Abstract
Purpose
To discuss and reach a consensus on the use of bevacizumab in women with advanced ovarian cancer in Indian settings.
Methods
An advisory board meeting comprising Indian oncologists was convened to review key literature available on the role of bevacizumab in the management of advanced ovarian cancer. Key recommendations were devised via consensus by the expert panel based on the analysis of available scientific evidence and clinical experience.
Results
The expert panel recommends the use of bevacizumab in patients with advanced ovarian cancer in first-line settings, as well as in recurrent settings.
Conclusion
This document summarizes key discussion points and recommendations provided by the advisory panel, which helps guide clinicians on the use of bevacizumab for managing advanced ovarian cancer in the Indian setting. It also acts as a pragmatic tool to assist clinicians in making appropriate treatment decisions with respect to advanced ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, ovarian cancer is the most fatal and the second most common gynecological malignancy affecting women [1]. India has the second highest burden of ovarian cancer globally, followed by the United States of America [2]. According to 2018 The Global Cancer Observatory (GLOBOCAN) data, ovarian cancer is the eighth most common cancer in India. In 2018 alone, there were 36,170 newly diagnosed ovarian cancer cases (cumulative risk: 0.58) and 24,015 deaths due to ovarian cancer (cumulative risk: 0.43) in India [3].
The classification of ovarian cancer is based on the International Federation of Gynecology and Obstetrics (FIGO) staging system, as described in Table 1 [4]. In patients with advanced-stage (III–IV) ovarian cancer, first-line systemic therapy options include conventional chemotherapy; or dose-dense, intraperitoneal, or conventional chemotherapy combined with anti-angiogenic agents such as bevacizumab [5]. Bevacizumab is a monoclonal antibody directed against vascular endothelial growth factor (VEGF) that has been approved by the European Medicines Agency (EMA) and United States Food and Drug Administration (US FDA) for the treatment of ovarian, fallopian tube, and peritoneal cancer [6,7,8,9]. In addition, it has been approved for a range of cancers, including metastatic or recurrent non-squamous non-small–cell lung cancer, metastatic renal cell carcinoma, metastatic colorectal cancer, and cervical cancer [8, 9].
In adult patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer, bevacizumab is indicated as the first-line therapy in combination with carboplatin and paclitaxel [5]. The 2019 National Comprehensive Cancer Network (NCCN) clinical practice guidelines on ovarian cancer and the 2019 guidelines by the European Society of Medical Oncology (ESMO) recommend the use of bevacizumab in patients with stages II–IV ovarian cancer. Despite the significant burden of ovarian cancer in India, there are no India-specific guidelines on the use of bevacizumab in patients with advanced ovarian cancer [10, 11]. Thus, an expert panel meeting was convened to develop a consensus document on optimizing the use of bevacizumab for the management of advanced ovarian cancer in the Indian setting.
Materials and Methods
The expert panel comprising 15 oncologists reviewed existing literature, including randomized clinical trials, systematic reviews, meta-analyses, and available guidelines relevant to ovarian cancer settings. Subsequently, during the advisory board meeting, the panelists discussed and debated available evidence and put forth clinical recommendations. The quality of evidence of the clinical recommendations was based on the Grading of Recommendations Assessment, Development and Evaluation system by Guyatt et al. (Table 2) [12].
This document summarizes the key consensus statements provided by the expert panel members.
