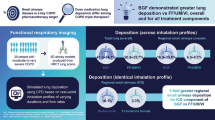
Abstract
Introduction
Drug deposition in the peripheral airways has been reported to differ between pressurized metered dose inhalers (pMDIs) and dry powder inhalers (DPIs). This study was conducted to determine the drug deposition fraction from three inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) combination products for inhalation in the peripheral airways.
Methods
An airway deposition model was constructed using computed tomography (CT) images obtained in patients with asthma. Functional respiratory imaging was used to determine the airway deposition of Flutiform® (Kyorin Pharmaceutical), Symbicort® (AstraZeneca and Astellas Pharma), and Relvar® (GlaxoSmithKline K.K.) without actual drug inhalation based on airway images from CT and computational fluid dynamics using drug particle size.
Results
The mean drug deposition fractions and standard deviations (SDs) in the peripheral airways for Flutiform, Symbicort, and Relvar were 30.33% ± 1.89%, 18.95% ± 2.03%, and 6.58% ± 0.98% for ICS, and 29.17% ± 1.81%, 17.24% ± 1.87%, and 11.30% ± 1.42% for LABA, respectively, showing that significantly higher proportions of ICS and LABA were deposited to the peripheral airways from Flutiform than from Symbicort and Relvar (p < 0.001). The mean drug deposition fractions and SDs in the entire lung for Flutiform, Symbicort, and Relvar were 46.18% ± 1.29%, 28.85% ± 0.78%, and 13.01% ± 0.66% for ICS, and 44.34% ± 1.23%, 26.32% ± 0.71%, and 20.02% ± 0.80% for LABA, respectively, showing that significantly higher proportions of ICS and LABA were deposited to the lung from Flutiform than from Symbicort and Relvar (p < 0.001).
Conclusions
Flutiform (pMDI) was shown to provide significantly higher drug deposition fraction to the central/peripheral airways than Symbicort (DPI) and Relvar (DPI). It is suggested that Flutiform may provide a potent therapeutic option for various asthma patients, including those who have difficulty using DPIs.
Trial registration
UMIN000022840.
Funding
Kyorin Pharmaceutical Co., Ltd.
Similar content being viewed by others
Introduction
Asthma is recognized as a chronic inflammatory disease of the airways, and is accompanied by airway remodeling resulting from persistent inflammation. While recent progress in techniques for objective assessment of airway obstruction and inflammation contribute to the understanding of pathology in each airway region, it has been shown that the peripheral airways in patients with asthma has uneven distribution of airway wall thickening caused by inflammation and luminal narrowing/obstruction caused by sputum, with more severe inflammation in the peripheral airways than in the central airway. In addition, profound correlation between peripheral airways lesions and nocturnal wheezing has been reported, revealing that inflammation is widely found in not only the central airway, but also the peripheral airways into alveoli in asthma patients [1].
From these pathological perspectives, inflammation control not only in the central airway, but also in the peripheral airways is important to achieve complete asthma control as defined by the Asthma Prevention and Management Guidelines (JGL2015) [2].
Inhaled corticosteroids (ICSs) are established as first-line therapy for long-term management of asthma, and the concomitant use of long-acting β2-agonists (LABAs) is recommended for asthma not well controlled by ICSs alone. In Japan, four different combinations of an ICS and a LABA are currently available and supplied in two dosage forms: a pressurized metered-dose inhaler (pMDI) and a dry powder inhaler (DPI). It has been shown that drug deposition in the peripheral airways differs between pMDIs and DPIs due to differences in particle size and flow rate, etc. [3], and is presumed difference of treatment effect.
The distribution research in airways and lungs of patients with pulmonary diseases was conducted by using single photon emission CT (SPECT) or computed tomography (CT). However, those methods used radiolabeled aerosols, and problems of radio-labeled exposure was bothered.
Meanwhile, functional respiratory imaging (FRI) is one of the computational fluid dynamics (CFD) technology, which is an imaging technology for analysis of drug deposition without actual drug inhalation based on airway images from CT and FRI using the factor such as drug particle size and flow rate, has recently been validated [4] and is being introduced for clinical use to determine the airway deposition of inhaled drugs [5].
