Abstract
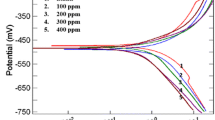
The inhibition by (4-tert-butyl-phenyl)-acetic acid hydrazide (TPAH), 5-(4-tert-butyl-benzyl)-[1,3,4] oxadiazole-2-thiol (TBOT) and 5-(4-tert-butyl-benzyl)-4H-[1,2,4] triazole-3-thiol (TBTT) of mild steel corrosion in 0.5 M HCl was investigated using gravimetric and electrochemical techniques. These inhibitors acted more effectively at higher concentration and at lower temperature; among these TBTT being the most efficient inhibitor, which showed highest efficiency of 92.6% at 303 K and 4.8 mM concentration. Adsorption of TPAH followed Freundlich isotherm, whereas TBOT and TBTT followed Langmuir isotherm. Energy of activation for corrosion increased after the addition of inhibitors. Free energy of adsorption showed that all the three inhibitors get adsorbed to the mild steel surface by both physical and chemical processes. EIS studies confirmed that all the inhibitors offered higher charge transfer resistance to the corrosion current and this led to decreased double-layer capacitance. Polarization studies showed that all inhibitors emerged as mixed type. Surface studies confirmed that the pits caused by corrosion were decreased by protective film of inhibitors. Corelation of experimental data with quantum chemical parameters like ELUMO, energy gap, dipole moment, hardness and softness confirmed the superior performance of TBTT as compared to TPAH and TBOT.












Similar content being viewed by others
References
Eddy NO, Stoyanov SR, Ebenso EE (2010) Fluoroquinolones as corrosion inhibitors for mild steel in acidic medium; experimental and theoretical studies. Int J Electrochem Sci 5:1127–1150
Migahed MA (2005) Electrochemical investigation of the corrosion behaviour of mild steel in 2 M HCl solution in presence of 1-dodecyl-4-methoxy pyridinium bromide. Mat Chem Phys 93:48–53
Zunita M, Wahyuningrum D, Buchari Bundjali B (2012) Investigation of corrosion inhibition activity of 3-butyl-2,4,5-triphenylimidazole and 3-butyl-2-(2-butoxyphenyl)-4,5-diphenylimidazole toward carbon steel in 1% NaCl solution. Int J Electrochem Sci 7:3274–3288
Bouklah M, Hammouti B, Benkaddour M, Benhadda T (2005) Thiophene derivatives as effective inhibitors for the corrosion of steel in 0.5 m H2SO4. J Appl Electrochem 35:1095–1101
Achary G, Sachin HP, Naik YK, Venkatesha TV (2008) The corrosion inhibition of mild steel by 3-formyl-8-hydroxy quinoline in hydrochloric acid medium. Mater Chem Phys 107:44–50
Karthik R, Vimaladevi G, Chen S, Elangovan A, Jeyaprabha B, Prakash P (2015) Corrosion inhibition and adsorption behavior of 4–amino-acetophenone pyridine 2-aldehyde in 1 M hydrochloric acid. Int J Electrochem Sci 10:4666–4681
Quraishi MA, Sardar R, Jamal D (2001) Corrosion inhibition of mild steel in hydrochloric acid by some aromatic hydrazides. Mat Chem Phys 71:309–313
Larabi L, Harek Y, Benali O, Ghalem S (2005) Hydrazide derivatives as corrosion inhibitors for mild steel in 1 M HCl. Prog Org Coat 54:256–262
Benabdellah M, Hammouti B, Warthan A, Al-Deyab SS, Jama C (2007) 2,5-Disubstituted 1,3,4-oxadiazole derivatives as effective inhibitors for the corrosion of mild steel in 2 M H3PO4 solution. Int J Electrochem Sci 7:3489–3500
Ouici HB, Benali O, Harek Y, Larabi L, Hammouti B, Guendouzi A (2013) The effect of some triazole derivatives as inhibitors for the corrosion of mild steel in 5% hydrochloric acid. Res Chem Intermed 39:3089–3103
Gurudatt DM, Mohana KN (2014) Synthesis of new pyridine based 1,3,4-oxadiazole derivatives and their corrosion inhibition performance on mild steel in 0.5 m hydrochloric acid. Ind Eng Chem Res 53:2092–2105
Chaitra TK, Mohana KN, Tandon HC (2015) Thermodynamic, electrochemical and quantum chemical evaluation of some triazole Schiff bases as mild steel corrosion inhibitors in acid media. J Mol Liq 211:1026–1038
Maxwell JR, Wasdahl DA, Wolfson AC (1984) Synthesis of 5-aryl-2h-tetrazoles, 5-aryl-2h-tetrazole-2-acetic acids, and [(4-phenyl-5-aryl-4h-1,2,4-triazol-3-yl)thio]acetic acids as possible superoxide scavengers and anti-inflammatory agents. J Med Chem 27:1565–1570
Tomi IHR, Al-Qaisi AHJ (2011) Synthesis, characterization and effect of bis-1,3,4-oxadiazole rings containing glycine moiety on the activity of some transferase enzymes. J King Saud Univ 23:23–33
Agrawal R, Pancholi SS (2011) Synthesis, characterization and evaluation of antimicrobial activity of a series of 1,2,4-triazoles. Der Pharma Chem 3:32–40
Bentiss F, Lebrini M, Legrenee M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros Sci 47:2915–2931
Ebenso EE (2003) Synergistic effect of halide ions on the corrosion inhibition of aluminium in H2SO4 using 2-acetylphenothiazine. Mat Chem Phys 79:58–70
Vracar L, Drazic DM (2002) Adsorption and corrosion inhibitive properties of some organic molecules on iron electrode in sulfuric acid. Corros Sci 44:1669–1680
