Abstract
Background and aim
Evidences suggest that androgen deficiency is associated with sudden cardiac death (SCD). Our purpose was to analyse some electrocardiographic (ECG) markers of repolarization phase in hypogonadal patients either at baseline or after testosterone replacement therapy (TRT).
Patients and Methods
Baseline and after 6 months of testosterone replacement therapy, 14 hypogonadal patients and 10 age-matched controls underwent a short-term ECG recordings at rest and immediately after a maximal exercise test. The following ECG parameters have been collected: QTe (the interval between the q wave the end of T wave), QTp (the interval between the q wave and the peak of T wave), and Te (the interval between the peak and the end of T wave).
Results
At baseline, in the hypogonadal patients, corrected QTe and QTp values were longer at rest than in the controls at rest (p < 0.05), whereas, during the recovery phase, only the QTp remained significantly longer (p < 0.05). After TRT, hypogonadal patients showed an improvement only in Te (p < 0.05). Conversely, any difference between hypogonadal patients and control subjects was found with respect to the markers of temporal dispersion of repolarization phases, except for a worse QTp → Te coherence (p = 0.001) obtained during the recovery phase.
Conclusions
In conclusion, at rest, hypogonadal patients suffer from a stable increase in the myocardial repolarization phase without an increase in its temporal dispersion and, hence, the SCD risk seems to be low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male hypogonadism is characterized by the presence of clinical symptoms of androgen deficiency (e.g. erectile dysfunction, delayed puberty, etc.) associated with low testosterone levels (< 12 nmol/L) [1]. This condition affects 6–12% of men aged between 40 and 69 years and it is strongly associated with cardiovascular disorders. Uncertain data are available about the effects of testosterone replacement therapy (TRT) on cardiovascular risk. The RHYME study clearly concludes that hypogonadal men receiving TRT did not show an increased cardiovascular risk [2]. Moreover, Corona et al. in a systematic review and misanalysis did not find a causal role between TRT and cardiovascular events [3]. Recently, it has been reported that low level of testosterone is associated with higher risk of sudden cardiac death (SCD) most likely due to a worsening in the myocardial repolarization phase [1, 4]. Supporting the hypothesis, gender- and hormone-related differences in myocardial repolarization phase length have been described and, hence, in the ECG-surface-derived QT interval. Indeed, in healthy condition, adult female subjects show a QT interval corrected for the heart rate significantly longer than male, this difference being absent before the puberty with a progressive QT interval shortening from 9 to 50 years old in male [5,6,7,8,9,10]. The abovementioned trend is thought to be related to progressive androgen level increase and, consistently, the opposite trend (i.e. QT interval increase) is detectable after the 60 years old [6,7,8]. Furthermore, the males after orchiectomy show QT interval longer than healthy age-matched male subjects and, even, the masculinized females have QT interval shorter than the normal ones. Moreover, the abuse of androgenic steroid in athletes is known to be related to the sudden cardiac death [11]. In this context, the analysis of temporal dispersion of myocardial repolarization might help in understanding some mechanisms underlying the impact of testosterone on arrhythmia propensity. Indeed, the myocardial repolarization phase, non-invasively studied on the surface electrocardiogram (ECG) by means of different QT segments measurement, short-term QT segment variability [12], QT/RR slope and QT-RR spectral coherence [12,13,14,15,16,17], yet imposed itself as a non-invasive marker of sudden cardiac death (SCD) risk in several cardiovascular and not cardiovascular conditions [12, 13, 18,19,20,21].
Therefore, the present experimental study sought to investigate non-invasively the myocardial repolarization phase and its temporal dispersion in a series of hypogonadal male patients either at baseline or after testosterone replacement therapy. All the ECG-derived parameters were studied both at rest and immediately after a maximal exercise test. Particularly, the evaluation of the ECG-derived parameters in the post-exercise phase aimed to study the repolarization in the absence of excessive muscle interferences, yet during intense autonomic nervous system imbalance [22,23,24] characterized by high vagal and sympathetic activity due to recovery from intense exercise.
