Abstract
In a clinical practice of the angiography, the blood vessel analysis is substantially important mainly in a sense of an objectification and modeling of the pathological spots such as the blood vessel calcifications. An amount of the calcification is commonly just estimated by naked eyes; therefore, the automatic modeling may be beneficial in a context of an extraction of the blood vessel features well representing a level of the blood vessel deterioration. In this work, we have proposed a fully automatic software environment (BloodVessCalc) for processing the blood vessel images acquired by the CT (computer tomography). The main function of the SW is the multiregional image segmentation allowing for an extraction of the physiological blood vessel location from the calcification spots. This model offers the calcium score calculation in a form of amount of the calcification. In the last part of our analysis, the predictive intervals of the average value and median for calcium score are calculated.
Similar content being viewed by others
1 Introduction
This journal paper is an extended version of conference paper entitled Segmentation of vascular calcifications and statistical analysis of calcium score. This paper significantly extends analysis and modeling of the blood vessel calcification with the goal automatic computing the calcium score, as one of the reliable predictors of blood vessel impairment [1].
Ischemic diseases such as ischemic heart disease (ICHS), chronical peripheral arterial disease (ICHDK), peripheral vascular disease, and ischemic stroke (CMP) are one of the main causes of the adult population morbidity and death rate across the world. All these diseases create themselves on a base of the atherosclerosis. It is a long-term process which can go without greater clinical signs by many years. This process causes a blood vessel deterioration by storing the fat in the blood and calcium salt. These sclerotic plaques narrow the vascular lumen, restrict blood flow, and can be basis for thrombosis. On a base of the aforementioned facts, it is obvious that early diagnosis and prediction of ischemic diseases are necessary for the clinical practice [2,3,4,5,6].
The first mutual sign of the ischemic diseases is an occurrence of the calcium plaques in a blood stream. Their amount can be found and assessed by computer tomography (CT). On a base of the CT data, it is needed to determine the calcium score (CS). This parameter allows for assessing of the calcium plaques occurrence in coronal arteries. One expression of CS is related to a density of the calcifications in given artery calculated in the Hounsfield units (HU) multiplied by the size of given area (mm2). Implicitly is calculated with only pixels having the HU bigger than 130, creating area ≥ 1 mm2. CS values are from the range: 0–400, and express deterioration level of calcium plaques, and probability of coronal arteries deterioration (Table 1) [7,8,9,10].
This clinical examination was stated by Artur Agatson; therefore, it is entitled as the Agatson calcium score [3]. In [4] published in 2008, a group of the authors verified an efficiency of the calcium score prediction on a sample of 6722 patients through characteristic and ethnical groups, and they proved the calcium score gives a relevant predictive information about a possible occurrence of the ICHS. Beside it, there are a lot of other studies dealing with the calcium score as a good response for ICHS. For instance in [7, 11], the calcium score was calculated by the electron beam CT and 64 multi-detector CT (MDCT) is compared.
An assessment of the CMP prediction is published in [12] dealing with the Essen Stroke Risk Score (ESRS). ESRS evaluates an occurrence of the CMP on a base of the patient’s age, arterial hypertension, diabetes mellitus, and smoking. In [13], they described a relationship between levels of the calcification occurrence. They sought 4814 patients during 8 years, and they proved that patients have undergone the CMP exhibited the calcium score initial values bigger than 100. There are other publications describing the ESRS which modify the calcium score by adding input clinical variables and parameters, as in [14].
2 State of the art: clinical strategies
Blood vessel calcifications and its treatment procedures are linked to the management of the discovered bone and mineral metabolism. In the clinical practice, there are many potential therapy procedures that directly focus to the calcification process [2, 3, 15]. Regarding efficacy and complete safety, two important issues can affect respective therapeutic procedure of the blood vessel calcification. First, we have to consider whether a treatment is preventive or it can serve for reverse calcifications. Second, can blood vessel calcification be treated without adversely affecting calcification in normal sites, such as bone and teeth? [8, 16]. The objectification and quantification of the blood vessel calcification is clinically complicated due to lack of reliable methods to quantify it. Furthermore, the sensitivity and precision of commonly used imaging modalities that are clinically used are relatively poor especially in the case of early calcifications. Lastly, none of the methods can reliably distinguish between atherosclerotic and medial calcification and, therefore, measure the combined changes in two different pathophysiological processes [4, 7]. On the base on the above stated reasons, the mathematical models which are able to autonomously detect and quantify of an amount of the blood vessel calcification are substantially important for the clinical practice.
