Abstract
Introduction
Light-chain (AL) amyloidosis is a rare, progressive, and typically fatal disease. Health-related quality of life (HRQoL) has been shown to be a significant prognostic factor associated with clinical outcomes such as survival and response to treatment. A better understanding of how patterns of HRQoL may be prospectively associated with costly healthcare resource utilization, such as emergency department (ED) visits and inpatient hospitalizations, is warranted.
Methods
A secondary data analysis of a non-interventional, longitudinal online study of patients with AL amyloidosis (n = 224) was conducted. Negative binomial regression models were used to examine whether initial HRQoL scores (as measured by the SF-36v2® Health Survey [SF-36v2], where higher scores reflect better HRQoL) and changes in HRQoL were associated with the number of ED visits and inpatient hospitalizations during a 12-month period. Incidence rate ratios were interpreted by 5-point decrements in initial HRQoL scores and minimally important changes in HRQoL change scores.
Results
There were significant inverse associations between initial SF-36v2 scores and subsequent rates of ED visits and inpatient hospitalizations across all domains and summary components (p < 0.05 for all). In contrast, changes in physical, but not mental, functioning were associated with rates of ED visits and inpatient hospitalizations during a 12-month period of observation.
Conclusion
Scores from patient-reported HRQoL surveys may be helpful in identifying patients at risk of future ED visits and hospital admissions, and may serve as a proxy for disease severity. Such information can provide stakeholders with insight into the humanistic and societal cost associated with AL amyloidosis.
Similar content being viewed by others
1 Introduction
Immunoglobulin light-chain (AL) amyloidosis is a rare and typically fatal disease caused by misfolded light chains that form soluble toxic aggregates and deposit fibrils (amyloid) in organs. Amyloid can lead to progressive failure of critical organs and systems (e.g., heart, kidneys, and nervous system), causing significant morbidity and mortality. Disease-related symptoms are often indicative of organ damage [2], suggesting that even patients who achieve an early diagnosis may still experience substantial organ dysfunction and incur high rates of costly healthcare resource utilization (HCRU).
In a recent analysis of commercially insured patients in the USA, the average annual healthcare costs of patients with AL amyloidosis was estimated to be US$101,855, with 50% of the sample having at least one hospitalization over the course of a year [3]. In a separate study, the estimated healthcare costs for patients in the first year following a relapse were US$139,143 [4], suggesting that patients who fail to respond to first-line treatment, or those with more severe manifestations of the disease, may incur even greater costs. These analyses reveal the substantial economic burden associated with the disease, motivating the need to better understand which patients may be at risk of requiring costly services.
In recognizing the substantial impact of this disease, and in working to improve the lives of patients with AL amyloidosis, it is necessary to identify those who are at the greatest risk for poor outcomes. While a variety of predictors of survival have been identified in patients with AL amyloidosis, including clinical assessments and biomarkers [5], less research has been conducted to explore other relevant outcomes, such as HCRU. Previous studies in other patient groups have shown a link between health-related quality of life (HRQoL) and HCRU [6,7,8,9,10], and certain measures of HRQoL have been shown to be sensitive to AL amyloidosis disease severity, suggesting candidates for a potential predictor of HCRU in AL amyloidosis [11].
To the best of our knowledge, examination of HCRU in patients with AL amyloidosis has been limited primarily to the aforementioned estimation of costs, while factors that predict HCRU have largely been unexplored. Analyses of HCRU are often based on claims data, which identify patients diagnosed with a disease of interest based on codes from either the 9th or 10th revision of the International Classification of Diseases, Clinical Modification (ICD-9-CM, ICD-10-CM, respectively). Conducting such analyses for patients with AL amyloidosis is complicated by the fact that no such code exists specifically for AL amyloidosis; therefore, clinical algorithms have to be developed with non-specific codes being used in conjunction with medical claims for treatments commonly used for the disease [3, 4]. As a complementary source to claims data, patient self-reported data may be useful to predict certain types of HCRU, such as emergency department (ED) visits and hospital admissions [12, 13].