Results
Use of Bevacizumab for First-Line Therapy in advanced Ovarian Cancer
Consensus Statements
-
Bevacizumab is an option for first-line treatment in combination with carboplatin/paclitaxel for newly diagnosed stages III and IV epithelial carcinoma of ovary/fallopian tube/primary peritoneal cancer (high quality)
-
Bevacizumab improves overall survival (OS) in patients with high-risk disease (sub-optimally debulked stage III disease and stage IV disease) (high quality)
-
The recommended dose of bevacizumab is 7.5 mg/kg every 3 weeks along with chemotherapy, followed by bevacizumab maintenance at a dose of 7.5 mg/kg every 3 weeks for 12 additional cycles (moderate quality)
-
Patients should be watched for side effects such as hypertension, proteinuria, bleeding, perforation, and fistula (high quality)
-
The use of bevacizumab alone as a maintenance treatment or in combination with intraperitoneal chemotherapy is not recommended (moderate quality)
-
Patients on bevacizumab scheduled to undergo surgery should wait for 6 weeks before surgery. Bevacizumab may be resumed 6 weeks after surgery (high quality)
Use of Bevacizumab in Platinum-Sensitive Recurrent Ovarian Cancer (PS-ROC)
Consensus Statements
-
The carboplatin/gemcitabine/bevacizumab regimen can be recommended for the first recurrence in all patients with platinum-sensitive (PS) high-grade serous disease, who have not previously received bevacizumab (high quality)
-
Bevacizumab may be continued as maintenance therapy if used previously as part of combination chemotherapy in patients who have achieved partial or complete remission following recurrence therapy for PS disease (high quality)
Use of Bevacizumab in Platinum-Resistant Recurrent Ovarian Cancer (PR-ROC)
Consensus Statements
-
Bevacizumab-naïve patients can be treated with chemotherapy (liposomal doxorubicin, topotecan, and paclitaxel) in combination with bevacizumab (high quality)
-
Bevacizumab has shown to improve progression-free survival (PFS) in platinum-resistant (PR) settings (high quality)
-
The recommended dose of bevacizumab is 15 mg/kg every 3 weeks while on chemotherapy followed by bevacizumab maintenance therapy at a dose of 15 mg/kg till progression/unacceptable toxicity (high quality)
-
There is inadequate evidence to re-challenge bevacizumab in patients with prior exposure to bevacizumab (moderate quality)
-
There is inadequate evidence to continue bevacizumab beyond progression (moderate quality)
Use of Bevacizumab in the Setting of Interval Debulking Surgery for Stages IIIB, IIIC, and IV Ovarian Cancer
Consensus Statements
-
There is inadequate evidence to use bevacizumab in the neoadjuvant setting (moderate quality)
-
Bevacizumab should be initiated only 6 weeks after surgery, as it can interfere with wound healing (high quality)
Discussion
Evidence Supporting the Recommendations
First-Line Treatment Options
Recommendations from the 2019 ESMO and the NCCN guidelines support use of bevacizumab in front-line settings [10, 11].
According to the 2019 ESMO guidelines, in front-line settings in patients with epithelial ovarian cancer (FIGO stages II–IV), bevacizumab (15 mg/kg or 7.5 mg/kg every 3 weeks for a maximum of 15 months) improves PFS in patients with stages III–IV ovarian cancer and should be considered in addition to carboplatin and paclitaxel (level of evidence I; strength of recommendation: A) [11].
The 2020 NCCN clinical practice guidelines on ovarian cancer recommend bevacizumab-containing regimens based on the International Collaboration on Ovarian Neoplasms (ICON7) and Gynecologic Oncology Group (GOG-0218) trials as one of the primary systemic therapy regimens in patients with stages II–IV ovarian cancer (Table 3) [10].
The GOG-0218 and the ICON7 trials provide evidence that the addition of bevacizumab to standard first-line chemotherapy improves PFS in patients with advanced ovarian cancer [13, 14].
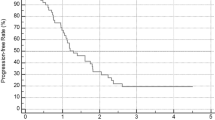
In the GOG-0218 trial, bevacizumab (15 mg/kg body weight) plus standard chemotherapy with carboplatin and paclitaxel followed by extended bevacizumab therapy up to 10 months significantly improved PFS (i.e., increased median PFS by 4 months), translating to a 28% reduction in the risk of progression versus chemotherapy alone (Fig. 1). This effect was consistent across all prognostic subgroups [13].
Primary analysis of PFS, according to treatment group [13]
In the ICON7 trial, bevacizumab (7.5 mg/kg body weight) administered concurrently with five or six cycles of platinum-based chemotherapy and continued for an additional 12 cycles improved PFS by about 2 months and increased the response rate by 20%. Although the OS data in this study were not final, the PFS (p < 0.001) and OS (hazard ratio [HR] for death: 0.64; p = 0.002) were greater in high-risk patients who received standard chemotherapy plus bevacizumab versus those received standard chemotherapy alone [14].
Based on these findings, the panel recommends the use of bevacizumab for first-line treatment in combination with carboplatin/paclitaxel in newly diagnosed stages III and IV epithelial carcinoma of ovary/fallopian tube/primary peritoneal cancer.
The findings from the extended follow-up of the GOG-0218 trial and the final OS analysis of the ICON7 trial support the use of bevacizumab for improving OS in patients with high-risk disease (sub-optimally debulked stages III and IV disease) [15, 16].
In the extended follow-up of the GOG-0218 study, among patients with stage IV disease, the relative HR for death was reduced in the bevacizumab-throughout group, compared to control therapy group (HR 0.774) [15]. In the final analysis of mature OS data from the ICON7 trial, a significant difference in OS was observed between women who received bevacizumab plus chemotherapy and those who received chemotherapy alone (restricted mean OS: 39.3 months vs. 34.5 months; p = 0.03), among high-risk patients with a poor prognosis. Although bevacizumab added to platinum-based chemotherapy did not increase OS in the overall study population, an OS benefit was recorded in poor-prognosis patients, concordant with the PFS results from the ICON7 and GOG-0218 trials—further supporting the use of bevacizumab in this patient population [16].
