Abstract
Objective
Our objective was to evaluate the cost effectiveness of the combination of nivolumab and ipilimumab, referred to as “Regimen”, as a first-line treatment for patients with advanced melanoma from the perspective of Canada’s public healthcare system.
Methods
We developed a partitioned-survival model (progression-free survival, post-progression survival, and death) to determine the clinical and economic outcomes of immunotherapy for advanced melanoma over a 20-year time horizon. Regimen was compared with nivolumab, ipilimumab, and pembrolizumab. Two treatment durations for pembrolizumab were considered: (1) maximum of 24 months or until progression or (2) no maximum duration, until progression. The model used data from CheckMate-067 (28 months’ follow-up) for treatments involving nivolumab and ipilimumab. The efficacy of pembrolizumab was estimated using indirect comparisons. A scenario looking at the cost of subsequent treatments following disease progression was examined.
Results
Regimen had better outcomes and was cost effective compared with all other immunotherapies at a threshold of $CAN100,000 per quality-adjusted life-year (QALY) gained. Compared with nivolumab and ipilimumab, the incremental cost-effectiveness ratios (ICERs) were $CAN47,119 and 66,750 per QALY, respectively. Compared with pembrolizumab with a treatment duration cap, the ICER was $CAN85,436. When assuming no duration cap, Regimen dominated pembrolizumab. With the inclusion of subsequent treatment costs following progression, Regimen’s ICER improved compared with all other comparators.
Conclusions
Despite the advent of effective new therapies for advanced melanoma, prognosis remains poor for some patients. Compared with other immunotherapies, Regimen offers marked benefit and may be a cost-effective treatment option.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study examined the economic value of the combination of nivolumab and ipilimumab (so-called Regimen) as a first-line treatment for advanced melanoma in Canada and showed that it may be a cost-effective alternative to all other immunotherapies. |
Relative to all other model parameters, drug costs were the primary driver of cost effectiveness, whereas adverse events had a minimal impact on model outcomes. |
1 Background
Advanced melanoma is the deadliest form of skin cancer, with an estimated 1250 deaths annually and an age-standardized mortality rate of 3.1 per 100,000 in Canada [1]. For 15–20% of patients, melanoma will progress to metastatic disease, resulting in a high mortality rate [2, 3].
Immune checkpoint-blocking antibodies and combination BRAF inhibitor therapies have now become the standard of care in Canada and have significantly improved progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) over chemotherapy [4,5,6,7,8]. However, BRAF inhibitors are effective in only the approximately 50% of the patient population with a BRAF V600 mutation [9]. Immunotherapies, on the other hand, can have lasting benefits regardless of BRAF status. The combination of nivolumab and ipilimumab, referred to as “Regimen”, represents the next generation in immuno-oncology treatment.
Regimen has demonstrated significantly longer PFS, higher ORR, and higher OS rates than ipilimumab alone in the CheckMate-067 and CheckMate-069 studies [10, 11]. In a descriptive analysis, Regimen resulted in a numerically greater median PFS (11.5 vs. 6.9 months) and ORR (58 vs. 44%) than nivolumab, including in patients with poor prognostic factors [11, 12] such as high lactate dehydrogenase (LDH) or stage M1c disease. Regimen was associated with increased toxicity compared with ipilimumab, as expected a priori, but toxicity was manageable and often addressed with discontinuation of the drug [12]. This did not appear to impact clinical benefit [11, 13, 14].
Health Canada issued a Notice of Compliance with Conditions in October 2016, based on reporting of the co-primary endpoint PFS from findings of the CheckMate-067 trial (database lock February 2015, minimum follow-up 12.2–12.5 months across all groups). An update on the second co-primary endpoint, OS, has recently been made available, showing improved survival with Regimen [15].
Following this approval, the next question to address is the health economic impact of Regimen compared with other treatments in the Canadian market. Therefore, the objective of this study was to evaluate the cost effectiveness of Regimen as a first-line treatment for previously untreated patients with unresectable stage IIIC or IV melanoma.