The objective of this study was to evaluate by FRI the difference of drug deposition between pMDI and DPIs using FRI in Japanese patients with asthma. Drug deposition fraction was imaged and calculated from three ICS/LABA combination products for inhalation, which are widely used in Japan, in the peripheral airways: Symbicort®, a combination of budesonide/formoterol fumarate hydrate (BUD/FM) via DPI, AstraZeneca and Astellas Pharma; Relvar®, a combination of fluticasone furoate/vilanterol trifenatate (FF/VI) via DPI, GlaxoSmithKline K.K.; and Flutiform®, a combination of fluticasone propionate/formoterol fumarate hydrate (FP/FM) via pMDI, Kyorin Pharmaceutical.
Methods
Subjects
Patients who met all of the following inclusion criteria were eligible for the study: an outpatient aged 20 years or older with a diagnosis of asthma; mild to moderate persistent asthma as defined by the Asthma Prevention and Management Guidelines 2015; good or better asthma control [Asthma Control Test (ACT) ≥20]; and able to communicate. Patients with evidence of chronic obstructive pulmonary disease (COPD) or respiratory infection and those who were, in the opinion of the investigator, inappropriate for the study were excluded from the study.
Data Collection
At enrollment (Visit 0), the following patient information was collected: gender, birth date, disease type and severity, duration of disease, treatment step, anti-asthmatic drugs used at enrollment, concurrent diseases, smoking, and asthma control level measured by ACT (25 points: complete control, 20–24 points: well control, <20 points: poor control). In addition, respiratory function testing was performed after inhalation of a bronchodilator only in the absence of asthma exacerbation (at least weekly occurrence of asthma symptoms or use of short-acting β2-agonists): spirometry [forced expiratory volume in 1 s (FEV)1, FEV1% predicted, FEV1/forced vital capacity (FVC), FVC, \({\dot{\text{V}}}\)50, \({\dot{\text{V}}}\)25, %\({\dot{\text{V}}}\)50, and %\({\dot{\text{V}}}\)25] and impulse oscillometry system (IOS) for measurement of airway resistance and reactance (R5, R20, R5–R20, X5, AX, Fres, R5 ex-in, R20 ex-in, R5–R20 ex-in, X5 ex-in, AX ex-in, and Fres ex-in).
Subjects with asthma exacerbation at Visit 1 were discontinued from the study without undergoing a CT scan. For subjects with no evidence of asthma exacerbation, chest CT images (tube voltage 120 kV, tube current 100–750 mA, slice thickness 0.625 mm, and pitch 1.375:1) associated with TLC and FRC were taken via USB spirometer (Spirostik; Geratherm Respiratory GmbH, Kissingen, Germany). CT scan data, flow pattern data collected via Spirostik, and CT scan form were anonymized and stored on a DVD before being sent to Fluidda NV (Kontich, Belgium) for data analysis via the clinical research support organization.
Spirostik, a USB spirometer not approved in Japan, but approved and used in 28 countries, was used, because (1) FRI analysis requires CT images associated with total lung capacity (TLC) and functional residual capacity (FRC), and thus CT scanning with flow patterns being monitored, and (2) a mouthpiece must be non-metal, while those for spirometers approved in Japan contain metals. The airway model at TLC was used for CFD flow stimulation by smoothing the airway wall. The TLC was after deep inspiration, and FRC was after expiration in lungs and lobes.
FRI Analysis
This study was conducted by Fluidda NV (Kontich, Belgium), investigated the lung deposition of inhaled particles emitted by Flutiform pMDI using FRI versus Symbicort and Relvar DPIs. In the FRI workflow, patient-specific anatomical images of CT scans are combined with functional information that is calculated using computational fluid dynamics. Patient-specific three-dimensional models of the airway and lungs were extracted from medical images and suitable boundary conditions were defined based on patient-specific internal airflow distribution. For both the inspiratory and expiratory scans, the patient-specific lung lobes have been reconstructed. All CT data were entered into software package (Mimics 15.0; Materialise, Leuven, Belgium), and lobar and airway segmentation were analyzed.