The dose of bevacizumab used for first-line therapy in the two landmark trials, GOG-0218 (15 mg/kg) and ICON7 (7.5 mg/kg), varied considerably [13, 14]. Furthermore, currently available guidelines do not specify the optimum dose of bevacizumab for the management of ovarian cancer in first-line settings [10, 11]. In view of the lack of specifications on the dosing, the panel recommends the use of bevacizumab at a dose of 7.5 mg/kg, in accordance with the ICON7 trial [14].
Evidence from clinical trials indicates that the most common side effects associated with the use of bevacizumab are hypertension (grade ≥ 2), proteinuria, bleeding, perforation, and fistula [13, 14, 17,18,19,20]. Accordingly, the panel recommends monitoring of patients on bevacizumab for these side effects.
According to the 2019 NCCN guidelines, bevacizumab-containing regimens should be used with caution before interval debulking surgery (IDS) due to potential interference with postoperative healing. If bevacizumab is being used as part of a neoadjuvant regimen, bevacizumab should be withheld from therapy for at least 6 weeks prior to IDS (category 2A) [10].
Platinum-Sensitive Recurrent Ovarian Cancer (PS-ROC) Settings
The Ovarian Cancer Study Comparing Efficacy and Safety of Chemotherapy and Anti-Angiogenic Therapy in Platinum-Sensitive Recurrent Disease (OCEANS) and GOG-0213 trials provide evidence on the benefit of bevacizumab in PS-ROC settings [17, 18].
In the OCEANS trial, the addition of bevacizumab to gemcitabine and carboplatin regimen was associated with a statistically significant increase in PFS (median PFS: 12.4 months vs. 8.4 months; HR 0.484) versus placebo [17]. Similarly, the GOG-0213 trial reported significantly longer median PFS with the addition of bevacizumab to standard chemotherapy regimen. The results were consistent across the subgroups of interest and after adjustment for the actual platinum-free interval [18].
According to the 2019 NCCN guidelines, the acceptable recurrence therapies for epithelial (including less common ovarian histologies)/fallopian tube/primary peritoneal cancer for patients with PS disease includes carboplatin/gemcitabine/bevacizumab and carboplatin/paclitaxel/bevacizumab (category 2A). Furthermore, the 2019 NCCN guidelines recommend that in certain circumstances, bevacizumab may be considered as a single-agent maintenance therapy if used previously as part of combination therapy, and if the patient has achieved partial or complete remission following [10]:
-
Primary therapy for stages II–IV disease or
-
Recurrence therapy for PS disease
Platinum-Resistant Recurrent Ovarian Cancer (PR-ROC) Settings
The AURELIA trial, an exploratory analysis of the AURELIA trial, and real-world evidence demonstrate the benefit of bevacizumab in PR-ROC settings [19,20,21]. They also support the panel recommendations on the addition of bevacizumab to single-agent chemotherapy (liposomal doxorubicin, topotecan, or paclitaxel) in recurrent ovarian cancer settings.
In the Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer (AURELIA) trial, the addition of bevacizumab to single-agent chemotherapy statistically significantly improved PFS and the objective response rate (ORR). The median PFS was 3.4 months with chemotherapy versus 6.7 months with bevacizumab plus chemotherapy. The benefit in PFS was consistently observed across all evaluated subgroups. The ORR was 12.6% with chemotherapy versus 30.9% with bevacizumab plus chemotherapy [19].
An exploratory analysis of the AURELIA trial evaluated the clinical impact of primary versus secondary platinum resistance (PPR vs. SPR) on treatment efficacy. Among patients in the bevacizumab group, SPR compared with PPR was associated with more favorable PFS (10.2 vs. 5.6 months; HR 0.45; p < 0.001) and OS (22.2 vs. 13.7 months; HR 0.46; p < 0.001). Further analyses within the SPR and PPR subgroups revealed that the addition of bevacizumab conferred a numerically more pronounced PFS benefit in the SPR subgroup (HR 0.30) than in the PPR subgroup (HR 0.55; treatment-subgroup interaction p = 0.07). Similarly, for OS, a non-significant direction of effect was observed (p for interaction 0.18) [21].