Methods
Patients and protocol
To test the hypothesis of the testosterone influence on the left ventricular repolarization, we planned a single-center pilot prospective study. The diagnosis of hypogonadism was based on the presence of clinical symptoms related to this condition (e.g. reduced libido or erectile dysfunction) and on the results of standard hormonal exams (total testosterone < 12 nmol/L). After 6 months of enrollment time, we selected 14 subjects with hypogonadism candidates to testosterone replacement therapy (TRT) and 10 eugonadal age-matched controls. Particularly, nine patients had post-surgical hypogonadotropic hypogonadism (nine pituitary adenomas), one post-surgical hypogonadism (testicular cancer), two patients had idiopathic congenital hypogonadotropic hypogonadism and two had naïve Kallmann syndrome (hypogonadotropic hypogonadism and anosmia). Patients with hypogonadism and controls underwent a complete myocardial repolarization phase non-invasive study at baseline and after 6 months from the first administration of testosterone undecanoate injectable (1000 mg i.m injection repeated time 0 and after 6 weeks as indicated by product indication schedule). Blood samples were collected at baseline: the testosterone level was measured before the repolarization studies and at the end of study observation period, after 6 months of follow-up (total of three injections). All blood samples were collected by venipuncture in fasting patients; serum concentrations of testosterone were measured by chemiluminescence.
The clinical assessment included physical examination, echocardiogram, 5 min of single-lead (D II) ECG recording at rest in supine position and 10 min of single-lead ECG recordings during the post-exercise recovery phase in sitting position on the bike. All subjects underwent Bruce protocol stress testing; patients with typical angina were excluded from the study as well as those with a ECG responses characterized by 1 mm or more horizontal or downsloping ST segment depression, measured at 80 ms sec after the J point. Tests were considered valid only if the subject reached at least 85% of the maximal age-corrected heart rate. All ECG registrations were collected baseline, and after 6 months from commencement of TRT.
Data processing
We used a custom-designed card (National Instruments USB-6008; National Instruments, Austin, TX, USA) to acquire and digitalize the ECG signals; the sampling frequency was 500 Hz. Points used for the ECG segment analysis were detected automatically by a classic adaptive derivative/threshold algorithm. We designed and produced a software for data acquisition, storage, and analysis with the LabView program (National Instruments). After a linear interpolation, an expert cardiologist (GP) checked the different points and, when needed, manually corrected the mistakes with an interactive software [15, 16, 22, 25,26,27]. All ECG data were analyzed in a single-blind fashion.
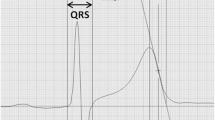
Beat-to-beat ECG intervals obtained at rest and during exercise recovery were: RR, QTe (the interval between the q wave the end of T wave), QTp (the interval between the q wave and the peak of T wave), and Te (the interval between the peak and the end of T wave) [15, 16, 22, 25,26,27,28] (Fig. 1). We, therefore, calculated mean and variance values for each of these intervals and then we used the original formula proposed by Berger et al. [29] to calculate three different QT variability indexes [15, 16, 22, 25,26,27] (Figs. 2, 3):
The same ECG intervals were also used for power spectral (autoregressive algorithm) and cross-spectral analysis (Fig. 4). Cross-spectral analysis indicated the influences on the different oscillations (coherence function) between RR, QTe, QTp and Te [12,13,14,15,16, 22, 25,26,27] (Fig. 4). Coherence expresses an index (from 0 to 1) of a linear association between the two signals [12,13,14,15,16, 22, 25,26,27] (Fig. 4).
Linear regression was used to calculate QTe-RR, QTp-RR and Te-RR slopes (Fig. 5) [14, 30,31,32,33]. This analysis was conducted only during exercise recovery because in rest the number of QT was not sufficient for significant linear regression [30].
From the ECG segments, the QTe, QTp, and Te intervals were corrected by the Bazett (QTe/RR0.5; QTp/RR0.5; Te/RR0.5), Fridericia (QTe/RR0.33; QTp/RR0.33; Te/RR0.33), Lilly (QTe/RR0.4; QTp/RR0.4; Te/RR0.4), and Framingham (QTe + [0.154 × {1000 − RR}]; QTp + [0.154 × {1000 − RR}]; Te + [0.154 × {1000 − RR}]) [15, 33] formulas. We calculated the repolarization corrected variables on the ECG overall length recordings at rest and during the recovery phase both at baseline and after testosterone replacement therapy. Moreover, we calculated manually the instantaneous corrected repolarization variables on three consecutive RR and on the following QRS-T (QTe, QTp and Te) intervals during the first minute at rest, at the exercise peak and, also, at the 1st, 3rd, 5th, 7th, 9th minutes during exercise recovery [24] with tangential method and using the ECG II lead. In particular, we measured the QTe interval as the time between QRS onset and the point at which the isoelectric line intersected a tangential line drawn at the maximal downslope of the positive T wave; instead the QTp was obtained measuring the interval from q and peak of T wave; finally Te interval was the difference between QTe and QTp. On the contrary, the QTe, QTp and Te data, obtained on baseline and after exercise recovery, were collected during the two whole recordings with the previous described and cited software. Finally, due the change of position (during supine or cycle) of patients could affect the amplitude and consequently the end of T wave, we checked possible variation of voltage of T wave [12].
