Abstract
Purpose of Review
To review the role of platelet transfusion in resuscitation for trauma, including normal platelet function and alterations in behavior following trauma, blood product transfusion ratios and the impact of platelet transfusion on platelet function, platelet function assays, risks of platelet transfusion and considerations for platelet storage, and potential adjunct therapies and synthetic platelets.
Recent Findings
Platelets are a critical component of clot formation and breakdown following injury, and in addition to these hemostatic properties, have a complex role in vascular homeostasis, inflammation, and immune function. Evidence supports that platelets are activated following trauma with several upregulated functions, but under conditions of severe injury and shock are found to be impaired in their hemostatic behaviors. Platelets should be transfused in balanced ratios with red blood cells and plasma during initial trauma resuscitation as this portends improved outcomes including survival. Multiple coagulation assays can be used for goal-directed resuscitation for traumatic hemorrhage; however, these assays each have drawbacks in terms of their ability to measure platelet function. While resuscitation with balanced transfusion ratios is supported by the literature, platelet transfusion carries its own risks such as bacterial infection and lung injury. Platelet supply is also limited, with resource-intensive storage requirements, making exploration of longer-term storage options and novel platelet-based therapeutics attractive. Future focus on a deeper understanding of the biology of platelets following trauma, and on optimization of novel platelet-based therapeutics to maintain hemostatic effects while improving availability should be pursued.
Summary
While platelet function is altered following trauma, platelets should be transfused in balanced ratios during initial resuscitation. Severe injury and shock can impair platelet function, which can persist for several days following the initial trauma. Assays to guide resuscitation following the initial period as well as storage techniques to extend platelet shelf life are important areas of investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following injury, platelets are an early and essential component of coagulation biology to control hemorrhage. While platelets were initially thought to be simple cellular fragments solely for mechanical obstruction in clot formation, research demonstrates these megakaryocyte fragments to be dynamic, active, complex cellular particles central to both clot formation and breakdown, as well as to mediation of cellular interactions critical for vascular homeostasis, inflammation, and immune function [1, 2]. We will review the role of platelets in mediating hemostasis, the evidence of mechanisms of dysregulation following injury, and multiple aspects of platelet transfusion in trauma resuscitation including platelet transfusion as part of hemostatic resuscitation, the effect of platelet transfusion on coagulation and platelet function assays, risks associated with platelet transfusion and storage considerations, and future novel platelet-based therapeutics including synthetic platelet analogs to expand the supply of these biologically central cellular mediators of vascular homeostasis.
Trauma-Induced Coagulopathy
Trauma-induced coagulopathy (TIC) develops in approximately one-quarter to one-third of severely injured and hemorrhaging trauma patients [3]. Importantly, these patients suffer from worse outcomes including a lower likelihood of survival [4,5,6,7]. Mechanistically, evidence supports that TIC is a multi-factorial failure in vascular homeostasis generally characterized by an early hypocoagulable state contributing to ongoing hemorrhage, followed by a later hypercoagulable and hyperinflammatory state predisposing survivors to thromboembolic complications and organ failure [8••, 9, 10]. Although TIC is often exacerbated by concomitant dilutional and consumptive coagulopathy, TIC develops prior to resuscitative efforts and is characterized by a spectrum of adaptive and maladaptive alterations to clot formation and breakdown and cellular behavior, including that of platelets [11].
Platelets in Normal Hemostasis
In normal hemostasis, following a vascular breach and exposure of sub-endothelial collagen surfaces, platelets are activated, change shape, interact with von Willebrand Factor (vWF) and fibrinogen, and degranulate their pro-coagulant contents in the process of formation of a platelet plug. This platelet-based nidus of clot formation is a catalyst for thrombin generation, growth of fibrin mesh, and the strengthening and expansion of clot [2, 12]. Platelets additionally have a balancing role in fibrinolysis through both release of plasminogen activator inhibitor-1 (PAI-1) and alpha-2 antiplasmin to counter natural fibrinolysis, as well as through the binding of activated platelet surfaces to plasminogen and plasminogen activators, providing a surface for local fibrinolysis [13]. Furthermore, platelets maintain endothelial integrity through direct interactions with endothelial cells and the release of growth factors that stabilize vascular endothelial junctions and promote angiogenesis [14]. Finally, platelets regulate inflammatory and immune responses through the release of hundreds of recognized proteins [15], and respond to damage-associated molecular patterns (DAMPs) as part of their innate immune response [16, 17]. The platelet transcriptome also responds to trauma with differential ribonucleic acid (RNA) expression and evidence of RNA editing after injury [18•, 19].
Platelet Behavior in Trauma
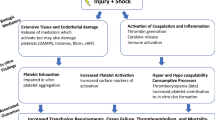
Many expected adaptive platelet-based hemostatic, endothelial, inflammatory, and immune regulatory behaviors have been described in the setting of injury. However, there is also evidence to support that in the setting of severe injury, hemorrhage, and associated malperfusion, even in the setting of normal platelet counts, platelets contribute to a maladaptive response worsening coagulopathy and exacerbating the risk of subsequent thrombotic and inflammatory complications (Fig. 1) [20•]. This includes data that demonstrate robust platelet activation — including increased expression of platelet activation surface markers, release of extracellular vesicles, platelet-leukocyte interactions, and cytokine release — yet functional alterations in adhesion, aggregation, calcium mobilization, vWF interactions, and changes to platelet composition (shed platelet surface glycoproteins, histone-driven platelet structural changes, and platelet transcriptomic changes) (Table 1) [2, 8••, 21,22,23]. Dysregulation of the endothelium after trauma may also impact platelet behavior; for example, elevated catecholamines after injury were found to be associated with endotheliopathy and higher mortality [24, 25], similar to the shock-induced endotheliopathy (SHINE) described in various types of critical illness [26]. Although it is commonly identified that platelets from injured patients are “dysfunctional” due to identified impairments in their aggregation responses to stimulation in ex vivo assays [21, 27], this may be mediated by the severity of injury and degree of shock as data suggest that minor injury results in increased activation of platelets; however, severe injury and combined shock are associated with reduced activation and aggregation [28•]. Furthermore, shock-induced soluble inhibitors may contribute to functional alterations in circulating platelets after injury [20•, 28•]. Platelets activated by trauma also have been shown to balloon and release microvesicles, which coat leukocytes and have procoagulant action promoted by DAMPs such as Histone H4. These platelet-leukocyte interactions persist for several days following injury and are associated with multisystem organ dysfunction as well [29••].
Platelet and endothelial interactions. Adapted with permission from: Moore, E.E., Moore, H.B., Kornblith, L.Z. et al. Trauma-induced coagulopathy. Nat Rev Dis Primers 7, 30 (2021). Springer Nature. https://doi.org/10.1038/s41572-021-00264-3. https://www.nature.com/articles/s41572-021-00264-3
Continued efforts to understand the post-injury behavior of platelets remains important in trauma resuscitation because although it is clear from clinical data that transfusion of platelets to injured and hemorrhaging trauma patients improves survival, it remains unclear what the central mechanisms of this are. This is because the biologic data supports that platelet counts and ex vivo platelet aggregation responses are relatively refractory to platelet transfusion [22], which can persist for several days following major trauma [23].