The Real-world effectiveness of bevacizumab based on AURELIA in platinum-resistant recurrent ovarian cancer (REBECA) study provides real-world evidence on the effectiveness of bevacizumab with single-agent chemotherapy (weekly paclitaxel, pegylated liposomal doxorubicin [PLD], or topotecan), based on the AURELIA trial, in women with PR ovarian cancer. The median PFS was 6.1 months in all the bevacizumab-containing therapy groups, 8.3 months in the weekly paclitaxel cohort, 5.4 months in the PLD cohort, and 7 months in topotecan cohort. The weekly paclitaxel cohort demonstrated better PFS when compared with the PLD cohort (p = 0.028); however, no significant difference was observed between the weekly paclitaxel and topotecan cohorts (p = 0.382), or between the PLD and topotecan cohorts (p = 0.070). The effectiveness results observed in this real-world study were generally consistent with those observed in phase 3 AURELIA trials [20].
Consistent with the dose of bevacizumab used in the AURELIA trial, the panel recommends bevacizumab 15 mg/kg every 3 weeks while on chemotherapy followed by bevacizumab 15 mg/kg maintenance therapy till progression/unacceptable toxicity.
According to the 2019 NCCN guidelines, the acceptable therapies for patients with PR disease include liposomal doxorubicin/bevacizumab and paclitaxel (weekly)/bevacizumab (category 2A) [10].
Use of Bevacizumab in the Setting of IDS for Stages IIIB, IIIC, and IV Ovarian Cancer
The 2019 ESMO guideline recommendations on the use of bevacizumab in the neoadjuvant setting are as follows [11]:
-
Bevacizumab in the neoadjuvant setting can be considered, although additional improvement in efficacy is not proven with level I evidence (level of evidence: II; strength of recommendation: B)
-
Bevacizumab can be safely administered in the neoadjuvant setting before and after IDS, provided the interval between surgery and administration is at least 4–6 weeks (level of evidence: II; strength of recommendation: B)
Two multicenter, open-label, phase II clinical trials, the Avastin Neoadjuvant Therapy in patients with Advanced ovarian cancer initialLY unresectAble (ANTHALYA) and GEICO 1205/NOVA, provide evidence on the administration of bevacizumab in the neoadjuvant setting [22, 23]. Both the trials included patients with unresectable stage III/IV ovarian cancer. Patients were randomized to four cycles of neoadjuvant carboplatin and paclitaxel chemotherapy either with bevacizumab (BCP group) or without three cycles of bevacizumab 15 mg/kg (CP group), followed by IDS [22, 23]. In the ANTHALYA trial, bevacizumab was stopped 4–5 weeks before IDS and resumed at cycle 6 after surgery [11, 22]. In the GEICO 1205/NOVA trial, bevacizumab was stopped 6 weeks before IDS and resumed 6 weeks after surgery [11]. In the ANTHALYA trial, the addition of bevacizumab in the neoadjuvant setting was associated with a significantly higher complete resection rate, the primary study endpoint, at IDS: 58.6% versus the reference rate of 45% [23]. The GEICO 1205/NOVA trial did not report any difference in complete macroscopic response rate at IDS with the addition of bevacizumab. However, the use of bevacizumab when compared with chemotherapy alone was associated with an improved surgical feasibility (66.7% vs. 88.6%; p = 0.029) at IDS [23]. These findings provide evidence only on the benefits of bevacizumab with regards to surgical feasibility, but not with regards to efficacy in improving the overall or PFS.
Biosimilar: The Need of the Hour
Biologics are important components of oncologic therapeutic regimens. However, they are expensive and, thereby, limit the patient’s access to cancer therapy. The development of biosimilars as alternatives to biologics has generated great interest in improving patient access to cancer therapies [24]. A biosimilar is defined as a biological medicine highly similar to another already approved biological medicine (the “reference medicine”) [25]. An accurately evaluated biosimilar can potentially offer cost savings for the healthcare system and thereby enhance patient access to treatment [26]. The development and availability of a biosimilar of innovator bevacizumab offer the opportunity to increase patient access to treatment and, thereby, improve clinical outcomes [7]. In view of the potential advantages, a biosimilar bevacizumab can be recommended as an alternative to innovator biologic bevacizumab.