Statistical analysis
We reported data as mean ± SD or as interquartile range, respectively, for normal and skewed distribution data. We used Student’s t test to compare data for the normally distributed variables; on the contrary, we used Mann–Whitney to compare non-normally distributed variables (as evaluated by Kolgomorov–Smirnov test). We used the paired t test, for the normally distributed variables, and Wilcoxon test, for non-normally distributed variables, to compare data during baseline and replacement therapy. We considered statistically significant p values < 0.05. For statistical analysis, we used SPSS-PC + [SPSS-PC + Inc, Chicago, Illinois].
Results
During TRT one patient interrupted the therapy for dysuria.
At baseline, general characteristics and echocardiographic data were similar between hypogonadal patients and normal subjects (Table 1). Furthermore, no difference was found regarding exercise data or voltage of T wave (Table 1). Naturally, testosterone levels were significant lower in the hypogonadal patients. Significant changes in PSA, hematocrit and waist circumference were reported in patients after 6 months testosterone undecanoate treatment (p < 0.05, data not shown) according to our previously published data obtained in hypogonadal men [34].
QTe and QTp were significantly longer in hypogonadal patients at rest (Table 2). QTe, but not QTeBazett (for an overestimation of Bazett’s formula during exercise), showed similar behavior during the recovery phase exercise recovery (Table 2). As regards, the instantaneous repolarization variables, the corrected and raw QTe measurements were significantly longer in hypogonadal patients only at rest at the 5th minute of the recovery phase (Table 3, Fig. 6a). Instead, QTp was often significantly longer, regardless the heart rate. In fact at rest, during the 3rd, 5th and 9th minutes QTp were longer in the hypogonadal patients (Table 3, Fig. 6a) than controls, but this behavior was not reported at the exercise peak and during the 1st minute when the heart rate was the highest.
a QTe, QTp, and Te with Fridericia correction and RR intervals at rest, during the peak, the first and the 10th minute of exercise recovery in hypogonadal (red) and control subjects (green). *p < 0.05 or **p < 0.001: hypogonadal patients vs controls. b QTe, QTp, and Te with Fridericia correction and RR intervals at rest, during the peak, the first and the 10th minute of exercise recovery in hypogonadal (baseline) and during replacement therapy (blue) in hypogonadal subjects. *p < 0.05 baseline vs control. These data are detected manually with tangential method
No significant differences were found with respect to the myocardial repolarization dispersion variables (Table 4), except for the QTp → Te coherence during the recovery phase (Table 4, Fig. 7a), where this parameter was lower in the hypogonadal subjects (p < 0.001).
a QTp → Te during exercise recovery in hypogonadal and control subjects. In the box plots, the central line represents the median distribution. Each box spans from 25th to 75th percentile points, and error bars extend from 10th to 90th percentile points. b Te-RR slope during exercise recovery at baseline and replacement therapy in hypogonadal subjects. In the box plots, the central line represents the median distribution. Each box spans from 25th to 75th percentile points, and error bars extend from 10th to 90th percentile points. These data are detected automatically with a custom software
After the TRT, the serum testosterone level was significant higher than baseline (from 3.86 ± 4.10 to 13.12 nmol/L, p < 0.001). Other general data (BMI, LVEF, LVMI, heart rate, blood pressure) at rest and during exercise (heart rate peak, systolic blood pressure peak, peak workload, exercise duration and rate pressure product) did not change. As far as this period, at rest and during the recovery phase most of the repolarization data were not significantly different. Conversely, non-corrected QTe (p < 0.05) and QTp (p < 0.05) at rest, Te with all corrections (p < 0.05) at the heart rate peak (Fig. 6b; Table 5) and Te-RR slope (p < 0.05) during recovery (Fig. 7b; Table 6) were significantly reduced with respect the baseline. We have found no difference between control group and hypogonadal subjects during TRT in ECG data. Finally, for a better understanding of the reported data, the instantaneous QRS-T data showed in the Tables 3 and 5 and Fig. 6 are obtained manually; on the contrary, data reported in the Tables 2, 4 and 6 and Fig. 7 are obtained automatically with the previous cited custom software.
