic minerals and discovery of zinc, lead, and mercury minerals in the Egyptian black sands. In: The third international conference on the geology of Africa, Assuit, Egypt, pp 153–171
Hammoud NS (1973) Physical and chemical properties of some Egyptian beach economic minerals in relation to their concentration problems. Ph.D. thesis, Cairo University, Cairo, Egypt
Rabie KA (2007) A group separation and purification of Sm, Eu and Gd from Egyptian beach monazite mineral using solvent extraction. Hydrometallurgy 85:81–86. https://doi.org/10.1016/j.hydromet.2005.12.012
Christie T, Brathwaite B, Tulloch A (1998) Mineral commodity report 17—rare earths and related elements. New Zealand Institute of Geological and Nuclear Sciences Ltd
Harben PW, Kuzvart M (1996) Global geology. Industrial Minerals Information Ltd., London, p 462
Gupta CK, Krishnamurthy N (2005) Extractive metallurgy of rare earths, 2nd edn. CRC Press, Boca Raton
Zachariou M (2008) Affinity chromatography: methods and protocols, 2nd edn. Humana Press, New York
Abdelfattah NA (2006) Recovery of pure valuble metals (lanthanides) from some local ore concentrates. Ph.D. thesis. Faculty of science, Zagazig University, Egypt
Kedari CS, Pandit SS, Ramanujam A (1999) Studies on the in situ electrooxidation and selective permeation of cerium (IV) across a bulk liquid membrane containing tributyl phosphate as the ion transporter. Sep Sci Technol 34:1907–1923. https://doi.org/10.1081/SS-100100746
Jose CG, Carlson PS (2005) Cerium extraction from cerite mineral using leaching and selective precipitation processes. In: 2nd Mercosur congress on chemical engineering, 4th Mercosur congress on process systems engineering, pp 1–5
Attallah MF, Hassan RS, Shady SA (2013) Chromatographic column separation of rare earth elements by esorcinol formaldehyde cationic exchanger resin. Arab J Nucl Sci Appl 46:18–29. Coden-Ajady-3211350/ISSN 1110-0451
Pourjavid MR, Rezaee M, Hosseini MH, Razavi T (2012) Monitoring of praseodymium(III) ions in aqueous solutions, soil and sediment samples by a PVC membrane sensor based on a furan-triazole derivative. Quim Nova 35:1973–1980
El-Dessouky SI, El-Sofany EA, Daoud JA (2007) Studies on the sorption of praseodymium(III), holmium(III) and cobalt(II) from nitrate medium using TVEX–PHOR resin. J Hazard Mater 143:17–23. https://doi.org/10.1016/j.jhazmat.2006.08.070
Akseli A, Kutun S (2000) Distribution coefficients and cation-exchange separation of rare earths in sodium trimetaphosphate media and application to monazite. Sep Sci Technol 35:561–571. https://doi.org/10.1081/SS-100100176
Mioduski T, Anhhao DH, Hoang HL (1989) Separation of cerium from other lanthanides by leaching with nitric acid rare earth(III) hydroxide-cerium(IV) oxide mixtures. J Radioanal Nucl Chem 132:105–113. https://doi.org/10.1007/BF02060982
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: With the authors’ decision to step back from Open Choice, the copyright of the article changed on 10 May 2019 to © Springer Nature Switzerland AG 2019 and the article is forthwith distributed under the terms of copyright.
Rights and permissions
About this article
Cite this article
Abdou, A.A., Abdelfattah, N.A. & Weheish, H.L. Development of a procedure for spectrophotometric determination of Pr(III) from rare earth elements (REEs) concentrate. SN Appl. Sci. 1, 479 (2019). https://doi.org/10.1007/s42452-019-0494-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0494-4