The aim of the current study was to examine the relationship between HRQoL and self-reported ED visits and inpatient hospitalizations in a sample of patients with AL amyloidosis. As already discussed, the data used in the study are entirely self-reported, allowing for a rich source of patient-level data, particularly with respect to patient-reported outcome measures related to HRQoL. Quantifying the link between HRQoL and HCRU can help provide a practical way of identifying patients at risk for future costly care, which may be particularly important for healthcare providers who wish to more efficiently allocate resources (e.g., supportive services or interventions) to patients at greatest risk for poor outcomes. We hypothesized that patients with greater deficits in HRQoL or a decline in HRQoL over time would have greater rates of ED visits and inpatient hospitalizations.
2 Methods
2.1 Sample/Study Procedures
Secondary data analyses were completed using data drawn from the AL Amyloidosis Patient Health-Related Quality of Life Study [1], a longitudinal, non-interventional observational study of patients with AL amyloidosis (n = 341) [11]. Patients were recruited for this study in the October to December of 2015 with the assistance of two patient advocacy groups (the Amyloidosis Support Groups and the Amyloidosis Foundation) using online recruitment strategies (i.e., study announcements on the patient advocacy websites and social media platforms). Interested patients were directed to a study home page to review additional study information and to complete both an informed consent and a study screener. Patients were deemed eligible if they (1) were at least 18 years of age; (2) self-reported receiving a diagnosis of AL amyloidosis from a physician; and (3) were willing and able to complete four online surveys administered over a 12-month period. Once eligibility was established, patients were immediately administered the initial online survey. Automated survey reminders were sent to each patient via e-mail indicating when follow-up assessments were due (1, 6, and 12 months following initial data collection). Patients were given the opportunity to complete each follow-up survey during a 17-day window; however, patients who failed to complete a follow-up survey were not excluded from completing future follow-up surveys. After completing each follow-up survey, patients were e-mailed a US$75 Amazon gift card as an honorarium. An extension study that included 18- and 24-month follow-up surveys was launched in 2017 following the same data collection protocol as the original study. Interested patients were re-consented prior to completing the additional surveys. All data for this study were based on patient self-report. All study materials, including the protocol and survey instruments, were approved by the New England Independent Review Board (IRB# 15-355).
2.2 Study Measures
Time-invariant sociodemographic information and diagnostic history were collected on the initial survey, whereas time-varying information, such as disease characteristics, treatments, HRQoL, and HCRU were collected at each timepoint. The following variables were used to describe the sample: age (in years); gender (male vs. female); race (white vs. non-white); educational attainment (≥ 4-year college degree vs. < 4-year college degree); indicator variables for being currently married and currently employed; insurance coverage (public only, private only, public and private, and unknown); country of residence (USA, UK, Canada, Australia, other); types (kidney, gastrointestinal system, nervous system, liver, and/or others) and number of organs (< 3 vs. ≥ 3 organs) affected by AL amyloidosis; duration of disease (time since diagnosis in years); and an indicator for complete hematologic response/complete remission.
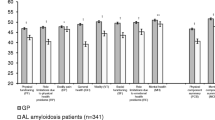
The SF-36v2® Health Survey (SF-36v2) with 4-week recall was used to measure general HRQoL burden at each timepoint [14]. The SF-36v2 assesses eight specific dimensions of functional health and well-being: Physical Functioning (PF), Role-Physical (RP; role limitations due to physical problems), Bodily Pain (BP), General Health Perceptions (GH), Vitality (VT), Social Functioning (SF), Role-Emotional (RE; role limitations due to emotional problems), and Mental Health (MH). Item responses were used to calculate scores for the eight scales. Weighted scores from the eight scales were subsequently used to compute two summary scores of physical and mental health (Physical Component Summary [PCS] and Mental Component Summary [MCS], respectively). All scale and summary scores were calculated using the developer’s scoring algorithm, which yields standardized norm-based distributions with a mean of 50 and a standard deviation (SD) of 10 for a nationally representative sample of US adults [14]. Higher SF-36v2 scores represent better functioning. Responses to all SF-36v2 items were required; therefore, missing score estimation procedures were not necessary in these analyses. To help ensure the integrity of survey responses, data quality was evaluated using the SF-36 Response Consistency Index (RCI) [14]. The RCI assesses individual-level responses to 15 paired items with specific hypothesized relationships, indicating a logically consistent pattern of responses. The RCI for each individual is based on the total sum of inconsistent pairs; therefore, the range of RCI scores is 0 (no inconsistent pairs) to 15 (all pairs answered inconsistently). Overall, approximately 95% of the sample used in this study had an RCI score of 0 on both the initial and 6-month follow-up surveys, indicating consistent responding to all 15 item pairs.