Discussion
The present study primarily confirms that corrected QTe and QTp at rest are longer in the hypogonadal subjects than age-matched normal controls. This finding confirms many other previous studies where it has been stated that the reduction or absence of testosterone levels prolongs the repolarization phases with a possible proarrhythmic effect [35,36,37,38]. Interestingly, in hypogonadals patients, we found that during the post-exercise recovery phase the QTe interval, corrected for the heart rate using all the available formulas except of the Bazett one [39,40,41], was longer and, contextually, there was significantly lower QTp → Te coherence. In fact, it has been previously demonstrated that the cubic root equation (Fridericia’s) might be more accurate than the square root (Bazett’s) or several complex formulas for correcting measured QT intervals for cardiac cycle length in middle-aged men [41]. Furthermore, the instantaneous QTp, obtained by the standard method (i.e. measuring three consecutive RR and the following QRS-T intervals), was longer at rest and in three over the six measurements of the recovery phase (3rd, 5th, 9th minute) regardless of the heart rate. Finally, since any differences of corrected Te both at rest or during the recovery phase was found, it is reasonable that the longer QTe was caused by an abnormal first part of the repolarization (i.e. QTp). Thus, our data suggest that the whole repolarization phase was altered by an abnormal duration of first part of QT at low level of heart rate and sympathetic activity. In this context, it might be possible to find a similarity between the effect of low level of testosterone and the congenital long QT type 3 syndromes (due to Na channel SN5) [42] where the arrhythmic events usually occur during the night at lower sympathetic activity and heart rate. Leptin increases sympathetic nerve activity in humans [43]; even if we did not evaluate leptin variations upon TRT, the maintenance of stable BP in our patients might have been explained by leptin reduction and amelioration of insulin sensitivity that usually occurs after short-term TRT in severe hypogonadal patients with metabolic syndrome [34, 44].
Indeed, the action potential duration of cardiac cells, corresponding to the surface QTe interval, is maintained by the perfect temporal activation and inactivation of the sodium, calcium and potassium current by means of specific ionic channels. Testosterone is able to shorten the action potential duration and, namely the QTe, especially influencing the L-type calcium channel (ICaL) and slow delayed rectifier K+ channel (IKs). Accordingly, testosterone could be able to reduce the QTe length throughout an inhibition of the ICaL and an increase of the IKs activities [42, 45,46,47,48], thus decreasing the calcium entry and increasing the potassium efflux. Consequently, we hypothesized that the absence of androgens in the hypogonadal subjects induces an increase of QTe by means of an increase of calcium influx and a lower action on specific ion channels [49].
Most of the myocardial temporal dispersion variables were similar between controls and hypogonadal patients both at rest and during the recovery phase. A possible explanation could be that these QT dynamic data are important to explain severe cardiac events [12] but they were not enough sensitive in subjects with almost normal cardiac function and, hence, a low relative risk of malignant ventricular arrhythmias. Thus, we hypothesize that, in case of further repolarization reserve reduction (myocardial ischemia, hypertrophy, heart failure, hypokalemia, genetic polymorphism of ionic channel etc.…) [49,50,51] also in a hypogonadal patient, these ECG parameters could be useful in disclosing an increased SCD risk.
The QTp → Te coherence during the recovery phase was the only myocardial temporal dispersion variable found to be different in hypogonadal patients with respect to the controls. The coherence between two oscillatory components expresses a strong linear coupling between QTp-Te interval fluctuation and the ability of two signals to have similar behavior in the time. We previously observed that, at rest, a reduction of QTp → Te coherence was associated with sustained ventricular tachycardia in patients with low ejection fraction and chronic heart failure [25]. In the present study, this parameter was altered (i.e. reduced) solely during the recovery phase, condition characterized by an autonomic nervous system imbalance with still high sympathetic activity. The underlying mechanisms for altering QTp → Te coherence are unknown and controversial [52,53,54]. Undoubtedly, QTp and Te have two different electrophysiological meanings. In some studies, QTp could be referred to action potential duration of the epicardial layer [55, 56]; on the contrary, Te predominantly is influenced by the M-cell layer repolarization and this last layer also showed the longer depolarization duration. Thus, these authors consider the Te reflecting the maximum difference in repolarization between the myocardial layers. For this reason, they have suggested it as a non-invasive marker of transmural dispersion of repolarization [54, 55]. Therefore, Te depends on IKr, IKs and IK1 function, whereas the QTp reasonably is influenced by the depolarization phase, so by Na currents, and by the early repolarization phase, both, mainly under the Ito control and by the sarcoplasmic reticulum Ca uptake (up) currents [17, 56, 57]. Definitely, the dysfunction of these ion channels’ network could alter one of these two QT segments, reducing their coherence and probably, increasing the ventricular arrhythmias risk’s [17, 57], especially during sympathetic stress. Given the abovementioned mechanisms, albeit merely speculative, the sympathetic stress might have increased the sensitivity of QTp → Te coherence so that low level of this parameter could be indicative of an intermediate risk of malignant ventricular arrhythmias.