Based on the values of mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) showed in Table S1, lognormal particle distributions were created. From this distribution, the cumulative mass of all the particles smaller than 5 μm in diameter was calculated, which was considered as the theoretical fine particle fraction (FPF), without any losses taken into account. In the next step, this distribution was scaled such that the theoretical FPF equals the measured FPF, due to possible losses in the inhaler and pre-separator. From MMAD and GSD, the lognormal particle size distribution was calculated and the geometries were reverse-engineered into 3D CAD models. For each of the patients, the patient-specific lower airway was coupled to an average upper airway from Fluidda’s database and the inhaler. On these models, the CFD simulations were performed. CFD simulations (Fluent, Ansys 14.0) were performed on the coupled 3D models using the following strategy: fully tetrahedral mesh (generated in TGrid, Ansys 14.0) with around four million cells; percentage of flow exiting the model towards a lobe equals the relative lobar expansion as obtained from the patient specific in- and expiratory lobar 3D models; selected inhalation profiles were applied at the inhaler inlet to take the turbulence that was generated by the device. Each device was tested under its most optimal conditions; at respective usual dosages and an inspiratory flow rate of 60 L/min for Symbicort (BUD 200 μg, FM 6 μg) and Relvar (FF 100 μg, VI 25 μg), and 30 L/min for Flutiform (FP 125 μg, FM 5 μg). Data of particle size distribution of each drug were obtained using Andersen Cascade Impactor (ACI) for Flutiform and Symbicort and Next Generation Impactor (NGI) for Relvar, since there were no data available for Relvar using ACI. The detailed method for FRI was previously reported [4]. The lobe definition was the same as that used with the CT image (right upper and right middle lobes, right lower lobe, left upper lobe, left lower lobe).
Patient-related variables concerning airway volume [total airway volume, peripheral airways volume and lung lobar airway volume (iVaw)] and airway resistance [total airway resistance, peripheral airways resistance, and lung lobar airway resistance (iRaw)], as well as drug-related FRI variables concerning deposition fraction (expressed as the percentage of the dose administered) and absolute deposition of each active ingredient from individual products in the lung, peripheral airways, and each lobe of the lung were determined.
Endpoints
The primary endpoint was the FRI-based drug deposition fraction in the peripheral airways. The secondary endpoint was the FRI-based drug deposition fraction in the lung and each lobe of the lung.
Target Sample Size and its Rationale
In a previous overseas study, the lung deposition of inhaled particles emitted by three metered-dose inhalers was compared in four subjects. Given the previously reported sample size, the target sample size of six subjects as those who successfully completed the study was selected for the present study to assess the peripheral airways deposition in asthma patients in an exploratory manner.
Statistical Analyses
Analysis of variance (ANOVA) was performed for calculated drug deposition from each product in the entire lung and peripheral airways to prepare boxplots with the median, interquartile range, maximum, and minimum. A whisker from the maximum to the minimum was less than 1.5 times the interquartile range, and outliers were individually plotted. The mean values and the standard deviations (SDs) were given for every drug. ANOVA tables were generated, in which the p values were generated based on Satterthwaite’s approximation for degrees of freedom. A multiple comparison z-test with confidence intervals was performed to test the differences in deposition between Flutiform and the other products. p values smaller than 0.05 were considered statistically significant.
Compliance with Ethics Guidelines
This study was conducted in compliance with the ethical and scientific principles based on the Declaration of Helsinki (revised in Fortaleza in 2013), the Ethical Guidelines for Medical and Health Research Involving Human Subjects (enforced on December 22, 2014), the Medical Exposure Guidelines 2006, and related laws and regulations, as well as in accordance with the protocol approved by the Ethics Committee of Kindai University Faculty of Medicine. For the management of conflict of interest, the study was also conducted in line with the Guidance for Management of Conflict of Interest (Health and Labour Sciences Research), the Transparency Guideline for the Relation between Corporate Activities and Medical Institutions (Japan Pharmaceutical Manufacturers Association), and the Problems Relating to Investigator-initiated Clinical Trials in Japan and Countermeasures (proposed by the Science Council of Japan) in such a manner that the study results would not be influenced by the interest of the research funder. While no Japanese laws or regulations, including the Medical Service Act, restrict the medical exposure dose, the radiation exposure dose in this study was lower than the exposure limit [volume CT dose index (CTDIvol) of 25 mGy] specified in the American College of Radiology Guidelines [6], indicating that participation in the study would involve no health hazards.