At each follow-up timepoint, patients were asked to report all-cause HCRU in terms of the total number of ED visits and inpatient hospitalizations they experienced during the previous 6 months. In reporting inpatient hospitalizations, patients were instructed not to include any time spent overnight in the ED; however, ED visits and inpatient hospitalizations were not necessarily mutually exclusive events, as it is possible that an ED visit that resulted in an inpatient hospitalization would be counted in both outcomes.
2.3 Statistical Analyses
Descriptive statistics, such as frequencies and proportions (for categorical variables) and means, SDs, medians, and ranges (for continuous variables) were used to describe the sample in terms of patient characteristics, HRQoL scores, and HCRU outcomes.
To examine the associations between HRQoL and rates of ED visits and inpatient hospitalizations, we prospectively examined the relationships between (1) initial HRQoL scores and (2) changes in HRQoL scores with subsequent HCRU using two sets of analyses.
In the first set of analyses, we examined the association between initial HRQoL scores and the cumulative rates of each of the two HCRU outcomes (ED visits, inpatient hospitalizations) during a 12-month observation period following the initial assessment. These analyses relied on an analytic sample (n = 224) comprised of patients who completed both the initial and the 6-month follow-up survey and were not missing data related to the two HCRU outcomes from the 6-month follow-up survey. The cumulative counts for each HCRU outcome were based on responses provided on the 6- and 12-month follow-up surveys (if available). Figure 1 illustrates the study design for these analyses.
The sample was heterogeneous in terms of disease severity risk factors (i.e., time since diagnosis, type and numbers of organs affected, and recent hematologic response to treatment). As a result, we anticipated a large amount of variability in our HCRU outcomes. Subsequently, negative binomial (NB) and zero-inflated NB (ZINB) models, adjusted by potential confounders, were used to account for count data with over-dispersion. Model diagnostics (i.e., the Vuong test and the Clarke Sign test) supported a preference for the NB models over the ZINB models for both outcomes; therefore, only the results from the NB models are reported. Demographic and disease characteristics that may confound the relationship between HRQoL and HCRU were considered for inclusion in the final models [11, 15, 16]. These potential covariates included gender, age, time since diagnosis, number and type of organs affected, and recent hematologic response to treatment. Final model selection was based on the Akaike information criterion (AIC) and the Bayesian information criterion (BIC); smaller values for both criteria indicate better model fit.
Patients who did not complete the 12-month follow-up survey were censored at their last point of contact by adding an offset to each model. The offset was derived by log transforming the number of months each patient was under observation to account for the shorter observation times among patients who did not complete the 12-month follow-up survey. To provide clinically meaningful interpretations, incidence rate ratios (IRRs) were calculated and reported in terms of 5-point deficits in each HRQoL score. This unit of interpretation is based on a minimally important difference (MID) threshold of half an SD.