Platelet Transfusion in Trauma
Hemostatic Resuscitation in Trauma
For patients with trauma-related hemorrhage who require large volume transfusion, decades of research support that banked platelets should be transfused early as part of a hemostatic resuscitation strategy, and in a 1:1:1 balanced manner with red blood cells (RBC) and plasma [30, 31, 32••]. These hemostatic strategies of resuscitation with balanced ratios of RBC:plasma and RBC:platelets lead to earlier correction of coagulopathies and lower hemorrhage-related mortality in injured patients requiring large volume transfusion [33,34,35,36,37,38]. Both the timing and ratios of blood products are important, as early platelet transfusion within the first 6 h after injury has been shown to be most beneficial to hemostasis and mortality, both in combat and civilian populations [39,40,41,42,43,44,45]. A recent study of the American College of Surgeons Trauma Quality Improvement Program (TQIP) database demonstrated that unbalanced platelet:RBC transfusion ratios are common with nearly twice as many patients receiving unbalanced ratios, which portended worse odds of mortality than unbalanced plasma:RBC transfusion ratios. This emphasizes the importance of delivering platelets along with initial RBC and plasma resuscitation [46•]. Most data for platelet transfusion comes from in-hospital studies, and given improvements in outcomes with early balanced resuscitation, expansion to the prehospital setting may have further positive impacts on mortality in a subset of massively hemorrhaging patients [47, 48]. Furthermore, the protocolized use of balanced resuscitation for patients requiring large-volume transfusion may even reduce overall blood product consumption, improve transfusion-related costs [49], and reduce waste [50, 51]. These protocols, often referred to as massive transfusion protocols (MTP), are becoming commonplace. Recent data from patients receiving very large volumes of transfusion after injury (≥ 20u RBC in the first 24 h), coined ultramassive transfusion (UMT), suggest these patients remain at risk of failed hemostatic resuscitation. In a modern multicenter study of 17 trauma centers, nearly half of patients receiving UMT were resuscitated with unbalanced ratios of RBC:plasma or RBC:platelets, with an associated increase in mortality, demonstrating ongoing areas for improvement in these practices [52]. On the other hand, the optimal transfusion ratio of platelet:RBC for non-massively transfused patients is elusive [50, 53, 54]. Outside of the massive transfusion practices for the hemorrhaging patient, transfusion thresholds of platelet counts < 50,000/μL for acute traumatic hemorrhage and < 100,000/μL for intracranial hemorrhage remain general recommendations [55], although the support for these practices in terms of clinical outcomes remains limited [54]. However, altered platelet function and biology in trauma is independent of platelet count.
Impact of Platelet Transfusion on Platelet Function in Trauma
While the use of hemostatic resuscitation with balanced ratios of platelets to other components has improved outcomes for injured patients including survival, the impact of platelet transfusion on platelet count and function is not as clear. There is evidence that severely injured patients receiving platelet transfusion have minimal increases in their platelet counts in the first 24 h after injury, and minimal to no improvements in the aggregation responses of circulating platelets in ex vivo assays [23]. Furthermore, injured patients receiving platelet transfusions at multiple timepoints during the first 12 units of RBC transfusion had similar platelet aggregation to those who did not receive platelet transfusions [56]. However, these investigators found that injured patients who received platelet transfusions did have a decrease in observed fibrinolysis, which they hypothesized to be related to increased PAI-1 from transfused platelets. Additionally, it has been found that dilution with autologous plasma used to deliver platelets, or allogenic plasma delivered in combination with transfused platelets also results in decreased platelet aggregation in in vitro studies [57]. Similar findings have been identified in patients with traumatic brain injury, where patients taking antiplatelet agents had no improvement in outcomes after platelet transfusion [58]. While some of the data are mixed, however, on reversal of platelet inhibition after traumatic brain injury, need for neurosurgical intervention, and mortality [59,60,61], a recent systematic review and meta-analysis found that across twelve studies, platelet transfusion after traumatic brain injury in patients taking antiplatelet agents had no significant reduction in mortality, progression of hemorrhage, or need for neurosurgical interventions [62].
While it is clearly important to include platelets as part of a hemostatic resuscitation strategy based on outcomes, overall, the combined data suggests that in the setting of severe injury and shock circulating platelets are altered in their functional behaviors [28•], and that even transfused platelets may be relatively refractory in their hemostatic behavior [23]. It may be that the circulating environment is inhibitory to platelet hemostatic behaviors for both native and transfused platelets, but that the driver of clinical benefit of transfused platelets is instead due to other activities of platelets including endothelial maintenance and immune and inflammation regulation [63]. Further investigations in this area are needed.
Role of Viscoelastic and Platelet-Function Assays in Transfusion for Trauma
Initial transfusion with balanced product ratios is associated with improved patient outcomes; however, a transition to tailored resuscitation including using real-time assays of clot formation and breakdown, to achieve earlier hemostasis, and to limit product consumption is now a critical piece of hemostatic resuscitation, often known as goal-directed resuscitation. Viscoelastic assays, such as thromboelastography (TEG, Haemonetics Corp) and Rotational Thromboelastometry (ROTEM, Tem International GmbH) (Table 2), are dynamic assays that provide information about initial clot formation, rate of growth of clot, strength of clot, and breakdown of clot in a sample of whole blood taken from an injured patient (Table 1) [64]. This information can inform targeted transfusion of deficient coagulation factors and platelets in real time [65, 66•, 67, 68]. When compared to conventional coagulation assays (international normalized ratio [INR], partial thromboplastin time [PTT], platelet and fibrinogen levels), severely injured patients resuscitated with viscoelastic-directed resuscitation have been found to receive less plasma and platelet transfusions, and have improved survival [65, 69,70,71,72]. However, data suggest use of both conventional assays and viscoelastic assays in combination may be most beneficial in goal-directed resuscitation to capture a broader range of coagulation abnormalities, and patients who are coagulopathic by conventional assays have higher injury scores and worse physiologic derangements as well as higher transfusion requirements and mortality [73]. In terms of the platelet-related parameters of these viscoelastic assays, the measure of clot strength relies on the contributions from fibrin and platelets, with platelets making up a majority of the contribution to clot strength following injury [12]. Reductions in clot strength may guide platelet transfusion in the post-injury period [66•]. While not originally developed for use in trauma, TEG platelet mapping (TEG-PM) has identified platelet impairments in aggregation behavior in trauma patients [74], and is particularly associated with the severity of traumatic brain injury [75,76,77]. However, this assay is designed for identifying effects of antiplatelet medications, and has not been shown to predict massive transfusion or platelet transfusion requirements well [78]. The utility in trauma may be to identify the degree of platelet inhibition in response to arachidonic acid and adenosine diphosphate (ADP) stimulation in patients taking aspirin or clopidogrel, respectively [79]. However, in traumatic brain injury patients, TEG-PM has also been used to show reduction in platelet inhibition after platelet transfusion both in patients taking aspirin [60] and those who were not taking antiplatelet agents [59, 61].
Other assays that have been used primarily in the trauma research setting to understand the effects of resuscitation and platelet transfusion include platelet aggregometer and microfluidic technologies. Multiple electrode impedance aggregometry, or Multiplate© (Verum Diagnostica GmbH, Munich, Germany), and Chronolog’s Whole Blood Aggregometer to measure the change in electrical impedance over time in whole blood in response to platelet stimulation by ADP, thrombin, collagen, or arachidonic acid, giving a proxy measurement for the degree of platelet aggregation in response to each stimulated pathway [80, 81]. These assays have been used in trauma populations to measure platelet aggregation responses following injury [21, 74, 82]. The Platelet Function Analyzer (PFA-100, Dade International Inc., Miami, FL) and Total Thrombus Formation Analysis System (T-TAS, Fujimori Kogyo, Tokyo, Japan) incorporate flow dynamics of whole blood across membranes coated with collagen and ADP or collagen and thromboplastin, respectively, to measure in vitro activity via the duration of time for a platelet plug to form and close a small aperture in the device (closure time, CT) [27, 83, 84]. The clinical role for these assays remains under investigation.