2 Methods
2.1 Overview
We developed a partitioned-survival model to determine the long-term clinical and economic outcomes of immunotherapy management for advanced melanoma over a 20-year time horizon. The model approach was selected because (1) it enables transparent representation of the health states, (2) clinical trial data can be used directly to capture key efficacy endpoints, and (3) it is commonly used for late-stage oncology models as treatments are often measured by their ability to delay the inevitable progression of disease in addition to prolonging survival. Partitioned-survival models have been previously used to evaluate the cost effectiveness of pembrolizumab in advanced melanoma [16, 17]. The choice of a 20-year time horizon was based on the pan-Canadian Oncology Drug Review (pCODR) for ipilimumab, which reported that a time horizon of 10–20 years is reasonable for patients with advanced melanoma receiving first-line treatment [18].
The model took the perspective of Canada’s public healthcare system (Ontario Ministry of Health and Long-Term Care), and only direct medical costs ($Can, year 2016 values) were captured. Life-years and quality-adjusted life-years (QALYs) were also captured. Both costs and outcomes were discounted at 5% annually, according to the Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines for economic evaluation of health technologies at the time the study was conducted [19].
Regimen was compared with nivolumab, pembrolizumab, and ipilimumab, as they are indicated for advanced melanoma and are the most commonly used immunotherapies in Canada.
2.2 Model Structure
The main health states in the model were PFS, post-progression survival (PPS), and death, and the time within each state was based on the PFS and OS observed in trials. The proportion of patients in the PPS state was simply OS minus PFS, and death was one minus OS (Fig. 1). Patients entered the model in the progression-free state, where they received first-line treatment until discontinuation or progression. These patients were divided by treatment response, which impacts quality of life [20].
A weekly cycle was used, which provided the flexibility to precisely assign drug costs for intravenous treatments with different dosing frequencies (e.g., the Regimen starting dose was every 3 weeks, and the maintenance dose was every 2 weeks). Although the cycle length is short, a half-cycle correction was still employed, in accordance with good modeling practice [21].
2.3 Overall Survival and Progression-Free Survival
OS for Regimen, nivolumab, and ipilimumab was informed by the CheckMate-067 trial (at least 28 months’ follow-up) and extrapolated over the model time horizon using standard statistical analyses. It is important to note that, at the time of this model analysis, the median OS had not been reached in the Regimen arm; therefore, the extrapolations should be interpreted with caution.
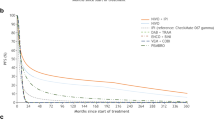
Different parametric functions, including Weibull, log-normal, log-logistic, exponential, generalized gamma, and Gompertz, were fitted to the observed OS data for Regimen, nivolumab, and ipilimumab (details are available in Appendix A in the Electronic Supplementary Material [ESM]). Goodness of fit was based on visual inspection, Akaike information criterion (AIC), and clinical validity. We determined that log-normal was the most reasonable function to extrapolate OS in all three treatments (Fig. 2).
Comparison of observed and projected overall survival. Source of KM: CheckMate-067 (28 months’ follow-up). Pembrolizumab KM not presented because projection is based on indirect comparison with ipilimumab. IPI ipilimumab, KM Kaplan–Meier, NA not available, NIVO nivolumab, NIVO/IPI Regimen, OS overall survival, PEM pembrolizumab
The mortality rate in each model cycle was checked against a background mortality based on the general population. The background mortality was based on the complete life table from Canada between 2010 and 2012, the most recent data available [22]. However, the model survival projection never met the background mortality at any point of the time horizon.
As for PFS for the treatments in CheckMate-067, we determined that a one-segment parametric function did not accurately capture the quickly changing hazard observed in the first 12 weeks, the point of the first evaluation for response. Therefore, a piecewise approach was followed and PFS up to 12 weeks was taken directly from the Kaplan–Meier (KM) data. A parametric function fitted to the post-12-week PFS was used for the long-term projection. Log-normal was found to have the most reasonable fit for this post-12-week period for all three treatments (Fig. 3).