For the second set of analyses, change in HRQoL was defined as the difference between the initial and the 6-month follow-up timepoints in SF-36v2 scale and summary scores. A negative change score indicated a decline in functioning, whereas a positive change score indicated an improvement in functioning. Similar to the first set of analyses, HCRU outcomes were defined as the cumulative count of self-reported ED visits and inpatient hospitalizations reported over a 12-month observation period. The 12-month interval for this set of analyses occurred between the 6-month and the 18-month follow-up surveys. As such, cumulative counts for each HCRU outcome were based on responses to the 12- and 18-month follow-up surveys. The analytic sample for these change analyses was comprised of patients who completed the first three surveys (initial, 6-month, and 12-month follow-up surveys) and responded to survey items pertaining to the HCRU outcomes of interest on the 12-month follow-up survey (n = 183). Patients who did not complete the 18-month follow-up survey were censored at their last point of contact. A schema of the study design is depicted in Fig. 2. Our modeling strategy for the second set of analyses was very similar to the approach described for the first set of analyses. NB models were used to regress each HCRU outcome (ED visits, inpatient hospitalizations) onto each SF-36v2 change score in ten separate models (i.e., one for each SF-36v2 domain and summary component). Each model included the initial SF-36v2 score as an independent variable and an offset (i.e., log months) to account for censoring. IRRs were calculated to reflect the percentage difference in HCRU rates between patients whose decline in HRQoL differed by an interval equivalent to the minimally important change (MIC) for each SF-36v2 domain and summary component. MICs for the SF-36v2 reflect the smallest change in functioning a patient would identify as clinically meaningful and range from 3.4 to 7.2 points. MICs for each SF-36v2 scale/summary are noted in tabled results.
A series of sensitivity analyses were conducted to look at the impact of both observations with response inconsistencies and observations from patients who received care outside of the US healthcare system. In terms of data quality, it is typically considered acceptable to include surveys with one or two inconsistent responses on the SF-36v2. To examine whether observations with potentially poor data quality influenced the results presented in this study, models were fit with and without the four patients with an SF-36v2 RCI score ≥ 2.
Although the sample was primarily comprised of patients living in the USA, 17% of the sample reported living in another country or did not provide a place of residence. Given the international differences in the use of healthcare services [17], a sensitivity analysis was conducted among the subgroup of patients who were living in the USA. This allowed us to better examine the relationship between HRQoL and HCRU among patients who were presumably treated within the US healthcare system.
All analyses were performed using SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA).
3 Results
3.1 Sample Characteristics
The demographic and disease characteristics for the initial HRQoL analytic sample (n = 224) are provided in Table 1. The mean age of patients was 60.7 years. The sample was comprised of more women than men, and the majority of patients were white (92%), married (83%), and had earned at least a college degree (64%). Patients were mainly from the USA (83%), but small proportions were also from the UK (8%), Canada (3%), Australia (3%), and other countries/regions (3%).
On average, patients were diagnosed 4.5 years prior to completing the initial survey. Nearly half of the sample reported at least three organs or systems affected by their AL amyloidosis. There was broad representation in terms of the different types of organs affected by the disease, including 66% of patients who experienced kidney involvement, 50% with cardiac involvement, 46% with gastrointestinal involvement, and 42% with nerve involvement. A sizeable proportion of the sample (44%) reported having achieved complete hematologic response or remission at the time of initial data collection.
The sample’s initial mean PCS score was 41.3, nearly 1 SD below the general population norm of 50, indicating severely impaired physical health. The sample’s initial mean MCS score, a measure of mental health, was 48.5, closer to the general population norm of 50.
The sample characteristics of patients included in the second set of analyses closely mirrored the characteristics already described (data not shown).
3.2 Utilization of Emergency Department and Inpatient Hospitalizations
Approximately one-quarter of all patients reported ≥ 1 ED visit and/or inpatient hospitalization during the 12-month observation period (Table 2; initial HRQoL analytic sample). Overall, 6% of patients reported ≥ 3 ER visits and 5% reported ≥ 3 inpatient hospitalizations. The maximum number reported by a single patient was 20 ED visits and inpatient hospitalizations.