The TRT in hypogonadal subjects seemed to have a weak effect on the duration and on the dynamic of repolarization phases. Indeed, after 6 months of therapy we did not have any difference of corrected QT in baseline and during exercise recovery. Although, in vitro, some of our previous studies reported a reduction of action potential duration in cardiac cells, few data existed on QT in the hypogonadal patients. In regard to the corrected QT, Charbit et al. found a reduction of 13.6 ± 2.8 ms between low and high levels of serum testosterone (low versus high testosterone level medians: 6 versus 52.6 nmol/L) [58]. On the contrary, Pecori Giraldi et al. did not find a reduction of corrected QT except for a small percentage with an abnormal corrected QT (> 440 ms) [36,37,38]. In our study, the level of testosterone reached was less than half of the Charbit study and none of our hypogonadal subjects had a corrected QT at rest higher of 440 ms; thus, we cannot make a definitive comparison with the previous cited studies. However, although our patients were older than the Pecori Giraldi study (for these reasons, we reported lower testosterone serum levels), we obtained the similar results with a corrected QT at rest unmodified by the TRT. On the contrary, other authors reported an improvement of QTpVI in hypogonadal subjects with spinal cord injury during TRT [59, 60]. Probably, the study is incomparable because our hypogonadal subjects were 20 years older than the previous patients of abovementioned study. Only corrected Te and Te-RR slopes (resulted from Te/RR relation) were significantly reduced after the TRT, the first one at the peak and the second during whole recovery phase. The reduction of corrected Te was consistent with the possible reduction malignant arrhythmias risk. The decrease of the Te-RR slope indicates a reduction of steepness of the regression line obtained for these two variables. Given that, a high steepness represents a major risk of sudden death in heart failure [14, 30,31,32,33]; therefore, the observed decrease of Te-RR slope might hypothetically support a possible reduction of the SCD risk.
One important study limitation consists in the operator-dependent evaluation of single patient EKGs may be considered weak; indeed, in our prior experience, this single-operator in-deep analysis led us to identify any important variation related to myocardial dispersion and repolarization to better predict QT variations [41]. Another limitation is represented by the limited number of subjects studied; we acknowledge the great ethical difficulties to maintain any hypogonadal subject without TRT and also for this reason we did not enrol a placebo-controlled treated group that was not permitted by our Ethical Committee. We tried to overcome this bias by using a control group in whom no treatment for hypogonadism was indicated. Finally, we recognize that expected changes in symptoms and signs of hypogonadism as well as in hormonal and body composition patterns are not presented since they were not in the aim and scope of the present study; they had been already reported in previous studies [34, 61].
In conclusion, an increase of QT duration not heart-rate related in hypogonadal patients has been observed, but most of the dynamic markers of myocardial temporal dispersion of repolarization were not altered neither at rest or during the post-exercise recovery phase. Therefore, it is likely that hypogonadism per se does not increase the risk of malignant ventricular arrhythmias. Nevertheless, some subtle modifications in the repolarization phase either at rest (stable QT prolongation) or during autonomic nervous system unbalance (reduced QTp → Te coherence during the recovery phase), as well as the improvement of some ECG variables after TRT (Te, Te/RR slope) might support the idea of a leading tendency to ventricular arrhythmias in hypogonadal patients and, hence, it claims for a close control of possible other conditions able to further reduce the repolarization reserve. Hypogonadal patients are generally considered at increased risk of major adverse cardiovascular events and sudden cardiac death. When considering the results of our EKG study, it seems appropriate to treat severe hypogonadism with TRT independently of age and comorbidities, preceding it by a thorough cardiologic counselling to avoid possible ventricular adverse event (QT) related to testosterone action on the repolarization phase.