Comparison of observed and projected progression-free survival. Source of KM: CheckMate-067 (28 months’ follow-up). Pembrolizumab KM not presented because projection is based on indirect comparison with ipilimumab. IPI ipilimumab, KM Kaplan–Meier, NIVO nivolumab, NIVO/IPI Regimen, PEM pembrolizumab, PFS progression-free survival
2.4 Indirect Comparison for Pembrolizumab
A simple chained indirect comparison using Bucher methodology was performed to determine the relative treatment effect of pembrolizumab (details can be found in Appendix B in the ESM). Ipilimumab served as the common comparator between CheckMate-067 and KEYNOTE-006. KEYNOTE-002 was added to this network to allow comparison with the dose of pembrolizumab indicated in Canada. The included trials were evaluated for similarity in patient characteristics and potential treatment-effect modifiers. It was assumed that proportional hazards were not violated and that a single hazard ratio (HR) could be used to describe the relative difference between the treatments. The analysis found the HRs for pembrolizumab 2 mg/kg every 3 weeks relative to ipilimumab for OS was 0.78 (95% confidence interval [CI] 0.55–1.10) and PFS was 0.67 (95% CI 0.49–0.92). At the time of this model analysis, the median OS for KEYNOTE-006 was not reached, so the HRs should be interpreted with caution.
2.5 Treatment Duration
Treatment durations for Regimen and for nivolumab were based on KM data of time to treatment discontinuation from CheckMate-067. Parametric functions were fitted to the KM data to extrapolate beyond the data. Log-logistic had the best fit for both treatments (details are available in Appendix A in the ESM). The ipilimumab component of Regimen was given in up to four doses (over a total of 3 months) or until discontinuation.
Time on treatment data for pembrolizumab from KEYNOTE-006 were not published and could not be modeled; instead, two scenarios for treatment duration were modeled based on clinician feedback. The first scenario assumed that pembrolizumab was administered for 24 months or until disease progression, according to the product monograph [23]. The second scenario assumed pembrolizumab would be administered until progression without any maximum duration. Ipilimumab was administered for up to four doses or until progression [24].
2.6 Best Objective Response
Best objective response rates to first-line treatment, used as predictors for quality-of-life estimates in the model, were collected from each comparator’s trial. The response rates for Regimen, nivolumab, and ipilimumab were 59, 45, and 19%, respectively [12].
The response rate for pembrolizumab 2 mg/kg was estimated through an indirect treatment comparison, utilizing the same simple network of trials used to estimate the relative PFS and OS for pembrolizumab. The analysis yielded a response rate of 28%.
2.7 Adverse Events
A list of common toxicities associated with the treatments incorporated in this analysis was compiled by examining the relevant clinical trial publications and the manufacturer’s product monographs for the comparators and by eliciting input from clinicians about adverse events (AEs) that were costly to manage or impacted utility. The resulting list, presented in Table 1, consists of treatment-related grade 3 and 4 AEs.
2.8 Value Clinical Outcomes
The model applied utilities for advanced melanoma health states elicited from the Canadian general public using the standard gamble method (Table 2) [20]. EuroQol–5 Dimensions, 3 Levels (EQ-5D-3L) data were collected during CheckMate-067 (Appendix C in the ESM) but were not used for the base-case analysis because the utilities from the Canadian study were considered to be more reflective of a Canadian population. However, the utility data from CheckMate-067 were examined in sensitivity analysis.
2.9 Resource Use and Costs
All costs used in the model are presented in Table 2. The dosing regimens were derived from the corresponding phase III clinical trials and product monographs. In calculating the drug cost per dose, we assumed that patients with advanced melanoma have an average weight of 70 kg and an average body surface area of 1.83 m2, based on average patient characteristics reported in recent pCODR submissions. These recent submissions for injectable immunotherapies in melanoma—namely ipilimumab, nivolumab, and pembrolizumab—consistently used an average weight of 70 kg to calculate doses [25,26,27]. Drug wastage was part of the base-case analysis, in which the cost of excess drug that may have been discarded was considered.