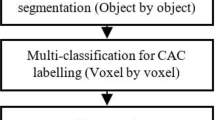
3 Multilevel thresholding algorithm for vascular calcifications
Segmentation using only one thresholding is not appropriate for blood vessel segmentation due to the fact that image data often contain image noise which cannot be properly classified. We use optimized Otsu method utilizing of multi-thresholding increasing sensitivity of blood vessel segmentation and extraction of vascular calcifications [1, 17, 18].
The core of the method is finding a specific intensity level on a base of the histogram distribution into evenly large areas. Specific thresholding level is used for each area. The analyzed image is consequently segmented according to the thresholding levels. Pixels having different shade levels are labeled by parameter L from the interval: \( \left[ {0, 1, 2, \ldots ,L} \right] \). Number of levels is indicated by p. A size of respective segmented area is given by the following equation:
The between class variance σ2 is calculated similarly as in the Otsu method:
Parameter W represents the weight, and average intensity is represented by µ. The number of separated histogram image regions is identical to a number of the thresholding levels p. Optimal number of the thresholding levels is calculated as:
It is necessary to a number of pixels in different shades of gray L would be equal to 256 × j. Parameter p must belong to the range: \( \left[ {2 \times j,4 \times j,8 \times j} \right] \), where j belongs to the range: \( \left[ {1,2, \ldots ,\infty } \right] \). The overall structure of the multilevel segmentation approach is depicted in Fig. 1 [19, 20].
4 Modeling of vascular calcifications
The algorithm was tested on the sample of the 90 patient’s records including the calcification plaques. Data were acquired from the CT angiography from the Hospital in Trinec. Example of analyzed records is depicted in Fig. 2.
In common physiological situation, the blood vessel system is represented by shade intensity without more significant intensity changes. The presence of the calcifications is represented by white color. Calcification changes are obviously observable by naked eye. In a case of the CT angiography, the calcification areas are usually represented by bright white color spectrum, while the physiological blood vessels are imagined in gray. Nevertheless, an important issue is a quantification of the calcification area in a form of the calcium score. We approached to extract of this parameter on a base of the mathematical model separating the physiological area of respective blood vessel and the vascular calcification (Fig. 3). The vascular model consequently allows for computing respective blood vessel area and calcification area (Fig. 4). As it is stated in the previous text, the whole image area does not have to be processed, but only smaller part of the image may represent region of the interest (Fig. 5). In this situation, the spatial image area is expanded to maximize the local blood vessel features and investigate a particular spot of the blood vessel.
5 Application for clinical assessment of blood vessel calcifications
In our work, the clinical SW application (BloodVessCalc) has been proposed. This application offers various tools for an assessment and visualization of the blood vessels affected by the calcification. An important issue of the clinical imaging of the blood vessels from the CT is a fact that various CT devices may generate the image data having different spatial features (contrast, resolution, etc.). It predetermines a fact that a result of the blood vessel modeling can be significantly affected by a lower image quality. In this context, the image pre-processing is important to achieve better image features. The BloodVessCalc allows for both basic editing operations with the native CT records and image transformation enhancing the image features. In the following text, the basic functions of the BloodVessCalc are present.
5.1 CT data editing
CT blood vessel data can be either loaded in various win forms or directly in the DICOM format. A great advantage of the SW application is the multiple image manipulation. User is offered either loading the single image or an image series. All the SW operations are intended for the multiple image processing. It brings a benefit for unified processing of the entire patient’s image series.
5.2 RoI and interpolation
User may work either with the whole image area or the image part in a form of the RoI. RoI specification brings benefit to expand the image spatial area. This operation is important especially in cases where we are focused on a tiny calcification spot which is baldy observable from the whole image record. Since RoI is linked with a phenomenon of a lower number pixels expanded to a bigger area having a consequence of worse image contrast, the image linear interpolation is employed. User may also additionally specify an order of the linear interpolation. A higher order ensures a better image quality; nevertheless, it significantly increases the time complexity. The SW application offers the following RoI shapes:
-
Free hand.
-
Polygon.
-
Rectangle.
-
Ellipse.
-
Circle.
An example of the RoI application is depicted on the Fig. 6.