3.3 Initial Health-Related Quality of Life (HRQoL) and Healthcare Resource Utilization (HCRU) Outcomes
There were significant inverse associations between initial SF-36v2 scores and rates of ED visits and inpatient hospitalizations across all domains and summary components, independent of time since diagnosis (p < 0.05 for all) (Table 3), indicating that patients with worse HRQoL had greater rates of HCRU. In examining the associations between HRQoL and ED visits, the effect estimates were larger for aspects of HRQoL related to physical well-being than for mental wellbeing. This is exemplified, for example, by examining the results for PCS (IRR = 1.47, 95% confidence interval [CI] 1.22–1.77) and MCS (IRR = 1.23, 95% CI 1.10–1.39). Every 5-point decrement in PCS score was associated with a 47% higher rate of ED visits, whereas a similar decrement in MCS score was associated with a 23% higher rate of ED visits. Based on the AIC and BIC values for this set of models, the model with PF as a covariate had the best model fit compared with those in which other SF-36v2 scales and summary component scores were included.
When we examined inpatient hospitalizations as the outcome, the effect estimates for physical and mental deficits were fairly similar. In this set of models, the AIC and BIC values both favored the model with SF as the best model candidate for inpatient hospitalizations.
Based on a series of sensitivity analyses, results for both outcomes were consistent after excluding (1) observations with potential data quality concerns (i.e., an RCI ≥ 2) and (2) patients who lived outside of the USA (data not shown).
3.4 Changes in HRQoL and HCRU Outcomes
Changes in physical, but not mental, functioning were associated with ED visits and inpatient hospitalizations during a 12-month period of observation (Table 4). More specifically, significant inverse associations were observed between RP, BP, GH, and PCS change scores and ED visits. Similarly, significant inverse associations were observed between RP, BP, VT, and PCS change scores and inpatient hospitalizations. For example, on average, patients with a decline in HRQoL equal to the MIC for PCS had a 35% greater rate of ED visits and a 38% greater rate of inpatient hospitalizations than in patients who reported stable PCS.
These findings were persistent for both outcomes even when patients with a pattern of inconsistent responses on the SF-36v2 (i.e., an RCI ≥ 2) were excluded from the analysis. However, when patients who lived outside of the USA. were excluded, most point estimates were slightly attenuated and, in some cases, associations that were significant in the full analytic sample no longer achieved significance. For example, when we restricted the analyses to US patients only, we did not observe a significant inverse association between GH and ED visits and nor did we observe significant inverse associations between BP, VT, and inpatient hospitalizations. In contrast, the associations between RP and PCS with each outcome were consistent with and without the inclusion of non-US patients.
4 Discussion
We observed significant associations between both physical and mental HRQoL impairments and higher rates of ED visits and inpatient hospitalizations, independent of how long the patient had AL amyloidosis. These findings were robust across all initial SF-36v2 scale and summary component scores—both those related to physical and mental well-being. However, a slightly different pattern arose when we examined the associations between changes in HRQoL and subsequent HCRU outcomes. Results from these analyses indicate that changes in physical well-being are associated with rates of ED visits and inpatient hospitalizations, while changes in mental well-being were not. Specifically, clinically meaningful declines in functioning related to physical problems, bodily pain, and overall physical health over a 6-month period were associated with greater rates of ED visits and inpatient hospitalizations in the following 12 months as compared with patients whose HRQoL scores in these areas remained stable.
These results contribute to the growing body of evidence highlighting the importance of assessing HRQoL in patients with AL amyloidosis. Patients’ overall HRQoL may be a significant prognostic factor for clinical outcomes such as survival [18] and response to treatment [19]. In fact, HRQoL measures may serve as proxies for disease severity and/or prognosis and may help clinicians to (1) identify patients who are at the greatest risk for poor health outcomes that could lead to the use of costly health care resources; and (2) target supportive treatments accordingly. Furthermore, elucidating the magnitude of HCRU in AL amyloidosis provides clinicians, scientists, and regulators with a broader understanding of the burden of disease patients with AL amyloidosis experience.