Prior to enrollment of patients into the study, written voluntary consent was obtained from each subject who received a detailed explanation of the study from the investigator based on an informed consent form approved by the ethics committee. In addition, a subject identification code was obtained by entering each subject’s name in a correspondence table to assure anonymity of subjects.
Results
Patient Characteristics
The characteristics of all six patients are shown in Table 1. The study population consisted of three males and three females. The disease type was atopic in three subjects and non-atopic in three subjects, the disease severity was mild persistent in four subjects and moderate persistent in two subjects, and the treatment was Step 2 in four subjects and Step 3 in two subjects. Two subjects had concurrent disease (allergic rhinitis in one subject and other disease in one subject), and five subjects had a smoking history.
Drug Deposition Fraction in the Peripheral Airways
The mean drug deposition fractions (and SDs) in the peripheral airways for Flutiform, Symbicort, and Relvar were 30.33% ± 1.89%, 18.95% ± 2.03%, and 6.58% ± 0.98% for ICS, respectively (Fig. 1a), and 29.17% ± 1.81%, 17.24% ± 1.87%, and 11.30% ± 1.42% for LABA, respectively (Fig. 1b), showing that significantly higher proportions of ICS and LABA were deposited to the peripheral airways from Flutiform than from Symbicort and Relvar (p < 0.001).
Drug Deposition Fraction in the Entire Lung
The mean drug deposition fractions (and SDs) in the entire lung for Flutiform, Symbicort, and Relvar were 46.18% ± 1.29%, 28.85% ± 0.78%, and 13.01% ± 0.66% for ICS, respectively (Fig. 2a), and 44.34% ± 1.23%, 26.32% ± 0.71%, and 20.02% ± 0.80% for LABA, respectively (Fig. 2b), showing that significantly higher proportions of ICS and LABA were deposited to the lung from Flutiform than from Symbicort and Relvar (p < 0.001).
Drug Deposition Fraction in Each Lobe of the Lung
For both ICS and LABA from all of the three products, the drug deposition fractions in each lobe of the lung were higher in the lower lobe in the right lung and the upper/lower lobe in the left lung than in the upper/middle lobe in the right lung. The mean drug deposition fractions (and SDs) in each lobe of the lung for Flutiform, Symbicort, and Relvar were 8.78% ± 4.98%, 5.45% ± 2.89%, and 2.36% ± 1.36% for ICS, respectively (Fig. 3a), and 8.43% ± 4.78%, 4.97% ± 2.64%, and 3.69% ± 2.05% for LABA, respectively (Fig. 3b), showing that significantly higher proportions of ICS and LABA were deposited from Flutiform than from Symbicort and Relvar (p < 0.001).
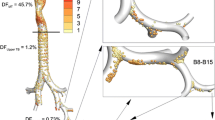
Particle Deposition Imaging of Each Product in the Upper, Central, and Peripheral Airways
Particle deposition images of ICS and LABA from Flutiform, Symbicort, and Relvar in the airway are provided in Fig. 4a, b. In these images of airways, red indicates a dense particle deposition (6.74E–03% of delivered dose), and blue indicates a sparse particle deposition (1.51E–08% of delivered dose). The colors in the lobes indicate >13.0% (red) and 0% (blue) of delivered dose. Values in the Figures represent the particle deposition fractions in the upper, central, and peripheral airways from top to bottom. The ICS/LABA deposition fractions in the upper airway were 52.53/54.45%, 72.11/74.56%, and 87.22/80.62% for Flutiform, Symbicort, and Relvar, respectively, showing that the drug deposition fractions in the upper airway were higher with Symbicort and Relvar than with Flutiform, with less than 30% of the dose being deposited to the central/peripheral airways. All data in Fig. 4 were from imaging in the same subject. Drug deposition imaging data of all six subjects are shown in the Figs. S1–6. In addition, airway deposition imaging with Symbicort at the same inspiratory flow rate (30 L/min) as that for Flutiform revealed a further decreased deposition to the central airway (Fig. S7).