The nivolumab component of Regimen was assigned the administration cost of a standard chemotherapy agent. Since ipilimumab requires an infusion that lasts for 90 min, an administration cost for special single-agent chemotherapy was employed.
Costs for routine follow-up care and unplanned medical care were assigned to each health state. To inform the inputs for frequency of medical resource use, we interviewed Dr. David Hogg, MD, FRCP(C), a clinical expert in melanoma and treatment patterns in Canada. He advised on the frequency of routine physician visits and consultations, imaging and diagnostic tests, and clinical laboratory tests. Unplanned medical resource use (MRU) consisted of hospitalizations, emergency room visits, healthcare provider visits, and radiotherapy. Data on unplanned MRU were from a Canadian chart review study (MELODY study) [28], with review and adjustments by a clinician. We assumed that unplanned MRU was the same across treatments, which the clinician considered reasonable.
Subsequent cancer treatments could have a significant impact on overall costs and, because of the uncertainty of the breakdown of treatments following pembrolizumab, we decided to not model the cost of these treatments in post-progression. This assumption is considered conservative, since OS implicitly captures the efficacy of subsequent treatments. Nevertheless, we evaluated the inclusion of subsequent cancer treatment costs in scenario analysis.
The costs of terminal care involved palliative care physician visits every 2 weeks for the last 2 months of life and hospice care [29].
The costs of treating AEs were calculated as weighted averages using a clinician’s assumptions of the split between inpatient and outpatient care in Canada. Inpatient and outpatient costs for each AE were identified through the Ontario Case Costing Initiative Tool. These costs were applied in the model as one-time costs upon initiating first-line treatment.
3 Results
3.1 External Validation
The projection of OS for Regimen and ipilimumab was compared with long-term OS data from a pooled analysis of ipilimumab, which included two phase III, five phase II, and three phase I/II trials and two observational studies [30]. With this level of variability in design, comparison of the study’s findings with the OS projection is not ideal but will provide some confirmation of the shape of the projected survival curves.
With up to 10 years of follow-up, the survival curve from the pooled analysis began to plateau around year 3, with a survival rate of 22%. This plateau is in line with the OS projections for Regimen, nivolumab, and ipilimumab (see Appendix D in the ESM), which are based on a log-normal distribution—this type of distribution has a long and gradually declining tail. Despite heterogeneity in the patient populations of the pooled analysis (e.g., different lines of treatment), the projected ipilimumab OS in the model was within 10% of the 10-year mark of the pooled analysis.
OS projections for Regimen and ipilimumab were also compared with results from CheckMate-069, which showed that, at a median follow-up of 24.5 months, OS was 63.8% (95% CI 53.3–72.6) for patients assigned to Regimen and 53.6% (95% CI 38.1–66.8) for those assigned to ipilimumab alone. Differences between arms were not statistically significant, but this is likely attributed to crossover, as 62% of patients in the ipilimumab arm of CheckMate-069 had crossed over to receive anti-programmed cell death 1 (PD1) treatment [10, 31]. Model projections for OS are in line with the observations from CheckMate-069, with 62.8% OS projected with Regimen and 44.9% with ipilimumab alone.
3.2 Base Case
Over a 20-year time horizon, patients treated with first-line Regimen were projected to have more life-years, progression-free life-years, and QALYs than those receiving nivolumab, ipilimumab, or pembrolizumab. Table 3 shows a breakdown of discounted costs by treatment phase among patients receiving Regimen and comparators. Patients receiving Regimen generally had higher costs in most of the cost categories, except for those associated with post-progression and terminal care because survival and PFS were higher with Regimen. When assuming a cost-effectiveness threshold of $CAN50,000–100,000 per QALY gained (incremental cost-effectiveness ratio [ICER]), Regimen was cost effective compared with the other treatments. When assuming treatment until progression, pembrolizumab was dominated by Regimen, with greater costs and lower clinical benefits.