The findings from this study should be interpreted with care. First, it is important to note that all data from this study were based on patient self-report, and thus are subject to recall bias. Second, the impact of the specific patient sample on the results must be considered. Through collaboration with patient advocacy groups, we were able to recruit a large number of patients with a rare disease. However, this sample included a large number of patients who were long-term survivors and whose disease was generally stable. Patients with a more acute disease status may be less likely to participate in the study overall and may be more likely to be lost to follow-up. The sample primarily comprised patients who were treated within the context of the US healthcare system. Subsequently, these analyses may have limited external generalizability and may provide conservative estimates of both HRQoL impairments and HCRU in patients with AL amyloidosis and may not be representative of the experience globally. Third, we were limited in exploring additional stratified analyses due to the small sample size. Stratifying the data by time since diagnosis, complete hematologic status, or other measures of disease severity may have allowed us to look at the relationships between HRQoL and HCRU among those with the highest rates of morbidity.
Finally, this was a secondary data analysis of an existing dataset. While the survey was designed to assess multiple dimensions of the patient experience, the module pertaining to HCRU did not exhaustively measure all aspects of HCRU. We chose to focus on two HCRU outcomes that are associated with acute care and high costs; however, we did not account for the total time spent in the hospital. It is important to note that the length of a hospital stay may be indicative of certain treatments, complications, and/or adverse events. While long hospital stays impact the total expense of a hospitalization, they also cause additional physical and emotional burden on patients and their families. Identifying risk factors of lengthy hospitalizations among patients with AL amyloidosis can provide insight into processes of care and strategies to mitigate these risks.
Although our analytic power was limited by the small absolute number of patients involved in the study, this dataset provided a relatively large sample size for such a rare condition. Furthermore, the dataset was a rich resource in terms of patient-reported outcomes and other important individual-level information that might not be available in large claims-based databases. Despite the novel aspects of these data, findings should be replicated with a larger sample of patients with newly diagnosed AL amyloidosis. Additional future research should also be extended to explore the relationship between HRQoL and other components of HCRU (outpatient visits, pharmaceuticals, other interventions) in this patient population and should explore whether interventions that improve physical HRQoL are associated with reductions in HCRU.
5 Conclusion
Scores from patient-reported HRQoL surveys may be helpful in identifying patients at risk of future ED visits and hospital admissions, and may serve as a proxy measure for disease severity. Such information can provide stakeholders insight into the humanistic and societal cost associated with AL amyloidosis.
References
Prothena Therapeutics Ltd., Quality of life (QOL) registry for patients with AL amyloidosis. 2015 [ClinicalTrials.gov identifier NCT02574676]. National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov/. Accessed 23 Jan 2019.
Merlini G, Wechalekar AD, Palladini G. Systemic light chain amyloidosis: an update for treating physicians. Blood. 2013;121:5124–30. https://doi.org/10.1182/blood-2013-01-453001.
Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Healthcare resource utilization and costs in amyloid light-chain amyloidosis: a real-world study using US claims data. J Comp Eff Res. 2018;7(6):549–59. https://doi.org/10.2217/cer-2017-0100.
Hari P, Lin HM, Asche CV, Ren J, Yong C, Luptakova K, et al. Treatment patterns and health care resource utilization among patients with relapsed/refractory systemic light chain amyloidosis. Amyloid. 2018;25:1–7. https://doi.org/10.1080/13506129.2017.1411796.
Bellavia D, Pellikka PA, Al-Zahrani GB, Abraham TP, Dispenzieri A, Miyazaki C, et al. Independent predictors of survival in primary systemic (Al) amyloidosis, including cardiac biomarkers and left ventricular strain imaging: an observational cohort study. J Am Soc Echocardiogr. 2010;23:643–52. https://doi.org/10.1016/j.echo.2010.03.027.
Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the veterans arthritis quality of life study. Semin Arthritis Rheum. 2005;34:755–65. https://doi.org/10.1016/j.semarthrit.2004.08.001.
Callaghan R, Prabu A, Allan RB, Clarke AE, Sutcliffe N, Pierre YS, et al. Direct healthcare costs and predictors of costs in patients with primary Sjogren’s syndrome. Rheumatology. 2007;46:105–11. https://doi.org/10.1093/rheumatology/kel155.