References
Isidori AM, Giannetta E, Lenzi A (2008) Male hypogonadism. Pituitary 11(2):171–180
Maggi M, Wu FC, Jones TH et al (2016) RHYME Investigators. Testosterone treatment is not associated with increased risk of adverse cardiovascular events: results from the Registry of Hypogonadism in Men (RHYME). Int J Clin Pract 70(10):843–852
Corona G, Maseroli E, Rastrelli G et al (2014) Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf 13(10):1327–1351
Narayanan K, Havmoeller R, Reinier K et al (2014) Sex hormone levels in patients with sudden cardiac arrest. Heart Rhythm 11(12):2267–2272
Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH (2007) The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol 156(5):595–602
Singh AB, Hsia S, Alaupovic P et al (2002) The effects of varying doses of testosterone on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab 87(1):136–143
Ng MK, Liu PY, Williams AJ et al (2002) Prospective study of effect of androgens on serum inflammatory markers in men. Arterioscler Thromb Vasc Biol 22(7):1136–1141
Nakhai-Pour HR, Grobbee DE, Emmelot-Vonk MH, Bots ML, Verhaar HJ, van der Schouw YT (2007) Oral testosterone supplementation and chronic low-grade inflammation in elderly men: a 26-week randomized, placebo-controlled trial. Am Heart J 154(6):1228.e1–1228.e7
Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH (2004) The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 89(7):3313–3318
Anker SD, Clark AL, Kemp M et al (1997) Tumor necrosis factor and steroid metabolism in chronic heart failure: possible relation to muscle wasting. J Am Coll Cardiol 30(4):997–1001
Frati P, Busardò FP, Cipolloni L, De Dominicis E, Fineschi V (2015) Anabolic Androgenic Steroid (AAS) related deaths: autoptic, histopathological and toxicological findings. Curr Neuropharmacol 13(1):146–159
Baumert M, Porta A, Vos MA et al (2016) QT interval variability in body surface ECG: measurement, physiological basis, and clinical value: position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace 18(6):925–944
Castro-Torres Y, Carmona-Puerta R, Katholi RE (2015) Ventricular repolarization markers for predicting malignant arrhythmias in clinical practice. World J Clin Cases 3(8):705–720
Sundaram S, Polito K, Kadish AH, Goldberger JJ (2008) Autonomic effects on QT-RR interval dynamics after exercise. Am J Physiol Heart Circ Physiol 294(1):H490–H497
Piccirillo G, Rossi P, Mitra M, Quaglione R et al (2013) Indexes of temporal myocardial repolarization dispersion and sudden cardiac death in heart failure: any difference? Ann Noninvasive Electrocardiol 18(2):130–139
Piccirillo G, Magrì D, Pappadà MA et al (2012) Autonomic nerve activity and the short-term variability of the Tpeak-Tend interval in dogs with pacing-induced heart failure. Heart Rhythm 9(12):2044–2050
Antzelevitch C (2007) Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm 4(7):964–972
Magrì D, Sciomer S, Fedele F et al (2008) Early impairment of myocardial function in young patients with beta-thalassemia major. Eur J Haematol 80(6):515–522
Magrì D, Piccirillo G, Bucci E et al (2012) Increased temporal dispersion of myocardial repolarization in myotonic dystrophy type 1: beyond the cardiac conduction system. Int J Cardiol 3:259–264
Magrì D, De Cecco CN, Piccirillo G et al (2014) Myocardial repolarization dispersion and late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Circ J 78(5):1216–1223
Magrì D, Santolamazza C, Limite L et al (2017) QT spatial dispersion and sudden cardiac death in hypertrophic cardiomyopathy: time for reappraisal. J Cardiol 70(4):310–315
Piccirillo G, Magrì D, Ogawa M et al (2009) Autonomic nervous system activity measured directly and QT interval variability in normal and pacing-induced tachycardia heart failure dogs. J Am Coll Cardiol 54(9):840–850
Wong JA, Gula LJ, Klein GJ, Yee R, Skanes AC, Krahn AD (2010) Utility of treadmill testing in identification and genotype prediction in long-QT syndrome. Circ Rhythm Electrophysiol 3(2):120–125
Aziz PF, Wieand TS, Ganley J et al (2011) Genotype- and mutation site-specific QT adaptation during exercise, recovery, and postural changes in children with long-QT syndrome. Circ Rhythm Electrophysiol 4(6):867–873
Piccirillo G, Moscucci F, Persi A et al (2014) Intra-QT spectral coherence as a possible noninvasive marker of sustained ventricular tachycardia. Biomed Res Int 2014:583035