Risks Associated with Platelet Transfusion in Trauma
Despite the benefit to hemostasis and survival, there are important potential associated complications of platelet transfusions in injured patients. Data support that injured patients receiving platelet transfusions have an increased risk of developing acute respiratory distress syndrome (ARDS) [85], both early after injury [86] and later [87]. Even without receiving large volume transfusion, blunt trauma patients who received platelet transfusions had a higher risk of developing ARDS compared with those who did not receive platelets [88]. As with other blood products, patients receiving platelet transfusions are also at risk of developing transfusion-related acute lung injury (TRALI) [89] and transfusion-associated circulatory overload (TACO) [90].
Relatively common but benign reactions associated with platelet transfusions include allergic and nonhemolytic febrile transfusion reactions [54, 91], which are more common after platelet transfusions than other blood products [92], and thought to be related to cytokine release [93]. In select populations known to have severe allergic reactions, the risk may be reduced by platelet washing [94, 95], use of an inert additive solution [96, 97], or concentrating the allogenic plasma in which platelets are delivered [98]. Finally, post-transfusion purpura after platelet transfusion is rare but can occur following multiple transfusions, more often from female donors [99, 100], and is likely due to circulating anti-HPA-1a antibodies in donated platelets [101]. In the PATCH Trial, platelet transfusion was also associated with increased thromboembolic events and complications of spontaneous intracranial hemorrhage, although this study did not include trauma patients [102, 103].
Bacterial infection after platelet transfusion, although rare, is more common than after other blood products, likely due to the standard warmer storage conditions of platelets compared to other components [54, 104]. Pathogen reduction methods such as photochemical reduction with psoralen and ultraviolet-A irradiation have demonstrated effectiveness in high-risk populations with no documented bacterial infection events [104]. Transfusion of pathogen-reduced platelets is associated with decreased post-transfusion corrected counts as opposed to non-treated platelets, but these patients have similar associated bleeding events so the clinical relevance of the reduced counts may be negligible [104,105,106]. Aside from chemical and energy methods of decontaminating banked platelets, colder storage temperatures may also reduce the risk of pathogen contamination [107,108,109].
Considerations for Platelet Storage
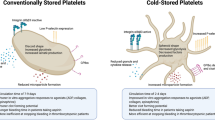
Historically, platelets have been stored at room temperature (22 °C for 5–7 days) [110]; however, other storage modalities such as cold storage or cryopreservation, while not yet widely used, have both been studied as possible methods to extend shelf life and even improve initial hemostasis of the transfused platelets [111, 112] (Table 3).
Cold storage (2 to 6 °C), compared with standard room temperature storage (20 to 24 °C) of platelets is another potential storage modality to extend platelet shelf life for up to 14 days [113•]. Cold-stored platelets have reduced circulation time after transfusion (approximately 3 days vs. 8 days for standard temperature storage) but appear to have improved hemostatic function [114, 115], with increased in vitro activation in response to stimulants [116,117,118] and reduced bleeding time in in vivo studies [119]. One pilot study of cold-stored platelet transfusion in cardiothoracic surgery patients demonstrated that post-transfusion platelet aggregation, transfusion requirements, and outcomes were similar as compared to the group receiving room temperature platelet transfusion [120••]. The CHIlled Platelet Study (CHIPS) is an ongoing randomized controlled trial to study the hemostatic efficacy of cold-stored platelet transfusion in cardiothoracic surgery patients [121••]. The Cold Stored Platelet Early Intervention in Hemorrhagic Shock (CriSP-HS) and a sub-study in traumatic brain injury (CriSP-TBI) are studying the feasibility of an early cold-stored platelet transfusion protocol, as well as impact on mortality and transfusion requirements in hemorrhagic shock and TBI, respectively [122••].
Cryopreservation of platelets can extend the shelf life of a unit of platelets from days to up to 4 years. There is relatively robust data demonstrating cryopreserved platelets (treated with dimethyl sulfoxide [DMSO] and stored at − 65 °C) can effectively establish hemostasis in bleeding patients and have not been implicated in major adverse events. However, they do result in lower post-transfusion platelet counts than with room temperature-stored platelets, including higher decrements at 24 h from transfusion [123,124,125,126]. The Cryopreserved vs. Liquid Platelet (CLIP) study in cardiothoracic surgery patients showed lower rates of significant postoperative hemorrhage, RBC transfusion, and no difference in postoperative complications for the patients who received cryopreserved platelets, although they did receive more platelet and plasma transfusions [127••]. Despite blood product shortages making the long shelf life of cryopreservation appealing, this method has yet to become commonplace in blood banking [128]. Another long-term storage method is freeze-dried lyophilization. Freeze-dried platelets, such as Thrombosomes® (CellPhire Therapeutics), are stabilized in paraformaldehyde or trehalose and then freeze-dried and stored at room temperature, where shelf life is up to 3 years, then reconstituted with saline prior to transfusion. The study of transfusion of Thrombosomes® in humans is ongoing [129, 130••] and in mouse models has been shown to contribute to clot function similarly to room temperature-stored platelets [131••].
Adjunct Therapies
The complex interactions of a plethora of other mediators with platelets during the coagulation process are also important considerations. Early interactions of platelets with von Willibrand factor promote platelet adhesion and aggregation [132]. The large increase in vWF following trauma may promote more platelet interactions but also may overwhelm the cleavage abilities of ADAMTS-13 metalloproteinase, contributing to coagulopathy by inappropriate binding of platelets to large vWF multimers [133•], reduction of ADAMTS-13 activity [134•], and downstream organ dysfunction [135, 136]. Desmopressin, or ddAVP, also increases vWF levels, promotes platelet-based hemostasis, and enhances endothelial function. Animal studies of treatment with ddAVP plus balanced blood product resuscitation after hemorrhagic shock demonstrated that ddAVP improved coagulation parameters including platelet function and clot strength, but did not impact organ damage [137•]. In human studies, administration of ddAVP has not demonstrated impact on progression of intracranial hemorrhage [138]; however, in a separate study of patients with severe traumatic brain injury, administration of ddAVP in lieu of platelet transfusion corrected platelet inhibition similarly to platelet transfusion alone [139•], suggesting that ddAVP may have similar impact on the correction of platelet dysfunction after trauma. Finally, higher serum calcium levels are associated with improved platelet activation, aggregation, and clot strength after injury [22], highlighting its importance as an adjunct during massive transfusion.
Future Directions
A deeper understanding of platelet biology, the platelet response to injury, and platelet-based therapies in trauma continues to inform new areas of study. For example, the platelet transcriptome in injured and uninjured populations is an area of ongoing exploration to identify the mechanistic and molecular bases of the commonly described post-injury platelet “dysfunction,” and uncover treatment targets [18•, 19]. Similarly, platelet-derived extracellular vesicles may also provide insights into trauma-induced coagulopathy, and recent consensus panels have provided guidance to standardize measurements to improve relevance of this test across centers [140, 141].