Conclusion
Ovarian cancer is a significant global burden, notably in India. Bevacizumab is a monoclonal antibody directed against VEGF. It has been approved by the US FDA and EMA for the treatment of ovarian, fallopian tube, and peritoneal cancer. The NCCN guidelines recommend the use of bevacizumab in combination with standard chemotherapy in the first-line and recurrent settings. The ESMO guidelines recommend the use of bevacizumab in combination with chemotherapy as first-line therapy specifically in high-risk patients. Clinical evidence indicates that bevacizumab when used in combination with chemotherapy, improves PFS and OS, especially in high-risk patients. Accordingly, the expert panel recommends the use of bevacizumab in patients with advanced ovarian cancer in first-line settings as well as in recurrent settings. Although the addition of bevacizumab to standard chemotherapy is beneficial, treatment costs would increase with reference bevacizumab. This may lead to a reduction in the number of treatment cycles planned for patients. The availability of bevacizumab biosimilars has the potential to improve access to treatment and, thereby, enhance treatment outcomes.
References
Desai A, Xu J, Aysola K, et al. Epithelial ovarian cancer: an overview. World J Transl Med. 2014;3:1–8.
World Ovarian Cancer Coalition 2018. The World Ovarian Cancer Coalition Atlas. 2018. https://worldovariancancercoalition.org/wp-content/uploads/2018/10/THE-WORLD-OVARIAN-CANCER-COALITION-ATLAS-2018.pdf. Accessed 9 Feb 2019.
IACR. Globocan 2018 India Factsheet. http://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf. Accessed 9 Feb 2019.
Berek JS, Kehoe ST, Kumar L, et al. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2018;143:59–78.
Santaballa A, Barretina P, Casado A, et al. SEOM clinical guideline in ovarian cancer. Clin Transl Oncol. 2016;18:1206–12.
Avastin SmPC. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf. Updated 28 Mar 2018. Accessed 9 Feb 2019.
Melosky B, Reardon DA, Nixon AB, et al. Bevacizumab biosimilars: scientific justification for extrapolation of indications. Future Oncol. 2018;14:2507–20.
US FDA. FDA approves bevacizumab in combination with chemotherapy for ovarian cancer https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm610664.htm. Accessed 9 Feb 2019.
EMA. EPAR summary for the public. https://www.ema.europa.eu/documents/overview/avastin-epar-summary-public_en.pdf. Accessed 9 Feb 2019.
NCCN. NCCN clinical practice guidelines in oncology. Ovarian cancer. 2020. https://www.nccn.org/professionals/physician_gls/pdf/ovarian_blocks.pdf. Accessed 30 Sept 2020.
Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO Ovarian Cancer Consensus Conference Working Group. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30:672–705.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83.
Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96.
Burger RA, Enserro D, Tewari KS, et al. Final overall survival (OS) analysis of an international randomized trial evaluating bevacizumab (BEV) in the primary treatment of advanced ovarian cancer: a NRG oncology/Gynecologic Oncology Group (GOG) study. J Clin Oncol. 2018;36:5517.
Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–36.
Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–45.
Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779–91.
Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–8.
Lee JY, Park JY, Park SY, et al. Real-world effectiveness of bevacizumab based on AURELIA in platinum-resistant recurrent ovarian cancer (REBECA): a Korean Gynecologic Oncology Group study (KGOG 3041). Gynecol Oncol. 2019;152:61–7.
Trillsch F, Mahner S, Hilpert F, et al. Prognostic and predictive effects of primary versus secondary platinum resistance for bevacizumab treatment for platinum-resistant ovarian cancer in the AURELIA trial. Ann Oncol. 2016;27:1733–9.
Rouzier R, Gouy S, Selle F, et al. Efficacy and safety of bevacizumab-containing neoadjuvant therapy followed by interval debulking surgery in advanced ovarian cancer: results from the ANTHALYA trial. Eur J Cancer. 2017;70:133–42.
Garcia YGD, Juan A, Mendiola C, et al. Phase II randomized trial of neoadjuvant (NA) chemotherapy (CT) with or without bevacizumab (Bev) in advanced epithelial ovarian cancer (EOC) (GEICO 1205/NOVA TRIAL). J Clin Oncol. 2017;35:5508.
Chopra R, Lopes G. Improving access to cancer treatments: the role of biosimilars. J Glob Oncol. 2017;3:596–610.
European Medicines Agency. Biosimilar medicines: overview. https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview. Accessed 19 Dec 2019.
Rugo HS, Rifkin RM, Declerck P, et al. Demystifying biosimilars: development, regulation and clinical use. Future Oncol. 2019;15:777–90.
Acknowledgements
We would like to thank BioQuest Solutions for providing editorial assistance.
Funding
Manuscript writing was funded by Mylan Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Advani, S.H., Doval, D.C., Koppikar, S.B. et al. Use of Bevacizumab in Advanced Ovarian Cancer: Consensus from an Expert Panel Oncologists. Indian J Gynecol Oncolog 19, 25 (2021). https://doi.org/10.1007/s40944-020-00485-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-020-00485-6