5.3 Image pre-processing
To enhance the spatial features, the image pre-processing is employed. An important SW tool is the image brightness and contrast transformation. This process is done by a fully automatic way via respective sliders allowing for a smooth adjustment of the brightness scale of the native CT images. In this regard, the contrast transformation is substantially important, by this operation, objects having lower contrast are suppressed, on the other hand by the contrast oversaturation; the image noise might be boosted leading to the image deterioration. The second important tool of the CT image pre-processing is the morphological operations. For this task, the image binarization is employed (Fig. 7). Image binarization allows for suppressing adjacent structures to keep the blood vessel structure by adjustable way via a slider.
In some cases, the blood vessel boundaries (image edges) may be imagined under a weaker contrast, or even missing. This unfavorable fact may lead to worse segmentation effectivity significantly deteriorating the resulting calcium score. This issue may be solved by the edge sharpening intuitively adjusting the blood vessel edges via a slider (Fig. 8).
6 Verification of blood vessel modeling
In this section, the multilevel blood vessel modeling is verified. Verification is done in two procedures. First, we have compared our model with the gold standard defined by the clinical experts. Second, a set of verification metrics have been adopted to compare our model with state-of-the-art segmentation methods. To make verification, the gold standard should be defined. In a field of the clinical blood vessel imaging, the manual expert segmentation is perceived as gold standard (Fig. 9). To prevent the subjective errors, the manual segmentation has been done three times by each clinical expert. Each of manual segmentation had been done by three clinical experts, and their results were averaged. The result was consequently averaged. As a representative blood vessel feature, size of the segmented blood vessel is taken.
In the verification procedure, a difference between the multilevel and manual segmentation is evaluated. We have tested 50 CT blood vessel images. On a base of the testing, the average difference 6.5% representing 21 pixels is achieved. Table 2 points out to an extract of the verification procedure.
In the second step of the verification procedure, a comparison of the blood vessel model with state-of-the-art models is done on the base of the three criterions. As alternative methods we have used:
-
Fuzzy C-means (FCM) performs segmentation using clustering.
-
The iterative thresholding segmentation (ITS).
-
Region growing (RE) performs regional segmentation.
The following scalar measures are considered for the retinal image quantitative comparison:
Rand index (RI): it measures a similarity between two data clusters. RI compares assignments between pairs of elements in two clusters on a base of a calculation of a fraction of the correctly classified elements against all the elements. RI definition for C1 and C2 clusters is following:
where N denotes a total number of the points, n11 denotes a number of the pairs that they are in the same cluster in C1 and C2 and n00 is a number of the pairs belonging to different clusters. The RI gives results in a range [0;1]. 0 indicates that the data clusters do not agree on any pair of the points, contrarily 1 indicates that the data clusters are completely same [21].
Variation of information (VI): it is a metric which measures distance between two segmentation results on the base of the average conditional entropy. VI is given by:
Mean squared error (MSE): it is an estimator measuring the average of the error squares between two segmentation results. The MSE represents a risk function which corresponds with the expected value of the squared or quadratic error loss [22].
The RI and VI measures in a sense greater are better, while lower values of the MSE indicates better results. The best result for each measurement is highlighted. Tables 3, 4, and 5 indicate the best results of RI, VI, and MSE for individual segmentation methods for each clinical expert.
We have performed an objective comparison of the proposed method for the blood vessel calcification segmentation against three conventional segmentation methods (FCM, ITS, and RE). The comparison evaluation is done on the base of three clinical expert’s manual segmentations. Results are reported in Tables 3, 4, and 5. Based on the results, the calcification model achieved best results on at least two evaluated parameters. This objective evaluation serves as an effective feedback representing effectivity of the segmentation procedure. On the other hand, we are aware that manual segmentation might be affected by the subjective error depending on the experience of the respective clinical expert. Furthermore, we have to take advantage the image noise influence may influence the pixels distribution.
7 Statistical evaluation of calcium score
From a view of the clinical practice, there is an absence of the clinical instrument automatically calculating calcium score from the native CT images, thus it would perform diagnosis of the blood vessel deterioration level by the calcification. The multilevel segmentation algorithm separates individual areas of the blood system into individual classes reflecting a state whether in the particular area it is calcification, or not. On a base of this model, we can calculate the calcification score:
where Rc corresponds with an area of the calcification, and Ro corresponds with the overall blood vessel area.
7.1 Testing of statistical significance of calcium score
Calcium score has greater ambitions to be used in a field of clinical radiology and angiography. Physician performs the diagnosis, usually, on a base of the subjective opinion and own experience. For one angiographic examination, MR commonly generates 150–200 patient records of region of interest. On a base of the clinical evaluation of three clinical experts, patients are clinically classified into three groups:
-
Calcified blood vessels (CBV)—blood vessels are completely calcified.