Particle deposition imaging of Flutiform, Symbicort, and Relvar in the upper, central and peripheral airways in the first patient. a. ICS, b. LABA. In the images, red indicates a dense particle deposition (6.74E–03 μg), and blue indicates a sparse particle deposition (1.51E–08 μg). Note: Since the dose levels of ICS/LABA differ among the three products, a drug administered at a higher dose level provides greater absolute deposition than another drug with the same particle distribution ratio. Accordingly, it may be necessary to take this into account for comparison of drug deposition shown by imaging. Nonetheless, since deposition is classified into 11 colors with an approximately 450,000-fold difference in deposition between the maximum indicated by red and the minimum indicated by blue, a maximum of twofold difference in the ICS dose and a maximum of fivefold difference in the LABA dose among the three products may be ignorable for color-based comparison of deposition among the products
Discussion
The imaging data using FRI model were used numerical number of monodisperse particle size and flow rate, it was suggested that pulmonary deposition of ICS/LABA was difference between clinical practice and FRI data. FRI data was important and interest because less-invasive technology.
FRI, which is used to determine alveolar hyperinflation, vascular density, ventilation, aerosol deposition, and bronchodilation in patients with respiratory disease [7], is a validated method for measuring airflow distribution and aerosol deposition in patients with mild asthma, with high correlation with SPECT/CT [4].
Using FRI, the present study showed that Flutiform, a FP/FM combination via pMDI, provided significantly greater drug deposition in the peripheral airways than Symbicort, a BUD/FM combination via DPI, and Relvar, a FF/VI combination via DPI. Flutiform also provided significantly greater drug deposition in the entire lung and each lobe of the lung than Symbicort and Relvar. Even though the pMDI was delivered about 30% to the periphery compared to the Symbicort DPI of 19%, the BUD dose delivered is essentially the same for asthma treatment. On the other hand, the particle deposition fraction in the upper airway was only approximately half of the dose delivered from Flutiform, but approximately 70–90% of the dose delivered from Symbicort and Relvar, revealing a decreased deposition to the central/peripheral airways with Symbicort and Relvar.
Since the results with Flutiform were better than we assumed compared with the other two devices, an additional FRI analysis was conducted for Spiriva Respimat® (Boehringer Ingelheim Japan, Inc.), Long-acting muscarinic antagonist (LAMA) delivered via a soft mist inhaler (SMI), a different inhalation device from a pMDI or a DPI. Data on physical property of Spiriva Respimat were obtained in six patients using ACI, while the same impactor used for Flutiform and Symbicort. The drug deposition fractions in the upper, central, and peripheral airways of Spiriva Respimat in the six subjects were in the ranges of 41.26–44.28, 13.82–22.64, and 34.61–42.35%, respectively (Fig. S8). And the mean deposition fractions and SDs of Spiriva Respimat in the entire lung and the peripheral airways were 57.09% ± 1.20% and 39.65% ± 2.84%. Although no statistical analysis was performed among the drugs, drug deposition fraction to the peripheral airways was the highest with the SMI, followed by the pMDI and then the DPI, indicating a favorable lung deposition profile of pMDIs as well as SMIs (Fig. S9).
It is said that the efficacy of inhaled drugs for asthma depends on the properties of drug particles [8], and studies in patients with mild to moderate asthma have shown that pMDIs were more readily accessible to the peripheral airways [9] because of smaller aerosol particle size and higher aerosolization efficiency compared with DPIs [10]. In addition, it has been shown that the optimal particle size for drug delivery to the central airway and alveoli is from 0.8 to less than 5 μm, because particles smaller than 0.8 μm are likely to be excreted in expired air [11], and a study assessing the aerodynamic diameter and its distribution showed that the proportions of ICS/LABA particles smaller than 5 μm at expiratory flow rates of 28.3 and 60.0 L/min were 41.2/39.1% and 43.7/42.1% for Flutiform, respectively, and 8.2/6.5% and 35.0/30.0% for Symbicort, respectively [12], showing that the proportions of particles smaller than 5 μm were higher with Flutiform than with Symbicort. The differences among the products in deposition to the entire lung, including the peripheral airways, in the present study may be partly explained by differences in particle size distribution, and Flutiform was verified through FRI to generate more particles with an effective particle size for deposition to the central/peripheral airways, regardless of the expiratory flow rate.