3.3 Multi-way Sensitivity and Scenario Analyses
Sensitivity analyses, which tested the effect of the high and low ranges of the model’s parameters on model results, were performed to identify key model drivers; results are presented in Appendix C in the ESM. Several scenarios in the sensitivity analyses required adjustments to several inputs simultaneously, making this a multi-way analysis. Key drivers included parameters associated with drug costs (e.g., treatment duration, patient weight, and drug wastage), parametric functions for projecting OS and PFS, relative treatment effect for pembrolizumab, time horizon, discounting, and inclusion of subsequent treatment costs. All scenarios yielded ICERs within the threshold of $CAN50,000–100,000 per QALY gained. The sensitivity analysis did not find that AEs influenced overall results. Although the incidence of AEs was higher with Regimen, the costs of AEs were relatively small compared with those for other medical resources.
The scenario examining subsequent treatments following disease progression assumed that the clinical benefits of these subsequent treatments were implicitly captured in the OS data; therefore, only the costs of these subsequent treatments were modeled. The distribution of the specific subsequent treatments was based on observations from CheckMate-067. Data on subsequent treatments were not available for pembrolizumab. Therefore, this was assumed to equal the distribution of subsequent treatments following nivolumab, with the exception of the proportion of patients continuing onto pembrolizumab; these patients were assigned the cost of nivolumab. Accounting for subsequent treatment costs led to an improvement in Regimen’s ICER vs. all comparators relative to the base case (vs. nivolumab: $CAN16,898 [64% decrease]; vs. ipilimumab: $CAN59,149 [11% decrease]; vs. pembrolizumab with a 24-month treatment cap: $CAN75,682 [11% decrease]), since a lower proportion of patients treated with Regimen continued on to high-cost subsequent systemic treatments such as ipilimumab and targeted therapies. Improved efficacy with Regimen, in terms of lower post-progression life-years, also affected the amount of time patients spent receiving subsequent treatments.
3.4 Probabilistic Sensitivity Analysis
A probabilistic sensitivity analysis (PSA) was conducted to account for multivariate and stochastic uncertainty in the model. The uncertainty in the individual parameters was characterized using probability distributions and analyzed using Monte Carlo simulation (1000 iterations). Mean incremental QALYs and costs were in line with base-case results (vs. nivolumab: 0.558 QALYs [95% CI − 0.135 to 1.204], $CAN26,961 [95% CI 9565–43,181]; vs. ipilimumab: 2.021 QALYs [95% CI 1.615–2.783], $CAN149,817 [95% CI 136,769–165,627]; vs. pembrolizumab with a 24-month treatment cap: 1.498 QALYs [95% CI 0.463–2.307], $CAN132,936 [95% CI 102,185–158,250]), suggesting that deterministic results were robust in light of uncertainty in all parameters. PSA was also conducted to determine the likelihood of a treatment being cost effective at different willingness-to-pay (WTP) thresholds when compared across comparators (Fig. 4). Regimen started to become the most cost-effective treatment among all comparators at the WTP level of $CAN89,000.
4 Discussion
Compared with all other immunotherapies, Regimen yielded ICERs within the threshold of $CAN50,000–100,000 per QALY gained. When following the product monograph for pembrolizumab, which recommends treat to progression, Regimen resulted in cost savings with improved survival, making it dominant over pembrolizumab. Regimen’s cost effectiveness compared with ipilimumab is in line with pembrolizumab in Canada. In a cost-effectiveness analysis of pembrolizumab in Canada sponsored by Merck, the ICER compared with ipilimumab was $CAN81,091 [17].
Relative to all other model parameters, drug cost was the primary driver of cost effectiveness. AEs had a minimal impact on model outcomes.
The scenario examining inclusion of subsequent treatment costs following progression showed an improvement in Regimen’s cost effectiveness compared with all other comparators. These results were driven by Regimen’s improved PFS (reducing need for subsequent therapies) and composition of subsequent treatments observed in CheckMate-067.
4.1 Study Limitations
An important limitation of this economic analysis is that the median for the OS data in CheckMate-067 has not yet been met, leading to uncertainty in the long-term projections. To address this, the standard practice of testing different parametric models for extrapolation beyond observed data was performed and extrapolations were verified with external data.