-
Partially calcified blood vessels (PCBV)—blood vessels are partially deteriorated by calcification.
-
Early calcification (EC)—blood vessels are not visually impaired but there is a predisposition of calcification.
Descriptive statistics of all the patient groups are summarized in Table 6. Calcification score was calculated on a base of the 90 patients. 30 patients were used for every group (nCBV = 30, nPCBV = 30 and nEC = 30).
On a base of the observation, it is evident that median and average values are significantly different for individual groups. On a base of the physician opinions, expected values of the calcification score should belong to the following intervals:
-
CBV: < 85 to 100 > [%].
-
PCBV: < 35 to 60 > [%].
-
EC: < 10 to 30 > [%].
7.1.1 Robust interval estimation of average value
For each type of the calcification deterioration (CBV, PCBV, EC), there are subjectively defined intervals by physicians where outputs of the CS calculated by the multilevel segmentation are expected. These subjectively defined intervals should be verified on a base of the median which is robust against outlying observations. The interval estimation of the median is proved on a base of the Gastwirth median estimation. Interval median estimation with reliability 95% is estimated from the interquartile range:
where xp represent 100p% selection quartiles.
7.1.2 Median estimation for calcified blood vessels (CBV)
Defining of parameters for median estimation is summarized in Table 7.
Median estimation for the calcified blood vessels is calculated:
7.1.3 Median estimation for partially calcified blood vessels (PCBV)
Defining of parameters for median estimation is summarized in Table 8.
Median estimation for partially calcified blood vessels is calculated:
7.1.4 Median estimation for early calcification (EC)
Defining of parameters for median estimation is summarized in Table 9.
Median estimation for partially early calcification is calculated:
7.1.5 Gastwirth median estimation
Gastwirth median also belongs among of robust average value estimation. This estimation is determined by the sample median, lower (\( x_{0.33} \)) and upper (\( x_{0.67} \)) decile. Gastwirth median estimation is given by the expression:
where
7.1.6 Gastwirth median estimation for calcified blood vessels (CBV)
Defining of parameters for the Gastwirth median estimation is summarized in Table 10.
Gastwirth median estimation for calcified blood vessels is calculated:
7.1.7 Gastwirth median estimation for partially calcification (PCBV)
Defining of parameters for the Gastwirth median estimation is summarized in Table 11.
Gastwirth median estimation for partially calcified blood vessels is calculated:
7.1.8 Gastwirth median estimation for early calcification (EC)
Defining of parameters for the Gastwirth median estimation is summarized in Table 12.
Gastwirth median estimation for the early calcification is calculated:
The following charts (Tables 13, 14) summarize median interval estimation for the calcium score of three tested groups of patients (CBV, PCBV, EC).
The important fact from a view of the calcium score is a confidence interval of the median for the individual analyzed groups of patients. In this context it is important, so that these interval estimations would correspond with estimated range of values proposed by physicians from clinical practice. The individual interval estimations lay inside of expected intervals of the CS. Interesting fact is a comparison of individual interval lengths. Calcified blood vessels have the narrowest interval of calcium score. The next important fact is also comparison between length of the estimated intervals and the average value estimation. These parameters are directly proportional. Gastwirth median exhibits minimal differences in a comparison with the median estimation. In a case of the CBV group, the Gastwirth median is identical to median estimation; in the other cases, observed differences are not perceived as statistically significant.
8 Discussion
The blood vessel calcification is absolutely important issue for a clinical diagnosis of blood vessel systems. The calcifications and other blood vessel diseases affecting the blood vessel permeability are commonly assessed by medical imaging modalities, such as the CT angiography. CT is capable to provide good images where physiological areas of the blood vessels are distinguished from the calcifications under great contrast. Nevertheless, commonly used clinical SW allows for only imaging of the blood vessel system and some basic image adjustment such as contrast or brightness transformations. Thus, such systems lack of procedures which would allow for autonomous modeling and objectification of the calcification amount which would predict further disease development.
Multiregional segmentation seems to be promising direction of the blood vessel modeling in a context of the blood vessel calcification. The proposed regional segmentation model is capable of distinguish physiological blood vessel area from areas of calcification spots. On the base of this procedure, we obtain a model reflecting whole blood vessel and second model reflecting only the calcifications. This blood vessel feature selection procedure serves as a solid basis for extraction parameters for objectification of the calcification amount.