Piccirillo G, Moscucci F, D’Alessandro G et al (2014) Myocardial repolarization dispersion and autonomic nerve activity in a canine experimental acute myocardial infarction model. Heart Rhythm 11(1):110–118
Piccirillo G, Ottaviani C, Fiorucci C, Petrocchi N et al (2016) Transcranial direct current stimulation improves the QT variability index and autonomic cardiac control in healthy subjects older than 60 years. Clin Interv Aging 11:1687–1695
Piccirillo G, Viola E, Bucca C et al (1999) QT interval dispersion and autonomic modulation in subjects with anxiety. J Lab Clin Med 133(5):461–468
Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF (1997) Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated Cardiomyopathy. Circulation 96(5):1557–1565
Piccirillo G, Germanò G, Quaglione R et al (2002) QT-interval variability and autonomic control in hypertensive subjects with left ventricular hypertrophy. Clin Sci (Lond) 102(3):363–371
Padfield GJ, Escudero CA, DeSouza AM et al (2016) Characterization of myocardial repolarization reserve in adolescent females with anorexia nervosa. Circulation 133(6):557–565
Ramírez J, Laguna P, Bayés de Luna A, Malik M, Pueyo E (2014) QT/RR and T-peak-to-end/RR curvatures and slopes in chronic heart failure: relation to sudden cardiac death. J Electrocardiol 47(6):842–848
Piccirillo G, Moscucci F, Pascucci M et al (2013) Influence of aging and chronic heart failure on temporal dispersion of myocardial repolarization. Clin Interv Aging 8:293–300
Aversa A, Bruzziches R, Francomano D, Spera G, Lenzi A (2010) Efficacy and safety of two different testosterone undecanoate formulations in hypogonadal men with metabolic syndrome. J Endocrinol Invest 33(11):776–783
van Noord C, Dorr M, Sturkenboom MC et al (2010) The association of serum testosterone levels and ventricular repolarization. Eur J Epidemiol 25:21–28
Pecori Giraldi F, Toja PM, Filippini B et al (2010) Increased prevalence of prolonged QT interval in males with primary or secondary hypogonadism: a pilot study. Int J Androl 22:e132–e138
Pecori Giraldi F, Manzoni G, Michailidis J, Scacchi M, Stramba-Badiale M, Cavagnini F (2011) High prevalence of prolonged QT interval in obese hypogonadal males. Obesity 19:2015–2018
Pecori Giraldi F, Toja PM, Michailidis G et al (2011) High prevalence of prolonged QT duration in male patients with Cushing’s disease. Exp Clin Endocrinol Diabetes 119:221–224
Salvi V, Karnad DR, Panicker GK, Kothari S (2010) Update on the evaluation of a new drug for effects on cardiac repolarization in humans: issues in early drug development. Br J Pharmacol 159(1):34–48
Vandenberk B, Vandael E, Robyns T et al (2016) Which QT correction formulae to use for QT monitoring? J Am Heart Assoc 5:e003264
Puddu PE, Jouve R, Mariotti S et al (1988) Evaluation of 10 QT prediction formulas in 881 middle-aged men from the seven countries study: emphasis on the cubic root Fridericia’s equation. J Electrocardiol 21:219–229
Shimizu W, Matsuo K, Kokubo Y et al (2007) Sex hormone and gender difference–role of testosterone on male predominance in Brugada syndrome. J Cardiovasc Electrophysiol 18(4):415–421
Simonds SE, Pryor JT, Cowley MA (2017) Does leptin cause an increase in blood pressure in animals and humans? Curr Opin Nephrol Hypertens 26(1):20–25
Morgentaler A et al (2016) Fundamental concepts regarding testosterone deficiency and treatment. Mayo Clin Proc 91(7):881–896
Yang P-C, Kurokawa J, Furukawa T, Clancy CE (2010) Acute effects of sex steroid hormones on susceptibility to cardiac arrhythmias: a simulation study. PLoS Comput Biol 6(1):e1000658
Odening KO, Koren G (2014) How do sex hormones modify arrhythmogenesis in long-QT syndrome?—Sex hormone effects on arrhythmogenic substrate and triggered activity. Heart Rhythm 11(11):2107–2115
Alexandre J, Milliez P, Rouet R et al (2015) Aldosterone and testosterone: two steroid hormones structurally related but with opposite electrophysiological properties during myocardial ischemia-reperfusion. Fundam Clin Pharmacol 29(4):341–351
Salem JE, Alexandre J, Bachelot A, Funck-Brentano C (2016) Influence of steroid hormones on ventricular repolarization. Pharmacol Ther 167:38–47
Kurokawa J, Kodama M, Clancy CE, Furukawa T (2016) Sex hormonal regulation of cardiac ion channels in drug-induced QT syndromes. Pharmacol Ther 168:23–28