There is further uncertainty in the efficacy in Regimen’s primary comparison, pembrolizumab at 2 mg/kg. This dosage of pembrolizumab was not studied in the first-line setting. Therefore, PFS and OS for pembrolizumab 2 mg/kg were estimated through an indirect comparison that captured the observed difference in efficacy between pembrolizumab 2 mg/kg and 10 mg/kg in KEYNOTE-002. Moreover, at the time of this model analysis, the median OS for KEYNOTE-006 was not reached, creating uncertainty in the projection of survival.
The model considered two scenarios for treatment duration of pembrolizumab in Canada based on separate recommendations from pCODR and the product monograph. However, whether pembrolizumab’s efficacy derived from clinical trials reflects the pCODR recommendation to administer until 24 months or until progression is uncertain. As data from CheckMate-067 did not show loss of response upon early discontinuation, it can be assumed that earlier discontinuation on pembrolizumab does not entirely affect clinical benefit. Moreover, evidence from the CA209-003 trial suggests continued response and durable survival with a 96-week (22-month) treatment cap [32].
4.2 Study Strengths
The model employed recent trial data to inform significant endpoints. PFS and OS for Regimen and ipilimumab alone were based on a minimum of 28 months of follow-up data from CheckMate-067, which was the most recent trial at the time of this study.
External validation (i.e., comparison of model projections with other data not used in the model) showed that the shape of the distributions used in the model to project OS were consistent with the 10-years follow-up data from the pooled analysis of ipilimumab studies (see Sect. 3.1). External validation of projections for OS with Regimen also showed consistency with OS data from CheckMate-069. At 24.5 months (median follow-up for CheckMate-069), the model estimated 63.6% OS compared with 63.8% in CheckMate-069.
The cost-effectiveness findings of our study for Regimen vs. ipilimumab are consistent with a separate cost-effectiveness study in the UK that examined different structural approaches to estimating survival [33]. The model utilized Canada-specific utilities in the base case, which makes the model findings more targeted to a Canadian population. Interestingly, in the multi-way sensitivity analyses, the model assessed the impact of using the utility derived from CheckMate-067 instead and found that this did not greatly affect results (10% increase in ICER vs. ipilimumab, 11% increase vs. nivolumab, 2% increase vs. pembrolizumab with 24-month treatment cap).
5 Conclusion
This study suggests that Regimen is a valuable and cost-effective first-line treatment for patients with advanced melanoma and is similar in cost effectiveness to currently listed immunotherapy options in Canada.
Data Availability Statement
The economic model used in this study is not publicly available in order to protect the commercial, confidential discounts that are in place for both nivolumab and ipilimumab.
References
Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2017. Table 2.2 on page 62. 2017. http://www.cancer.ca/~/media/cancer.ca/CW/publications/Canadian/Cancer/Statistics/Canadian-Cancer-Statistics-2017-EN.pdf.
Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–34.
Petrella T, Ernst S, Spatz A, Claveau J, Wong R, Smylie M. Canadian perspective on the clinical management of metastatic melanoma. New Evid Oncol. 2012;2012(September):108–20.
pan-Canadian Oncology Drug Review (pCODR). pCODR Expert Review Committee (pERC) Final recommendation for Nivolumab (Opdivo). 2016. https://cadth.ca/sites/default/files/pcodr/nivolumab_opdivo_mm_fn_rec.pdf. Accessed 5 Oct 2018.
pan-Canadian Oncology Drug Review (pCODR). pCODR Expert Review Committee (pERC) Final Recommendation for Ipilimumab (Yervoy). 2012. https://www.cadth.ca/sites/default/files/pcodr/pcodr-yervoy-adv-mel-fn-rec.pdf. Accessed 5 Oct 2018.