Beyond a better understanding of the biology, and improved assays, there are many platelet-based therapeutics of interest. For example, the transfusion of other blood-based therapies such as platelet-derived extracellular vesicles may promote thrombin-mediated clot formation in hemorrhaging patients, as well as attenuate endothelial injury following trauma [142, 143]. Furthermore, in vitro production of platelets to enhance the supply is also an area of interest. Induced pluripotent stem cells can be used to generate megakaryocytes which could create a renewable and genetically modifiable source of platelets. This opens the door to platelets that are HLA-deleted and safe against blood-borne pathogens [144]. The ex vivo production of platelets from stem cells has been explored; however, current methods are too low yield to permit clinical utility [145, 146].
Synthetic platelet analogs or “bioinspired artificial platelets” are also investigatory microparticle templates that can be modified to replicate the adhesion and aggregation mechanisms of platelet hemostasis [147•, 148]. Infusible platelet membrane (IPM, Cypress Bioscience), albumin microparticles with fibrinogen additives (Synthocyte) and liposomal microparticles with vWF-binding peptides, collagen-binding peptides, and active GPIIb-IIIa–binding fibrinogen-mimetic peptides (SynthoPlate) are a few examples of synthetic platelet analogs undergoing testing [149, 150]. While these synthetic analogs are attractive, it remains challenging to simply mimic the hemostatic, adhesive, aggregative, and other cellular functions of platelets, when these mechanisms are not fully understood in the endogenous platelet response to trauma [151].
Summary and Conclusions
Platelets are a critical component of hemostasis after trauma, both due to their mechanical hemostatic effects in formation of a platelet plug and their dynamic involvement in clot formation and breakdown, but also their biochemical activity and interaction with the endothelium and inflammatory and immune cells. While this biology after injury remains incompletely understood, platelet function is seen to be altered after trauma, particularly in the setting of severe injury and shock. Including platelet transfusions in hemostatic resuscitation with the aim of balanced transfusion is recommended to achieve the best outcomes in terms of early hemostasis and reduced mortality. Despite overall improved outcomes with early platelet transfusion in injury and hemorrhage, transfusion of platelets is associated with risk of development of ARDS, bacterial infection, and allergic and immune reactions; however, these can be mitigated with improved decontamination procedures and additive solutions in special populations. Cold storage, cryopreservation, synthetic platelets, and platelet-based adjuncts that can expand the supply and deliver safe, effective platelet-based hemostasis are current areas of investigation. Platelets are dynamic and essential components of coagulation biology and mediate a number of cellular interactions following trauma. As research continues to advance our knowledge of these complex cellular fragments, we work toward an ever more tailored and nuanced understanding of how to best diagnose and treat coagulopathy following trauma to improve patient outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Brass LF, Zhu L, Stalker TJ. Minding the gaps to promote thrombus growth and stability. J Clin Invest. 2005;115(12):3385–92.
Hoffman M, Monroe DM. A cell-based model of hemostasis. Thromb Haemost. 2001;85(6):958–65.
Kunitake RC, Howard BM, Kornblith LZ, Christie SA, Conroy AS, Cohen MJ, et al. Individual clotting factor contributions to mortality following trauma. J Trauma Acute Care Surg. 2017;82(2):302–8.
Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30.
MacLeod JBA, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44.
Niles SE, McLaughlin DF, Perkins JG, Wade CE, Li Y, Spinella PC, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64(6):1459–63; discussion 1463-1465.
Cap A, Hunt BJ. The pathogenesis of traumatic coagulopathy. Anaesthesia. 2015;70 Suppl 1:96–101; e32-34.
Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, et al. Trauma-induced coagulopathy. Nat Rev Dis Primer. 2021;7(1):30. Comprehensive overview of trauma-induced coagulopathy including mechanisms and management.
Cardenas JC, Wade CE, Holcomb JB. Mechanisms of trauma-induced coagulopathy. Curr Opin Hematol. 2014;21(5):404–9.
Chang R, Cardenas JC, Wade CE, Holcomb JB. Advances in the understanding of trauma-induced coagulopathy. Blood. 2016;128(8):1043–9.
Kornblith LZ, Moore HB, Cohen MJ. Trauma-induced coagulopathy: the past, present, and future. J Thromb Haemost. 2019;17(6):852–62.
Kornblith LZ, Kutcher ME, Redick BJ, Calfee CS, Vilardi RF, Cohen MJ. Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014;76(2):255–6; discussion 262-263.
Field TS, Castellanos M, Weksler BB, Benavente OR. 63 - Antiplatelet therapy for secondary prevention of stroke. In: Grotta JC, Albers GW, Broderick JP, Day AL, Kasner SE, Lo EH, et al., editors. Stroke (Seventh Edition). Philadelphia: Elsevier; 2022 [cited 2022 Apr 10]. p. 912–931.e4. Available from: https://www.sciencedirect.com/science/article/pii/B9780323694247000636. Accessed 4/10/2022
Nachman RL, Rafii S. Platelets, petechiae, and preservation of the vascular wall. N Engl J Med. 2008;359(12):1261–70.
Maynard DM, Heijnen HFG, Horne MK, White JG, Gahl WA. Proteomic analysis of platelet alpha-granules using mass spectrometry. J Thromb Haemost. 2007;5(9):1945–55.
Cognasse F, Laradi S, Berthelot P, Bourlet T, Marotte H, Mismetti P, et al. Platelet inflammatory response to stress. Front Immunol. 2019;10:1478.
Lewandrowski U, Wortelkamp S, Lohrig K, Zahedi RP, Wolters DA, Walter U, et al. Platelet membrane proteomics: a novel repository for functional research. Blood. 2009;114(1):e10-19.
Fields AT, Lee MC, Mayer F, Santos YA, Bainton CMV, Matthay ZA, et al. A new trauma frontier: exploratory pilot study of platelet transcriptomics in trauma patients. J Trauma Acute Care Surg. 2022;92(2):313–22. Comparison of platelet ribonucleic acid (RNA) expression between nine injured and five healthy patients found differential alternative splicing of platelet RNA in platelets from injured patients.
Kornblith LZ, Bainton CMV, Fields AT, Matthay ZA, Magid NT, Nunez-Garcia B, et al. A journey upstream: fluctuating platelet-specific genes in cell-free plasma as proof-of-concept for using ribonucleic acid sequencing to improve understanding of postinjury platelet biology. J Trauma Acute Care Surg. 2020;88(6):742–51.
Vulliamy P, Kornblith LZ, Kutcher ME, Cohen MJ, Brohi K, Neal MD. Alterations in platelet behavior after major trauma: adaptive or maladaptive? Platelets. 2021;32(3):295–304. Review of platelet biology alterations following trauma, including platelet-endothelial and platelet-leukocyte interactions, and response to transfusions.
Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73(1):13–9.
Matthay ZA, Fields AT, Nunez-Garcia B, Patel MH, Cohen MJ, Callcut RA, et al. Dynamic effects of calcium on in vivo and ex vivo platelet behavior after trauma. J Trauma Acute Care Surg. 2020;89(5):871–9.
Kornblith LZ, Decker A, Conroy AS, Hendrickson CM, Fields AT, Robles AJ, et al. It’s about time: transfusion effects on postinjury platelet aggregation over time. J Trauma Acute Care Surg. 2019;87(5):1042–51.
Johansson PI, Henriksen HH, Stensballe J, Gybel-Brask M, Cardenas JC, Baer LA, et al. Traumatic endotheliopathy: a prospective observational study of 424 severely injured patients. Ann Surg. 2017;265(3):597–603.