Guan NM, Xueming L, Fei L (2004) Synergistic inhibition between o-phenanthroline and chloride ion on cold rolled steel corrosion in phosphoric acid. Mater Chem Phys 86:59–68
Singh AK (2010) Inhibiting effects of 5-substituted isatin-based Mannich bases on the corrosion of mild steel in hydrochloric acid solution. J Appl Electrochem 40:1293–1306
Noor EA (2009) Evaluation of inhibitive action of some quaternary N-heterocyclic compounds on the corrosion of Al–Cu alloy in hydrochloric acid. Mater Chem Phys 114:533–541
Umoren SA, Gasem ZM, Obot IB (2013) Natural products for material protection: inhibition of mild steel corrosion by date palm seed extracts in acidic media. Ind Eng Chem Res 52:14855–14865
Behpour M, Ghoreishi SM, Soltani N, Salavati-Niasari M, Hamadanian M, Gandomi A (2008) Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corros Sci 50:2172–2181
Bentiss F, Legrenee M, Traisnel M, Hornez JC (1999) The corrosion inhibition of mild steel in acidic media by a new triazole derivative. Corros Sci 41:789–803
Martinez S, Stern I (2002) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulfuric acid system. Appl Surf Sci 199:83–89
Gopi D, Sherif EM, Manivannan V, Rajeshwari D, Surendiran M, Kavitha L (2014) Corrosion inhibition of mild steel in groundwater at different temperatures by newly synthesized benzotriazole and phosphono derivatives. Ind Eng Chem Res 53:4286–4294
Motamedi M, Tehrani-Bagha AR, Mahdavian MA (2011) Comparative study on the electrochemical behavior of mild steel in sulfamic acid solution in the presence of monomeric and gemini surfactants. Electrochim Acta 58:488–496
Ahamed I, Prasad R, Quraishi MA (2010) Experimental and theoretical investigations of adsorption of fexofenadine at mild steel/hydrochloric acid interface as corrosion inhibitor. J Solid State Electrohem 14:2095–2105
Singh AK (2012) Inhibition of mild steel corrosion in hydrochloric acid solution by 3-(4-((Z)-Indolin-3-ylideneamino) phenylimino) indolin-2-one. Ind Eng Chem Res 51:3215–3223
Karthik R, Muthukrishnan P, Chen S, Jeyaprabha B, Prakash P (2015) Anti-corrosion inhibition of mild steel in 1 M hydrochloric acid solution by using tiliacoraaccuminata leaves extract. Int J Electrochem Sci 10:3707–3725
Quafsaoui W, Blanc CH, Bebere N, Srhiri A, Mankowski G (2000) Study of different triazole derivative inhibitors to protect copper against pitting corrosion. J Appl Electrochem 30:959–966
Obot IB, Obi-Egbedi NO (2011) Anti-corrosive properties of xanthone on mild steel corrosion in sulphuric acid: experimental and theoretical investigations. Curr Appl Phys 11:382–390
Dubey AK, Singh G (2007) Corrosion inhibition of mild steel using Brij-30. Port Electrochim Acta 25:205–219
Ferreira ES, Giancomlli C, Giacomlli FC, Spinelli A (2004) Evaluation of the inhibitor effect of l-ascorbic acid on the corrosion of mild steel. Mater Chem Phys 83:129–134
Udhayakala P, Rajendiran TV, Gunasekaran S (2012) Theoretical approach to the corrosion inhibition efficiency of some pyrimidine derivatives using DFT method. J Comput Methods Mol Des 2:1–15
Ayta A, Bilgic S, Gece G, Ancin N, Oztas SG (2012) Experimental and theoretical study of the inhibition effects of some Schiff bases as corrosion inhibitors of aluminium in HCl. Mater Corros 63:729–734
Yurt A, Ulutas S, Dal H (2006) Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases. Appl Surf Sci 253:919–925
Ashassi-Sorkhabi H, Shabani B, Aligholipour B, Seifzadeh D (2006) The effect of some Schiff bases on the corrosion of aluminum in hydrochloric acid solution. Appl Surf Sci 252:4039–4047
Udhayakala P, Jayanthi A, Rajendiran TV (2011) Adsorption and quantum chemical studies on the inhibition potentials of some formazan derivatives. Der Pharma Chem 3:528–539
Obi-Egbedi NO, Obot IB, El-Khaiary MI, Umoren SA, Ebenso EE (2011) Computational simulation and statistical analysis on the relationship between corrosion inhibition efficiency and molecular structure of some phenanthroline derivatives on mild steel surface. Int J Electrochem Sci 6:5649–5675
Hasanov R, Sadikglu M, Bilgic S (2007) Electrochemical and quantum chemical studies of some Schiff bases on the corrosion of steel in H2SO4 solution. Appl Surf Sci 253:3913–3921
Ansari KR, Yadav DK, Ebenso EE, Quraishi MA (2012) Novel and effective pyridyl substituted 1,2,4-triazole as corrosion inhibitor for mild steel in acid solution. Int J Electrochem Sci 7:4780–4799
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chaitra, T.K., Mohana, K.N. & Tandon, H.C. Experimental and Theoretical Studies on the Corrosion Inhibition Performance of Molecules Containing Tert-Butyl Benzyl Group on Mild Steel in Acid Media. J Bio Tribo Corros 4, 25 (2018). https://doi.org/10.1007/s40735-018-0141-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0141-4