Cavrini G, Broccoli S, Puccini A, Zoli M. EQ-5D as a predictor of mortality and hospitalization in elderly people. Qual Life Res. 2012;21:269–80. https://doi.org/10.1007/s11136-011-9937-0.
Chen T, Li L. Influence of health-related quality of life on health service utilization in addition to socio-demographic and morbidity variables among primary care patients in China. Int J Public Health. 2009;54:325–32. https://doi.org/10.1007/s00038-009-0057-3.
Mathews WC, May S. EuroQol (EQ-5D) measure of quality of life predicts mortality, emergency department utilization, and hospital discharge rates in HIV-infected adults under care. Health Qual Life Outcomes. 2007;5:5. https://doi.org/10.1186/1477-7525-5-5.
Bayliss M, McCausland KL, Guthrie SD, White MK. The burden of amyloid light chain amyloidosis on health-related quality of life. Orphanet J Rare Dis. 2017;12:15. https://doi.org/10.1186/s13023-016-0564-2.
Carroll M, Sutherland G, Kemp-Casey A, Kinner SA. Agreement between self-reported healthcare service use and administrative records in a longitudinal study of adults recently released from prison. Health Justice. 2016;4:11. https://doi.org/10.1186/s40352-016-0042-x.
Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev. 2006;63:217–35. https://doi.org/10.1177/1077558705285298.
Maruish ME. User’s manual for the SF-36v2 health survey. 3rd ed. Lincoln: QualityMetric, Inc.; 2011.
Bertakis KD, Azari R, Helms LJ, Callahan EJ, Robbins JA. Gender differences in the utilization of health care services. J Fam Pract. 2000;49:147–52.
Lin HM, Gao X, Cooke CE, Berg D, Labotka R, Faller DV, et al. Disease burden of systemic light-chain amyloidosis: a systematic literature review. Curr Med Res Opin. 2017;33:1017–31. https://doi.org/10.1080/03007995.2017.1297930.
OECD. Health at a glance 2017: OECD indicators. Paris: OECD Publishing; 2017. https://doi.org/10.1787/health_glance-2017-en.
Warsame R, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, et al. Hematology patient reported symptom screen to assess quality of life for AL amyloidosis. Am J Hematol. 2017. https://doi.org/10.1002/ajh.24676.
Seldin DC, Anderson JJ, Sanchorawala V, Malek K, Wright DG, Quillen K, et al. Improvement in quality of life of patients with AL amyloidosis treated with high-dose melphalan and autologous stem cell transplantation. Blood. 2004;104:1888–93. https://doi.org/10.1182/blood-2004-01-0089.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded Prothena Biosciences Inc. A full-time employee of the study sponsor, Prothena Biosciences Inc., served as an author on this manuscript.
Conflict of interest
At the time of the original submission, KLM, AAR, MKW, and MSB were full-time employees of Optum, Inc., which publishes the SF-36v2®, and received research funding from Prothena Biosciences Inc. to conduct the study. TPQ is a full-time employee of the study sponsor, Prothena Biosciences Inc.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol, informed consent, and measures for the study were reviewed and approved by the New England Institutional Review Board (IRB# 15-355).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Data availability statement
The datasets from the study are available from the corresponding author on reasonable request.
Author contributions
KLM, MKW, and MB contributed to the study design. KLM performed analyses. KLM and AAR wrote the manuscript. All authors contributed to the conceptualization of the research question and the analytic plan and revised the manuscript critically for important intellectual content and gave final approval of the version to be published.
Additional information
Data for these analyses were drawn from the AL Amyloidosis Patient Health-Related Quality of Life Study [1].
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
McCausland, K.L., Rizio, A.A., White, M.K. et al. Associations between Health-Related Quality of Life and Self-Reported Emergency Room Department Visits and Inpatient Hospitalizations: Insights from a Secondary Data Analysis of Patients with Light-Chain (AL) Amyloidosis. PharmacoEconomics Open 3, 367–375 (2019). https://doi.org/10.1007/s41669-019-0122-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-019-0122-7