In this study, distribution imaging of each product in the upper, central, and peripheral airways showed that the drug deposition fractions in the upper airway were higher with Symbicort and Relvar than with Flutiform. The study is of great significance in that the additional data obtained through imaging supports the previous finding of high efficiency of Flutiform in drug deposition to the peripheral airways. In clinical practice, it has been reported that Flutiform significantly improved asthma control in Japanese asthma patients with remaining eosinophilic inflammation in the peripheral airways [13].
In addition, the greatest difference between DPIs and pMDIs is the required inspiratory flow rate. Given a mean required inspiratory flow rate of 30 L/min for pMDIs and 60 L/min for DPIs [14], the deposition fractions were measured at an inspiratory flow rate of 30 L/min for Flutiform and 60 L/min for Symbicort and Relvar in the present study. Since the usual inspiration rate during safe breathing is approximately 30 L/min, it is of concern as to whether an asthma patient can generate the inspiratory flow rate required for a DPI, and Flutiform, a pMDI, may therefore provide a potent therapeutic option for various asthma patients, including those who have difficulty using DPIs.
Limitations
Data of particle size distribution of each drug were obtained using two different cascade impactors; ACI for Flutiform, Symbicort, and Spiriva Respimat, and NGI for Relvar, since there were no data available for Relvar using ACI, which might have affected the fine particle fraction of each drug and the deposition data.
Also, imaging data in FRI model in this study were expressed by numerical number of monodisperse particle size and flow rate.
Static volume distribution is a limitation of FRI technology. Functional residual capacity (FRC) imaging is closer to normal inhalation, but due to use of CT resolution, in order to further segment the airways, the TLC model was used.
Conclusions
In the present study using FRI, Flutiform, a FP/FM combination via pMDI, was shown to provide significantly higher drug deposition fraction to the central/peripheral airways than Symbicort, a BUD/FM combination via DPI, and Relvar, a FF/VI combination via DPI. It is suggested that Flutiform may provide a potent therapeutic option for various asthma patients, including those who have difficulty using DPIs.
References
Matsunaga K, Hirano T, Oka A, et al. Progression of irreversible airflow limitation in asthma: correlation with severe exacerbations. J Allergy Clin Immunol Pract. 2015;3:759–64.
Asthma Prevention and Management Guideline preparation committee. Asthma prevention and management guidelines. Tokyo: Kyowa Kikaku; 2015.
Longest PW, Tian G, Walenga RL, Hindle M. Comparing MDI and DPI aerosol deposition using in vitro experiments and a new stochastic individual path (SIP) model of the conducting airways. Pharm Res. 2012;29:1670–88.
De Backer JW, Vos WG, Vinchurkar SC, et al. Validation of computational fluid dynamics in CT-based airway models with SPECT/CT. Radiology. 2010;257:854–62.
De Backer J, Van Holsbeke C, Vos W, et al. Assessment of lung deposition and analysis of the effect of fluticasone/salmeterol hydrofluoroalkane (HFA) pressurized metered dose inhaler (pMDI) in stable persistent asthma patients using functional respiratory imaging. Expert Rev Respir Med. 2016;10:927–33.
American College of Radiology. ACR–AAPM practice parameter for diagnostic reference levels and achievable doses in medical x-ray imaging; 2013. https://www.acr.org/~/media/0DAB1CD6FFC44F09A05E0BD0FCA175F8.pdf.
Vos W, Hajian B, De Backer J, et al. Functional respiratory imaging to assess the interaction between systemic roflumilast and inhaled ICS/LABA/LAMA. Int J COPD. 2016;11:263–71.
Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc. 2004;1:315–20.