An important aspect of the blood vessel modeling is data pre-processing. Since the input CT image records are sometimes deteriorated by noise, or artifacts. Such additive image signals are often linked either with human’s body or adjacent technical resources. Such signals influence native image information. Especially blood vessel boundaries may be impaired or completely missing. Therefore, it is beneficial to consider image pre-processing which would improve image features. In our clinical application (BloodVessCalc), we have employed several functions for image adjustment. Those functions are well usable in cases where image data have a lower resolution. Thus, objects of interest are badly observable and detectable. On the other hand, we have to realize that each image pre-processing procedure has a certain impact on image structure and original clinical information is modified.
The proposed segmentation model of the blood vessels allows for features extraction which reflects real state of the calcifications and their future prediction. Since physicians in the clinical practice are focused on a permeability level of the respective blood vessels, we have selected features quantifying an amount of the calcification. In this regard, we are focused on area of the calcifications. Eventually, we have defined a calcification score indicating a proportion of the calcification in the respective blood vessel. By this parameter, prediction and tracking of the blood vessel calcification can be carried out. Nevertheless, we have done an analysis of spatial features representing a cross section of the blood vessels. In this regard, we estimate an amount of the calcifications in one or more blood vessel slices. This estimation is correct when we assume that the blood vessels are completely symmetrical and do not have any significant geometric variations. In the future time, we are going to focus on volumetric modeling when the segmentation model would be simultaneously applied to individual CT slices to make a 3D model. Such 3D model performs volumetric measurement and the volumetric calcification amount.
9 Conclusion
We proposed a method performing the blood vessel system modeling, consequently allowing for a calculation of the calcium score. The model is generated on a base of the multilevel thresholding method separating the physiological and calcification part of a respective blood vessel. Furthermore, the proposed model utilizes the color mapping of originally monochromatic areas where the blood vessels are clearly recognizable from the calcification plaques. In the context of the testing, patients were divided into three groups (CBV, PCBV, EC) according to the clinical expert’s opinion. For prediction of method utilization in the clinical practice, it is important distribution of calcium score for the individual groups regards to expected intervals given by physicians. Across of the groups, there are not outlying values. Interval estimations shall demonstrate a reliability of the CS measurement.
References
Kubicek, J., Bryjova, I., Valosek, J., Penhaker, M., Augustynek, M., Cerny, M., Kasik, V.: Segmentation of vascular calcifications and statistical analysis of calcium score. In: Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics), 10192 LNAI, pp. 455–464 (2017)
Wilson, P.W.F., D’agostino, R.B., Levy, D., Belanger, A.M., Silbershatz, H., Kannel, W.B.: Prediction of coronary heart disease using risk factor categories. Circulation 97(18), 1837–1847 (1998). https://doi.org/10.1161/01.CIR.97.18.1837. (ISSN 0009-7322)
Agatston, A.S., Warren, R., Janowitz, F., Hildner, J., Zusmer, N.R., Viamonte, M., Detrano, R.: Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. [online] 15(4), 827 (1990). https://doi.org/10.1016/0735-1097(90)90282-t. (ISSN 07351097)
Detrano, R., Guerci, J., Jeffrey, C., et al.: Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. [online] 358(13), 1336–1345 (2008). https://doi.org/10.1056/NEJMoa072100. (ISSN 0028-4793)
Meershoek, A., van Dijk, R.A., Verhage, S., Hamming, J.F., van den Bogaerdt, A.J., Bogers, A.J.J.C., Schaapherder, A.F., Lindeman, J.H.: Histological evaluation disqualifies IMT and calcification scores as surrogates for grading coronary and aortic atherosclerosis. Int. J. Cardiol. 224, 328–334 (2016)
Lee, W.-C., Fang, H.-Y., Wu, C.-J.: Coronary artery perforation and acute scaffold thrombosis after bioresorbable vascular scaffold implantation for a calcified lesion. Int. J. Cardiol. 222, 620–621 (2016)