Johansson PI, Sørensen AM, Perner A, Welling KL, Wanscher M, Larsen CF, et al. High sCD40L levels early after trauma are associated with enhanced shock, sympathoadrenal activation, tissue and endothelial damage, coagulopathy and mortality. J Thromb Haemost. 2012;10(2):207–16.
Johansson P, Stensballe J, Ostrowski S. Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care. 2017;21:25.
Jacoby RC, Owings JT, Holmes J, Battistella FD, Gosselin RC, Paglieroni TG. Platelet activation and function after trauma. J Trauma. 2001;51(4):639–47.
Starr NE, Matthay ZA, Fields AT, Nunez-Garcia B, Callcut RA, Cohen MJ, et al. Identification of injury and shock driven effects on ex vivo platelet aggregometry: a cautionary tale of phenotyping. J Trauma Acute Care Surg. 2020;89(1):20–8. Single center study on the association of injury severity and shock on platelet activation and aggregation.
Vulliamy P, Gillespie S, Armstrong PC, Allan HE, Warner TD, Brohi K. Histone H4 induces platelet ballooning and microparticle release during trauma hemorrhage. Proc Natl Acad Sci U S A. 2019;116(35):17444–9. First study in humans to demonstrate platelet membrane ballooning in injured patients, implicating this mechanism in trauma-induced thrombotic states.
Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–13.
Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma Inj Infect Crit Care. 2006;60(Supplement):S51–8.
Rijnhout TWH, Duijst J, Noorman F, Zoodsma M, van Waes OJF, Verhofstad MHJ, et al. Platelet to erythrocyte transfusion ratio and mortality in massively transfused trauma patients. A systematic review and meta-analysis. J Trauma Acute Care Surg. 2021;91(4):759–71. Systematic review and meta analysis of 59 articles demonstrate improved mortality with higher platelet:red blood cell transfusion ratios at multiple timepoints in the first 24 h of resuscitation, supporting balanced transfusion ratios.
Kutcher ME, Kornblith LZ, Vilardi RF, Redick BJ, Nelson MF, Cohen MJ. The natural history and effect of resuscitation ratio on coagulation after trauma: a prospective cohort study. Ann Surg. 2014;260(6):1103–11.
Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–58.
Hallet J, Lauzier F, Mailloux O, Trottier V, Archamblault P, Zarychanski R, et al. The use of higher platelet: RBC transfusion ratio in the acute phase of trauma resuscitation: a systematic review. Crit Care Med. 2013;41(12):2800–11.
Gunter OL Jr, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65(3):527–34.
Holcomb JB, Zarzabal LA, Michalek JE, Kozar RA, Spinella PC, Perkins JG, et al. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318-328.
Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471.
Cardenas JC, Zhang X, Fox EE, Cotton BA, Hess JR, Schreiber MA, et al. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv. 2018;2(14):1696–704.
Cap AP, Spinella PC, Borgman MA, Blackbourne LH, Perkins JG. Timing and location of blood product transfusion and outcomes in massively transfused combat casualties. J Trauma Acute Care Surg. 2012;73(2 Suppl 1):S89-94.
Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–36.
Nguyen M, Pirracchio R, Kornblith LZ, Callcut R, Fox EE, Wade CE, et al. Dynamic impact of transfusion ratios on outcomes in severely injured patients: targeted machine learning analysis of the Pragmatic, Randomized Optimal Platelet and Plasma Ratios randomized clinical trial. J Trauma Acute Care Surg. 2020;89(3):505–13.
Meneses E, Boneva D, McKenney M, Elkbuli A. Massive transfusion protocol in adult trauma population. Am J Emerg Med. 2020;38(12):2661–6.
Hamada SR, Garrigue D, Nougue H, Meyer A, Boutonnet M, Meaudre E, et al. Impact of platelet transfusion on outcomes in trauma patients. Crit Care. 2022;26(1):49.
del Junco DJ, Holcomb JB, Fox EE, Brasel KJ, Phelan HA, Bulger EM, et al. Resuscitate early with plasma and platelets or balance blood products gradually: findings from the PROMMTT study. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S24-30.
Gallastegi AD, Naar L, Gaitanidis A, Gebran A, Nederpelt CJ, Parks JJ, et al. Don’t forget the platelets: the independent impact of RBC: PLT ratio on mortality in massively transfused trauma patients. J Trauma Acute Care Surg. 2022;93(1):21–29. Analysis of the American College of Surgeons Trauma Quality Improvement database demonstrates that unbalanced platelet:red blood cell transfusion ratios are more common than unbalanced plasma:red blood cell ratios and have increased odds of mortality.
Stephens CT, Gumbert S, Holcomb JB. Trauma-associated bleeding: management of massive transfusion. Curr Opin Anaesthesiol. 2016;29(2):250–5.
Camazine MN, Hemmila MR, Leonard JC, Jacobs RA, Horst JA, Kozar RA, et al. Massive transfusion policies at trauma centers participating in the American College of Surgeons Trauma Quality Improvement Program. J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S48-53.
Nardi G, Agostini V, Rondinelli B, Russo E, Bastianini B, Bini G, et al. Trauma-induced coagulopathy: impact of the early coagulation support protocol on blood product consumption, mortality and costs. Crit Care. 2015;19:83.
McDaniel LM, Etchill EW, Raval JS, Neal MD. State of the art: massive transfusion. Transfus Med. 2014;24(3):138–44.
Dudaryk R, Hess AS, Varon AJ, Hess JR. What is new in the blood bank for trauma resuscitation. Curr Opin Anaesthesiol. 2015;28(2):206–9.
Matthay ZA, Hellmann ZJ, Callcut RA, Matthay EC, Nunez-Garcia B, Duong W, et al. Outcomes after ultramassive transfusion in the modern era: an Eastern Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg. 2021;91(1):24–33.
Murphy CH, Hess JR. Massive transfusion: red blood cell to plasma and platelet unit ratios for resuscitation of massive hemorrhage. Curr Opin Hematol. 2015;22(6):533–9.
Etchill EW, Myers SP, Raval JS, Hassoune A, SenGupta A, Neal MD. Platelet transfusion in critical care and surgery: evidence-based review of contemporary practice and future directions. Shock. 2017;47(5):537–49.
Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176(3):365–94.
Vulliamy P, Gillespie S, Gall LS, Green L, Brohi K, Davenport RA. Platelet transfusions reduce fibrinolysis but do not restore platelet function during trauma hemorrhage. J Trauma Acute Care Surg. 2017;83(3):388–97.
Henriksen HH, Grand AG, Viggers S, Baer LA, Solbeck S, Cotton BA, et al. Impact of blood products on platelet function in patients with traumatic injuries: a translational study. J Surg Res. 2017;214:154–61.
Wolff C, Muakkassa F, Marley R, El-Khatib A, Docherty C, Muakkassa L, et al. Routine platelet transfusion in patients with traumatic intracranial hemorrhage taking antiplatelet medication: Is it warranted? Can J Surg. 2022;65(2):E206–11.
Miles MVP, Hicks RC, Parmer H, Brown C, Edwards A, Stewart K, et al. Traumatic brain injury patients with platelet inhibition receiving platelet transfusion demonstrate decreased need for neurosurgical intervention and decreased mortality. J Trauma Acute Care Surg. 2022;92(4):701–7.
Holzmacher JL, Reynolds C, Patel M, Maluso P, Holland S, Gamsky N, et al. Platelet transfusion does not improve outcomes in patients with brain injury on antiplatelet therapy. Brain Inj. 2018;32(3):325–30.