Goldin JG, Tashkin DP, Kleerup EC, et al. Comparative effects of hydrofluoroalkane and chlorofluorocarbon beclomethasone dipropionate inhalation on small airways: assessment with functional helical thin-section computed tomography. J Allergy Clin Immunol. 1999;104:S258–67.
Tamura G, Sakai H, Fujino S. Study on aerosols emitted from inhaler devices of corticosteroid. Allergy. 2009;58:790–7 (article in Japanese).
Aerosol Consensus Statement. Consensus conference on aerosol delivery. Chest. 1991;100:1106–9.
Johal B, Howald M, Fischer M, Marshall J, Venthoye G. Fine particle profile of fluticasone propionate/formoterol fumarate versus other combination products: the DIFFUSE study. Comb Prod Ther. 2013;3:39–51.
Hozawa S, Terada M, Hozawa M. Comparison of the treatment efficacy of FP/FM-pMDI versus FP/SM-DK in asthma patients with remaining eosinophilic inflammation in the peripheral airway. Allergy Immunol. 2014;21:1932–40 (article in Japanese).
Haida M, Kuroki H. Correct comprehension of inhaled drugs and device usage—instruction points on a device-by-device bases. Chozai to joho. 2014;20:304–11 (article in Japanese).
Acknowledgements
Sponsorship for this study and article processing charges was funded by Kyorin Pharmaceutical Co., Ltd. FRI analysis and statistical analysis were done by Mebix, Inc. and Fluidda NV. Assistance in the submission of this manuscript was provided by WILL Medical Communications Inc. These assistance was funded by Kyorin Pharmaceutical Co., Ltd. The study was performed following the guidelines on Good Publication Practice initiated by the International Committee of Medical Journal Editors (ICMJE) criteria.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Yuji Tohda wrote the manuscript and all authors read and approved the final manuscript to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Yuji Tohda has received speaker’s fees from Kyorin Pharmaceutical and Teijin, and an honorarium from the advisory boards of Kyorin Pharmaceutical and Teijin. Takashi Iwanaga, Takenori Kozuka, Junko Nakanishi, Koji Yamada, Osamu Nishiyama, Hiroyuki Sano, and Takamichi Murakami have no conflicts of interest.
Compliance with Ethics Guidelines
This study was conducted in compliance with the ethical and scientific principles based on the Declaration of Helsinki (revised in Fortaleza in 2013), the Ethical Guidelines for Medical and Health Research Involving Human Subjects (enforced on December 22, 2014), the Medical Exposure Guidelines 2006, and related laws and regulations, as well as in accordance with the protocol approved by the Ethics Committee of Kindai University Faculty of Medicine. For the management of conflict of interest, the study was also conducted in line with the Guidance for Management of Conflict of Interest (Health and Labour Sciences Research), the Transparency Guideline for the Relation between Corporate Activities and Medical Institutions (Japan Pharmaceutical Manufacturers Association), and the Problems Relating to Investigator-initiated Clinical Trials in Japan and Countermeasures (proposed by the Science Council of Japan) in such a manner that the study results would not be influenced by the interest of the research funder. While no Japanese laws or regulations, including the Medical Service Act, restrict the medical exposure dose, the radiation exposure dose in this study was lower than the exposure limit [volume CT dose index (CTDIvol) of 25 mGy] specified in the American College of Radiology Guidelines [6], indicating that participation in the study would involve no health hazards.Prior to enrollment of patients into the study, written voluntary consent was obtained from each subject who received a detailed explanation of the study from the investigator based on an informed consent form approved by the ethics committee. In addition, a subject identification code was obtained by entering each subject’s name in a correspondence table to assure anonymity of subjects.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Additional information
Enahnced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/B6F7F060184583CE.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Iwanaga, T., Kozuka, T., Nakanishi, J. et al. Aerosol Deposition of Inhaled Corticosteroids/Long-Acting β2-Agonists in the Peripheral Airways of Patients with Asthma Using Functional Respiratory Imaging, a Novel Imaging Technology. Pulm Ther 3, 219–231 (2017). https://doi.org/10.1007/s41030-017-0036-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-017-0036-4