pan-Canadian Oncology Drug Review (pCODR). pCODR Expert Review Committee (pERC) Final Recommendation for Dabrafenib (Tafinlar) in combination with Trametinib (Mekinist). 2015. https://www.cadth.ca/sites/default/files/pcodr/pcodr_tafinlar_mekinist_metmelanoma_fn_rec.pdf. Accessed 5 Oct 2018.
pan-Canadian Oncology Drug Review (pCODR). pCODR Expert Review Committee (pERC) Final Recommendation for Vemurafenib (Zelboraf). 2012. https://www.cadth.ca/sites/default/files/pcodr/pcodr-zelboraf-adv-mel-fn-rec.pdf. Accessed 5 Oct 2018.
pan-Canadian Oncology Drug Review (pCODR). pCODR Expert Review Committee (pERC) Final Recommendation for Trametinib (Mekinist). 2013. https://www.cadth.ca/sites/default/files/pcodr/pcodr-mekinist-mm-fn-rec.pdf. Accessed 5 Oct 2018.
Colombino M, Capone M, Lissia A, et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J Clin Oncol. 2012;30(20):2522–9.
Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17(11):1558–68.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Wolchok J, Chiarion Sileni V, Gonzalez R, et al. Updated results from a Phase III trial of nivolumab combined with ipilimumab in treatment-naïve patients with advanced melanoma (Checkmate 067). ASCO annual meeting; June 3–7, 2016; Chicago, IL, USA.
Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17.
Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33.
Stenger M. Overall survival with nivolumab/ipilimumab, or nivolumab alone, vs ipilimumab in advanced melanoma. The ASCO Post. 2017. http://www.ascopost.com/News/58065. Accessed 4 Jun 2018.
National Institute of Health and Care Excellence (NICE). Melanoma (unresectable, metastatic, ipilimumab naive)—pembrolizumab [ID801]: Final appraisal determination committee papers for NICE Technology Appraisal 366 (Pembrolizumab for advanced melanoma not previously treated with ipilimumab). 2015. https://www.nice.org.uk/guidance/ta366/documents/committee-papers. Accessed 23 Mar 2017.
Wang J, Chmielowski B, Pellissier J, Xu R, Stevinson K, Liu FX. Cost-effectiveness of pembrolizumab versus ipilimumab in ipilimumab-Naive patients with advanced melanoma in the United States. J Manag Care Spec Pharm. 2017;23(2):184–94.
pan-Canadian Oncology Drug Review (pCODR). Final economic guidance report: Pembrolizumab (Keytruda) for metastatic melanoma. 2015. https://www.cadth.ca/sites/default/files/pcodr/pcodr_pembrolizumab_keytruda_mm_fn_egr.pdf. Accessed 23 Mar 2017.
Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada [3rd Edition]. 2006. https://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf. Accessed 23 Mar 2017.
Hogg D, Osenenko K, Szabo SM, et al. Standard gamble utilities for advanced melanoma health states elicited from the Canadian general public. Abstracts of melanoma 2010 congress, the 7th international melanoma research congress, and the 4th melanoma and skin cancer centres meeting, November 4–7, 2010. Sydney, Australia. Pigment Cell Melanoma Res. 2010;23(6):876–1004.
Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Value Health. 2012;15(6):812–20.
Statistics Canada. Life Tables, Canada, Provinces and Territories 2010 to 2012. 2016. http://www.statcan.gc.ca/pub/84-537-x/84-537-x2016006-eng.htm. Accessed 12 Nov 2016.
Merck Canada. Keytruda (pembrolizumab) Product Monograph for Canada. 2016; www.merck.ca/assets/en/pdf/products/KEYTRUDA-PM_E.pdf. Accessed 28 Nov 2016.
Bristol-Myers Squibb Canada. Product monograph of ipilimumab published in Canada (“Dosage and Administration” section on page 23 of the monograph). 2017.
Canadian Agency for Drugs and Technologies in Health (CADTH). Yervoy for advanced melanoma—details. 2012. https://www.cadth.ca/yervoy-advanced-melanoma-details. Accessed 23 Mar 2017.