Furay E, Daley M, Teixeira PG, Coopwood TB, Aydelotte JD, Malesa N, et al. Goal-directed platelet transfusions correct platelet dysfunction and may improve survival in patients with severe traumatic brain injury. J Trauma Acute Care Surg. 2018;85(5):881–7.
Alvikas J, Myers SP, Wessel CB, Okonkwo DO, Joseph B, Pelaez C, et al. A systematic review and meta-analysis of traumatic intracranial hemorrhage in patients taking prehospital antiplatelet therapy: is there a role for platelet transfusions? J Trauma Acute Care Surg. 2020;88(6):847–54.
Fields AT, Matthay ZA, Nunez-Garcia B, Matthay EC, Bainton RJ, Callcut RA, et al. Good platelets gone bad: the effects of trauma patient plasma on healthy platelet aggregation. Shock. 2021;55(2):189–97.
Gurbel PA, Bliden KP, Tantry US, Monroe AL, Muresan AA, Brunner NE, et al. First report of the point-of-care TEG: a technical validation study of the TEG-6S system. Platelets. 2016;27(7):642–9.
da Luz LT, Nascimento B, Rizoli S. Thrombelastography (TEG®): practical considerations on its clinical use in trauma resuscitation. Scand J Trauma Resusc Emerg Med. 2013;21:29.
Neal MD, Moore EE, Walsh M, Thomas S, Callcut RA, Kornblith LZ, et al. A comparison between the TEG 6s and TEG 5000 analyzers to assess coagulation in trauma patients. J Trauma Acute Care Surg. 2020;88(2):279–85. Comparison of the newer TEG 6S device with conventional TEG 5000 analyzer shows the TEG 6S results are reliable for use in trauma patients.
Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, Wade C, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma. 2011;71(2):407–14; discussion 414-417.
Driessen A, Schäfer N, Bauerfeind U, Kaske S, Fromm-Dornieden C, Stuermer EK, et al. Functional capacity of reconstituted blood in 1:1:1 versus 3:1:1 ratios: a thrombelastometry study. Scand J Trauma Resusc Emerg Med. 2015;23:2.
Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–9.
Johansson PI, Oliveri RS, Ostrowski SR. Hemostatic resuscitation with plasma and platelets in trauma. J Emerg Trauma Shock. 2012;5(2):120–5.
Johansson PI, Sørensen AM, Larsen CF, Windeløv NA, Stensballe J, Perner A, et al. Low hemorrhage-related mortality in trauma patients in a level I trauma center employing transfusion packages and early thromboelastography-directed hemostatic resuscitation with plasma and platelets. Transfusion (Paris). 2013;53(12):3088–99.
Leemann H, Lustenberger T, Talving P, Kobayashi L, Bukur M, Brenni M, et al. The role of rotation thromboelastometry in early prediction of massive transfusion. J Trauma. 2010;69(6):1403–8; discussion 1408-1409.
Sumislawski JJ, Christie SA, Kornblith LZ, Stettler GR, Nunns GR, Moore HB, et al. Discrepancies between conventional and viscoelastic assays in identifying trauma-induced coagulopathy. Am J Surg. 2019;217(6):1037–41.
Sirajuddin S, Valdez C, DePalma L, Maluso P, Singhal R, Schroeder M, et al. Inhibition of platelet function is common following even minor injury. J Trauma Acute Care Surg. 2016;81(2):328–32.
Kay AB, Morris DS, Collingridge DS, Majercik S. Platelet dysfunction on thromboelastogram is associated with severity of blunt traumatic brain injury. Am J Surg. 2019;218(6):1134–7.
Davis PK, Musunuru H, Walsh M, Cassady R, Yount R, Losiniecki A, et al. Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit Care. 2013;18(2):201–8.
Nekludov M, Bellander BM, Blombäck M, Wallen HN. Platelet dysfunction in patients with severe traumatic brain injury. J Neurotrauma. 2007;24(11):1699–706.
Stettler GR, Moore EE, Moore HB, Nunns GR, Huebner BR, Einersen P, et al. Platelet adenosine diphosphate receptor inhibition provides no advantage in predicting need for platelet transfusion or massive transfusion. Surgery. 2017;162(6):1286–94.
Swallow RA, Agarwala RA, Dawkins KD, Curzen NP. Thromboelastography: potential bedside tool to assess the effects of antiplatelet therapy? Platelets. 2006;17(6):385–92.
Cohen MJ, Christie SA. New understandings of post injury coagulation and resuscitation. Int J Surg. 2016;33(Pt B):242–5.
Brass L. Understanding and evaluating platelet function. Hematol Am Soc Hematol Educ Program. 2010;2010:387–96.
Solomon C, Traintinger S, Ziegler B, Hanke A, Rahe-Meyer N, Voelckel W, et al. Platelet function following trauma. A multiple electrode aggregometry study. Thromb Haemost. 2011;106(2):322–30.
Harrison P, Robinson M, Liesner R, Khair K, Cohen H, Mackie I, et al. The PFA-100: a potential rapid screening tool for the assessment of platelet dysfunction. Clin Lab Haematol. 2002;24(4):225–32.
Crescente M, Di Castelnuovo A, Iacoviello L, Vermylen J, Cerletti C, de Gaetano G. Response variability to aspirin as assessed by the platelet function analyzer (PFA)-100. A systematic review. Thromb Haemost. 2008;99(1):14–26.
Kornblith LZ, Robles AJ, Conroy AS, Redick BJ, Howard BM, Hendrickson CM, et al. Predictors of postinjury acute respiratory distress syndrome: lung injury persists in the era of hemostatic resuscitation. J Trauma Acute Care Surg. 2019;87(2):371–8.
Hendrickson CM, Howard BM, Kornblith LZ, Conroy AS, Nelson MF, Zhuo H, et al. The acute respiratory distress syndrome following isolated severe traumatic brain injury. J Trauma Acute Care Surg. 2016;80(6):989–97.
Howard BM, Kornblith LZ, Hendrickson CM, Redick BJ, Conroy AS, Nelson MF, et al. Differences in degree, differences in kind: characterizing lung injury in trauma. J Trauma Acute Care Surg. 2015;78(4):735–41.
Kasotakis G, Starr N, Nelson E, Sarkar B, Burke PA, Remick DG, et al. Platelet transfusion increases risk for acute respiratory distress syndrome in non-massively transfused blunt trauma patients. Eur J Trauma Emerg Surg. 2019;45(4):671–9.
Blumberg N, Heal JM, Gettings KF, Phipps RP, Masel D, Refaai MA, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion (Paris). 2010;50(12):2738–44.
Raval JS, Mazepa MA, Russell SL, Immel CC, Whinna HC, Park YA. Passive reporting greatly underestimates the rate of transfusion-associated circulatory overload after platelet transfusion. Vox Sang. 2015;108(4):387–92.
Savage WJ, Savage JH, Tobian AAR, Thoburn C, Hamilton RG, Schroeder JT, et al. Allergic agonists in apheresis platelet products are associated with allergic transfusion reactions. Transfusion (Paris). 2012;52(3):575–81.
Heddle NM, Klama LN, Griffith L, Roberts R, Shukla G, Kelton JG. A prospective study to identify the risk factors associated with acute reactions to platelet and red cell transfusions. Transfusion (Paris). 1993;33(10):794–7.