Carr, J., Jeffrey, J., Nelson, C., Wong, N.D., et al.: Calcified coronary artery plaque measurement with cardiac ct in population-based studies: standardized protocol of multi-ethnic study of atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) Study 1. Radiology [online] 234(1), 35–43 (2005). https://doi.org/10.1148/radiol.2341040439. (ISSN 0033-8419)
Huang, C.L., Wu, I.H., Wu, Y.W.: Association of lower extremity arterial calcification with amputation and mortality in patients with symptomatic peripheral artery disease. PLoS One. (2014). https://doi.org/10.1371/journal.pone.0090201
Burgers, L.T., Redekop, W.K., Al, M.J., Lhachimi, S.K., Armstrong, N., Walker, S., Rothery, C., Westwood, M., Severens, J.L.: Cost-effectiveness analysis of new generation coronary CT scanners for difficult-to-image patients. Eur. J. Health Econ. 1–12 (2016)
Penhaker, M., Stula, T., Cerny, M.: Automatic ranking of eye movement in electrooculographic records, pp. 456–460 (2010)
Mao, S., Raveen, S., Pal, S., Mckay, Ch., et al.: Comparison of coronary artery calcium scores between electron beam computed tomography and 64-multidetector computed tomographic scanner. J. Comput. Assist. Tomogr. [online]. 33(2), 175–178 (2009). https://doi.org/10.1097/RCT.0b013e31817579ee. (ISSN 0363-8715)
Weimar, C., Diener, H.-C., Alberts, M.J., Steg, P.G., Bhatt, D.L., Wilson, P.W.F., Mas, J.-L., Rother, R.: The Essen stroke risk score predicts recurrent cardiovascular events: a validation within the REduction of Atherothrombosis for Continued Health (REACH) Registry. Stroke [online]. 40(2), 350–354 (2009). https://doi.org/10.1161/strokeaha.108.521419. (ISSN 0039-2499)
Hermann, D.M., Gronewold, J., Lehmann, N., Moebus, S., Jockel, K.-H.M., Bauer, K.-H., Erbel, R.: Coronary artery calcification is an independent stroke predictor in the general population. Stroke [online]. 44(4), 1008–1013 (2013). https://doi.org/10.1161/STROKEAHA.111.678078. (ISSN 0039-2499)
Sumi, S., Hideki, O., Kiyohiro, H., et al.: A modified Essen stroke risk score for predicting recurrent cardiovascular events: development and validation. Int. J. Stroke [online]. 8(4), 251–257 (2013). https://doi.org/10.1111/j.1747-4949.2012.00841.x. (ISSN 17474930)
Cook, N.R., Paynter, N.P., Eaton, C.B., et al.: Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the multiethnic women’s health initiative. Circulation [online] 125(14), 1748–1756 (2012). https://doi.org/10.1161/CIRCULATIONAHA.111.075929. (ISSN 0009-7322)
Van Gils, M.J., Bodde, M.C., Cremers, L.G.M., Dippel, D.W.J., Van Der Lugt, D.W.J.: Determinants of calcification growth in atherosclerotic carotid arteries; a serial multi-detector CT angiography study. Atherosclerosis [online] 227(1), 95–99 (2013). https://doi.org/10.1016/j.atherosclerosis.2012.12.017
Kubicek, J., Penhaker, M., Bryjova, I., Augustynek, M.: Classification method for macular lesions using fuzzy thresholding method. In: Kyriacou, E., Christofides, S., and Pattichis, C.S. (eds.) Xiv Mediterranean Conference on Medical and Biological Engineering and Computing 2016, pp. 239–244 (2016)
Peterek, T., Krohova, J., Smondrk, M., Penhaker, M.: Principal component analysis and fuzzy clustering of SA HRV during the orthostatic challenge, pp. 596–599 (2012)
Kubicek, J., Valosek, J., Penhaker, M., Bryjova, I., Grepl, J.: Extraction of blood vessels using multilevel thresholding with color coding. Lect. Notes Electr. Eng. 362, 397–406 (2016)
Kubicek, J., Valosek, J., Penhaker, M., Bryjova, I.: Extraction of chondromalacia knee cartilage using multi slice thresholding method. In: Lecture Notes of the Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering, LNICST, vol. 165, pp. 395–403 (2016)
Rand, W.M.: Objective criteria for the evaluation of clustering methods. J. Am. Stat. Assoc. 66, 846–850 (1971)
Al-Najjar, Y.A.Y., Soong, D.C.: Comparison of image quality assessment: PSNR, HVS, SSIM, UIQI. Int. J. Sci. Eng. Res. 3(8), 1 (2012)
Acknowledgements
This article has been supported by financial support of TA ČR, PRE SEED Fund of VSB-Technical university of Ostrava/TG01010137. The work and the contributions were supported by the project SV4506631/2101 ‘Biomedicínské inženýrské systémy XII’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kubicek, J., Bryjova, I., Valosek, J. et al. Modeling and objectification of blood vessel calcification with using of multiregional segmentation. Vietnam J Comput Sci 5, 279–289 (2018). https://doi.org/10.1007/s40595-018-0122-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40595-018-0122-z