Canadian Agency for Drugs and Technologies in Health (CADTH). Keytruda for metastatic melanoma—details. 2015. https://www.cadth.ca/keytruda-metastatic-melanoma-details. Accessed 23 Mar 2017.
Canadian Agency for Drugs and Technologies in Health (CADTH). Opdivo for metastic melanoma—details. 2015. https://www.cadth.ca/opdivo-metastatic-melanoma-details. Accessed 23 Mar 2017.
Johnston K, Levy AR, Lorigan P, et al. Economic impact of healthcare resource utilisation patterns among patients diagnosed with advanced melanoma in the United Kingdom, Italy, and France: results from a retrospective, longitudinal survey (MELODY study). Eur J Cancer. 2012;48(14):2175–82.
Home Care Ontario. Facts and figures—publicly funded home care. 2015. http://www.homecareontario.ca/home-care-services/facts-figures/publiclyfundedhomecare. Accessed 23 Mar 2017.
Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–94.
Hassel JC. Ipilimumab plus nivolumab for advanced melanoma. Lancet Oncol. 2016;17(11):1471–2.
Hodi FS, Kluger HM, Sznol M, et al. Durable, long-term survival in previously treated patients with advanced melanoma who received nivolumab monotherapy in a Phase I trial. Abstract CT011. http://cancerres.aacrjournals.org/content/76/14_Supplement/CT001. AACR 107th annual meeting; April 16–20, 2016; New Orleans, LA. Accessed 23 Mar 2017.
Lee D, Amadi A, Sabater J, et al. Can we accurately predict cost effectiveness without access to overall survival data? The case study of nivolumab in combination with ipilimumab for the treatment of patients with advanced melanoma in England. https://doi.org/10.1007/s41669-018-0080-5. Pharmacoecon Open. 2018. Accessed 23 Mar 2017.
Bristol-Myers Squibb (BMS). Final clinical study report for study CA209067: a phase 3, randomized, double-blind study of nivolumab monotherapy or nivolumab combined with ipilimumab versus ipilimumab monotherapy in subjects with previously untreated unresectable or metastatic melanoma. 2016.
Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–18.
Ontario Ministry of Health and Long Term Care. Schedule of benefits. Physician Services under the Health Insurance Act (effective March 1, 2016). 2016. http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20160401.pdf. Accessed 23 Mar 2017.
Guerriere DN, Zagorski B, Fassbender K, Masucci L, Librach L, Coyte PC. Cost variations in ambulatory and home-based palliative care. Palliat Med. 2010;24(5):523–32.
Acknowledgements
The authors thank Margarita Schultz for her technical support throughout the course of conducting this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Bristol-Myers Squibb, of Quebec, Canada, provided the funding for the study described in this manuscript and for the manuscript itself.
Conflicts of interest
At the time the study or analysis was conducted, Amir Abbas Tahami Monfared was an employee and shareholder of Bristol-Myers Squibb, Canada. This study was sponsored by Bristol-Myers Squibb. Peter Quon, Ying Xiao, and Sonja Sorensen are all employees of Evidera, which provides consulting and other research services to pharmaceutical, medical device, and other organizations. In their salaried positions, they work with a variety of companies and are precluded from receiving payment or honoraria directly from these organizations for services rendered. Evidera received funding from Bristol-Myers Squibb, of Quebec, Canada, for the involvement of their employees.
Author contributions
PQ, YX, and SS designed and built the model, conducted the analyses, and interpreted the data. AATM and SS conceived the study and were in charge of overall direction and planning. All authors contributed to drafting the manuscript and approving the final version for submission. PQ serves as overall guarantor for this study and manuscript.
Ethical approval
For this type of study, formal consent is not required, so we did not submit our study to an independent ethics committee or institutional review board. This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Quon, P.L., Xiao, Y., Sorensen, S. et al. Economic Evaluation of Nivolumab Plus Ipilimumab Combination as First-Line Treatment for Patients with Advanced Melanoma in Canada. PharmacoEconomics Open 3, 321–331 (2019). https://doi.org/10.1007/s41669-018-0112-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-018-0112-1