Stack G, Snyder EL. Cytokine generation in stored platelet concentrates. Transfusion (Paris). 1994;34(1):20–5.
Buck SA, Kickler TS, McGuire M, Braine HG, Ness PM. The utility of platelet washing using an automated procedure for severe platelet allergic reactions. Transfusion (Paris). 1987;27(5):391–3.
Silvergleid AJ, Hafleigh EB, Harabin MA, Wolf RM, Grumet FC. Clinical value of washed-platelet concentrates in patients with non-hemolytic transfusion reactions. Transfusion (Paris). 1977;17(1):33–7.
Capocelli KE, Dumont LJ. Novel platelet storage conditions: additive solutions, gas, and cold. Curr Opin Hematol. 2014;21(6):491–6.
van der Meer PF. PAS or plasma for storage of platelets? A concise review. Transfus Med. 2016;26(5):339–42.
Tobian AAR, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion (Paris). 2011;51(8):1676–83.
McCrae KR, Herman JH. Posttransfusion purpura: two unusual cases and a literature review. Am J Hematol. 1996;52(3):205–11.
Mueller-Eckhardt C. Post-transfusion purpura. Br J Haematol. 1986;64(3):419–24.
Pavenski K, Webert KE, Goldman M. Consequences of transfusion of platelet antibody: a case report and literature review. Transfusion (Paris). 2008;48(9):1981–9.
Baharoglu MI, Cordonnier C, Salman RAS, de Gans K, Koopman MM, Brand A, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387(10038):2605–13.
Baharoglu MI, Al-Shahi Salman R, Cordonnier C, Koopman MM, Manson L, Susen S, et al. PATCH trial: explanatory analyses. Blood. 2020;135(16):1406–9.
Butler C, Doree C, Estcourt LJ, Trivella M, Hopewell S, Brunskill SJ, et al. Pathogen-reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev. 2013;3:CD009072.
McCullough J, Vesole DH, Benjamin RJ, Slichter SJ, Pineda A, Snyder E, et al. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT Trial. Blood. 2004;104(5):1534–41.
Cid J, Escolar G, Lozano M. Therapeutic efficacy of platelet components treated with amotosalen and ultraviolet A pathogen inactivation method: results of a meta-analysis of randomized controlled trials. Vox Sang. 2012;103(4):322–30.
Apelseth TO, Cap AP, Spinella PC, Hervig T, Strandenes G. Cold stored platelets in treatment of bleeding. ISBT Sci Ser. 2017;12(4):488–95.
Currie LM, Harper JR, Allan H, Connor J. Inhibition of cytokine accumulation and bacterial growth during storage of platelet concentrates at 4 degrees C with retention of in vitro functional activity. Transfusion (Paris). 1997;37(1):18–24.
Vostal JG, Mondoro TH. Liquid cold storage of platelets: a revitalized possible alternative for limiting bacterial contamination of platelet products. Transfus Med Rev. 1997;11(4):286–95.
Garraud O, Cognasse F, Tissot JD, Chavarin P, Laperche S, Morel P, et al. Improving platelet transfusion safety: biomedical and technical considerations. Blood Transfus Trasfus Sangue. 2016;14(2):109–22.
Milford EM, Reade MC. Comprehensive review of platelet storage methods for use in the treatment of active hemorrhage. Transfusion (Paris). 2016;56 Suppl 2:S140-148.
Waters L, Cameron M, Padula MP, Marks DC, Johnson L. Refrigeration, cryopreservation and pathogen inactivation: an updated perspective on platelet storage conditions. Vox Sang. 2018;113(4):317–28.
Cohn CS, Shaz BH. Warming up to cold-stored platelets. Anesthesiology. 2020;133(6):1161–3. Summary of research to date on cold-stored platelets supporting further investigation and rationale for clinical use.
Becker GA, Tuccelli M, Kunicki T, Chalos MK, Aster RH. Studies of platelet concentrates stored at 22 C nad 4 C. Transfusion (Paris). 1973;13(2):61–8.
Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability–deleterious effect of refrigerated storage. N Engl J Med. 1969;280(20):1094–8.
Johnson L, Tan S, Wood B, Davis A, Marks DC. Refrigeration and cryopreservation of platelets differentially affect platelet metabolism and function: a comparison with conventional platelet storage conditions. Transfusion (Paris). 2016;56(7):1807–18.
Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk CG, Aden JK, Ramasubramanian AK, et al. Hemostatic function of apheresis platelets stored at 4°C and 22°C. Shock. 2014;41 Suppl 1:54–61.
Montgomery RK, Reddoch KM, Evani SJ, Cap AP, Ramasubramanian AK. Enhanced shear-induced platelet aggregation due to low-temperature storage. Transfusion (Paris). 2013;53(7):1520–30.
Valeri CR. Circulation and hemostatic effectiveness of platelets stored at 4 C or 22 C: studies in aspirin-treated normal volunteers. Transfusion (Paris). 1976;16(1):20–3.
Strandenes G, Sivertsen J, Bjerkvig CK, Fosse TK, Cap AP, Del Junco DJ, et al. A pilot trial of platelets stored cold versus at room temperature for complex cardiothoracic surgery. Anesthesiology. 2020;133(6):1173–83. Pilot study of cold-stored platelets and one of the first in-human trials demonstrating the efficacy of transfusing cold-stored platelets after cardiothoracic surgery with similar bleeding and platelet aggregation parameters to room temperature-stored platelets.
Spinella P. CHIlled Platelet Study. clinicaltrials.gov; 2022 [cited 2022 Mar 8]. Report No.: NCT04834414. Available from: https://clinicaltrials.gov/ct2/show/NCT04834414. Accessed 3/8/2022. Ongoing randomized controlled trial of cold-stored versus room temperature-stored platelet transfusion in cardiothoracic surgery patients to determine hemostatic efficacy and chest tube output.
Sperry J. Cold Stored Platelet Early Intervention in Hemorrhagic Shock (CriSP-HS) Trial. clinicaltrials.gov; 2022 [cited 2022 Mar 8]. Report No.: NCT04667468. Available from: https://clinicaltrials.gov/ct2/show/NCT04667468. Accessed 3/8/2022. Ongoing multicenter randomized controlled trial of cold-stored versus room temperature-stored platelet transfusion in patients with hemorrhagic shock.
Slichter SJ, Jones M, Ransom J, Gettinger I, Jones MK, Christoffel T, et al. Review of in vivo studies of dimethyl sulfoxide cryopreserved platelets. Transfus Med Rev. 2014;28(4):212–25.
Cohn CS, Williams S. Cryopreserved platelets: the thaw begins … (Article, p. 2794). Transfusion (Paris). 2019;59(9):2759–62.
Pidcoke HF, Spinella PC, Ramasubramanian AK, Strandenes G, Hervig T, Ness PM, et al. Refrigerated platelets for the treatment of acute bleeding: a review of the literature and reexamination of current standards. Shock. 2014;41 Suppl 1:51–3.
Mack JP, Miles J, Stolla M. Cold-stored platelets: review of studies in humans. Transfus Med Rev. 2020;34(4):221–6.
Reade MC, Marks DC, Bellomo R, Deans R, Faulke DJ, Fraser JF, et al. A randomized, controlled pilot clinical trial of cryopreserved platelets for perioperative surgical bleeding: the CLIP-I trial (Editorial, p. 2759). Transfusion (Paris). 2019;59(9):2794–804. One of the first published trials of cryopreserved platelet transfusion in cardiothoracic surgery patients, demonstrating fewer red blood cell transfusions and improved hemostasis in the cryopreserved platelet group.