Roden DM (2006) Long QT syndrome: reduced repolarization reserve and genetic link. J Intern Med 259:59–69
Roden DM, Abraham RL (2011) Refining repolarization reserve. Heart Rhythm 8(11):1756–1757
Jost N, Virág L, Comtois P et al (2013) Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs. J Physiol 591(17):4189–4206
Xia Y, Liang Y, Kongstad O, Liao Q, Holm M, Olsson B, Yuan S (2005) In vivo validation of the coincidence of the peak and end of the T wave with full repolarization of the epicardium and endocardium in swine. Heart Rhythm 2(2):162–169
Xia Y, Liang Y, Kongstad O, Holm M, Olsson B, Yuan S (2005) Tpeak-tend interval as an index of global dispersion of ventricular repolarization: evaluations using monophasic action potential mapping of the epi- and endocardium in swine. J Intervent Card Electrophysiol 14(2):79–87
Opthof T, Coronel R, Wilms-Schopman FJG et al (2007) Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm 2:341–348
Antzelevitch C, Shimizu W, Yan G-X et al (1999) The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol 10(8):1124–1152
Aiba T, Tomaselli GF (2010) Electrical remodeling in the failing heart. Curr Opin Cardiol 25(1):29–36
Charbit B, Christin-Maître S, Démolis JL, Soustre E, Young J, Funck-Brentano C (2009) Effects of testosterone on ventricular repolarization in hypogonadic men. Am J Cardiol 103(6):887–890
Narayan SM, Lindsay BD, Smith JM (1999) Demonstration of the proarrhythmic preconditioning of single premature extrastimuli by use of the magnitude, phase, and distribution of repolarization alternans. Circulation 108(18):1887–1893
La Fountaine MF, Wecht JM, Cirnigliaro CM, Kirshblum SC, Spungen AM, Bauman WA (2013) Testosterone replacement therapy improves QTpVI in hypogonadal men with spinal cord injury. Neuroendocrinology 97(4):341–346
Aversa A, Bruzziches R, Francomano D et al (2010) Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 7:3495–3503
Funding
Funded by University “La Sapienza”; ClinicalTrials.gov number, NCT03126656. This study was supported and funded by University of Rome “Sapienza” and registered in ClinicalTrials.gov number NCT03126656.
Author information
Authors and Affiliations
Contributions
Gianfranco Piccirillo, MD, PhD: project leader, statistical analysis, manuscript writer; Federica Moscucci, MD, data collection, manuscript writer; Riccardo Pofi, MD, data collection, manuscript reviewer; Gaetana D’Alessandro, MD, data collection, manuscript reviewer; Marianna Minnetti, MD, data collection, manuscript reviewer; Andrea M. Isidori, MD, project leader, manuscript reviewer; Davide Francomano, MD, data collection; Andrea Lenzi, MD, project leader, manuscript reviewer; Paolo E. Puddu, MD, PhD, project leader, manuscript reviewed; Joachim Alexandre, MD, PhD, manuscript reviewer; Damiano Magrì, MD, PhD, manuscript reviewer; Antonio Aversa, MD, project leader, manuscript draft and comments to the reviewers.
Corresponding author
Ethics declarations
Conflict of interest
There are neither competing conflicts of interest in connection with the submitted article, nor industry relationship; so authors deny any competing financial interest related to this study.
Ethical approval
All the participants were cardiologic therapy free and did not change basal therapies during the protocol, provided written informed consent to participate and to publish data. Written informed consent was obtained for publication. The bioethical committee of Policlinico Umberto I, Rome, Italy, approved the study. The research was carried out according to the principles of the Declaration of Helsinki, in accordance with the relevant guidelines and ethical regulations in research involving human participants. Data and materials are stored in personal “Sapienza” database, following the current privacy policy.
Informed consent
Written informed consent was obtained for publication. The bioethical committee of Policlinico Umberto I, Rome, Italy, approved the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Piccirillo, G., Moscucci, F., Pofi, R. et al. Changes in left ventricular repolarization after short-term testosterone replacement therapy in hypogonadal males. J Endocrinol Invest 42, 1051–1065 (2019). https://doi.org/10.1007/s40618-019-01026-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01026-5