Marks DC. Cryopreserved platelets: are we there yet? Transfusion (Paris). 2018;58(9):2092–4.
Cellphire Therapeutics, Inc. Evaluation of the safety and immunogenicity of autologous Thrombosomes® in healthy human subjects; a microdose escalation study (cohorts 1 - 4) and repeat microdose immunogenicity study (cohort 5). clinicaltrials.gov; 2017 [cited 2022 May 4]. Report No.: NCT02223117. Available from: https://clinicaltrials.gov/ct2/show/NCT02223117. Accessed 5/4/2022
Cellphire Therapeutics, Inc. A phase I, multi-center, open-label, dose escalation study of Thrombosomes® in bleeding thrombocytopenic patients in three cohorts. clinicaltrials.gov; 2019 [cited 2022 May 4]. Report No.: NCT03394755. Available from: https://clinicaltrials.gov/ct2/show/NCT03394755. Accessed 5/4/2022. Single center ongoing trial on safety of synthetic platelet (Cellphire Thrombosomes) administration safety.
Trivedi A, Potter DR, Miyazawa BY, Lin M, Vivona LR, Khakoo MA, et al. Freeze-dried platelets promote clot formation, attenuate endothelial cell permeability, and decrease pulmonary vascular leak in a murine model of hemorrhagic shock. J Trauma Acute Care Surg. 2021;90(2):203–14. Study of administration of freeze-dried human platelets administered to mice in a hemorrhagic shock state found that freeze-dried platelets contribute to clot formation similarly to fresh platelets.
Ruggeri ZM, Mendolicchio GL. Interaction of von Willebrand factor with platelets and the vessel wall. Hamostaseologie. 2015;35(3):211–24.
MacArthur TA, Goswami J, Moon Tasson L, Tischer A, Bailey KR, Spears GM, et al. Quantification of von Willebrand factor and ADAMTS-13 after traumatic injury: a pilot study. Trauma Surg Acute Care Open. 2021;6(1):e000703. Study measuring serum concentrations of von Willebrand factor (vWF) antigen and a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS-13) antigen after injury found that increased serum vWF may overwhelm ADAMTS-13 activity, leading to multimer vWF that aggregate platelets and contribute to trauma associated thromboses.
Dyer MR, Plautz WE, Ragni MV, Alexander W, Haldeman S, Sperry JL, et al. Traumatic injury results in prolonged circulation of ultralarge von Willebrand factor and a reduction in ADAMTS13 activity. Transfusion (Paris). 2020;60(6):1308–18. Single center study found that injured patients had high levels of vWF and ultralarge vWF multimers as well as reduced ADAMTS-13 activity, which correlated with coagulopathy.
Plautz WE, Haldeman SH, Dyer MR, Sperry JL, Guyette FX, Loughran PA, et al. Reduced cleavage of von willebrand factor by ADAMTS13 is associated with microangiopathic acute kidney injury following trauma. Blood Coagul Fibrinolysis. 2022;33(1):14–24.
Kleinveld DJB, Simons DDG, Dekimpe C, Deconinck SJ, Sloos PH, Maas MAW, et al. Plasma and rhADAMTS13 reduce trauma-induced organ failure by restoring the ADAMTS13-VWF axis. Blood Adv. 2021;5(17):3478–91.
Wirtz MR, Roelofs JJ, Goslings JC, Juffermans NP. Treatment with ddAVP improves platelet-based coagulation in a rat model of traumatic hemorrhagic shock. Trauma Surg Acute Care Open. 2022;7(1):e000852. Study demonstrating desmopressin administered to rats improved coagulation parameters and reversed shock compared to controls, suggesting that desmopressin may be a useful adjunct in early reversal of coagulopathy.
Kim DY, O’Leary M, Nguyen A, Kaji A, Bricker S, Neville A, et al. The effect of platelet and desmopressin administration on early radiographic progression of traumatic intracranial hemorrhage. J Neurotrauma. 2015;32(22):1815–21.
Furay EJ, Daley MJ, Satarasinghe P, Lara S, Aydelotte JD, Teixeira PG, et al. Desmopressin is a transfusion sparing option to reverse platelet dysfunction in patients with severe traumatic brain injury. J Trauma Acute Care Surg. 2020;88(1):80–6. Administration of desmopressin in patients with blunt trauma and severe traumatic brain injury showed similar platelet dysfunction to patients who received platelet transfusions, suggesting this may be an alternative therapy to correct platelet dysfunction.
Lacroix R, Robert S, Poncelet P, Kasthuri RS, Key NS, Dignat-George F, et al. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2010;8(11):2571–4.
Cointe S, Judicone C, Robert S, Mooberry MJ, Poncelet P, Wauben M, et al. Standardization of microparticle enumeration across different flow cytometry platforms: results of a multicenter collaborative workshop. J Thromb Haemost. 2017;15(1):187–93.
Miyazawa B, Trivedi A, Togarrati PP, Potter D, Baimukanova G, Vivona L, et al. Regulation of endothelial cell permeability by platelet-derived extracellular vesicles. J Trauma Acute Care Surg. 2019;86(6):931–42.
Lopez E, Srivastava AK, Pati S, Holcomb JB, Wade CE. Platelet-derived microvesicles: a potential therapy for trauma-induced coagulopathy. Shock. 2018;49(3):243–8.
Sugimoto N, Eto K. Platelet production from induced pluripotent stem cells. J Thromb Haemost. 2017;15(9):1717–27.
Avanzi MP, Mitchell WB. Ex vivo production of platelets from stem cells. Br J Haematol. 2014;165(2):237–47.
Strassel C, Gachet C, Lanza F. On the way to in vitro platelet production. Front Med. 2018;5:239.
Luc NF, Rohner N, Girish A, Didar Singh Sekhon U, Neal MD, Sen Gupta A. Bioinspired artificial platelets: past, present and future. Platelets. 2022;33(1):35–47. A comprehensive review of platelet storage methods, artificial and bioinspired platelet analogs with descriptions of platelet surrogate types and studies of each.
Kim HW, Greenburg AG. Toward 21st century blood component replacement therapeutics: artificial oxygen carriers, platelet substitutes, recombinant clotting factors, and others. Artif Cells Blood Substit Immobil Biotechnol. 2006;34(6):537–50.
Girish A, Sekhon U, Sen GA. Bioinspired artificial platelets for transfusion applications in traumatic hemorrhage. Transfusion (Paris). 2020;60(2):229–31.
Modery-Pawlowski CL, Tian LL, Ravikumar M, Wong TL, Sen GA. In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials. 2013;34(12):3031–41.
Modery-Pawlowski CL, Tian LL, Pan V, McCrae KR, Mitragotri S, Sen GA. Approaches to synthetic platelet analogs. Biomaterials. 2013;34(2):526–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
NS, ZM, and AF have nothing to disclose. MN is supported by NIH R35GM119526 and an editorial board member for Current Trauma Reports. LZK is supported by NIH 1K23GM130892-01, is a member of the scientific advisory board for Cerus, Gamma Prime, and a consultant for University of Maryland and BARDA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Resuscitation in Trauma
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Starr, N., Matthay, Z., Fields, A. et al. Platelet Transfusion for Trauma Resuscitation. Curr Trauma Rep 8, 147–159 (2022). https://doi.org/10.1007/s40719-022-00236-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40719-022-00236-2