Abstract
Purpose of Review
Zeolite is an aluminosilicate compound having a wide spectrum of applications in medicine and dentistry. Several articles were published combining zeolites with various other elements for different applications in dentistry. This review aims to provide a detailed review on the origin of zeolites, their physical and chemical properties and possible applications as dental materials.
Recent Findings
Zeolite-based hybrid films can be used for detection of oral cancers. Silver zeolite can be added in restorative materials and dental liners. In cases of root canal irrigation, chlorhexidine zeolite is used owing to its antibacterial properties. For dental implants, a zeolite coating can improve the osseointegration.
Summary
Due to its microporous structure, application-driven zeolitic frameworks can be prepared by sieving in various cations and antibacterial compounds. This review helps improve our understanding regarding the uses of zeolites as a material in different aspects of dentistry along with possible further improvements as a dental material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zeolites, aluminosilicates with a tetrahedral crystal structure, have a wide application range varying from ecology to dentistry [1, 2]. Zeolites are particularly useful in these areas because of their distinct porous structure, which provides negatively charged channels and cavities capable of accommodating cations, hydroxyl groups, and water molecules [1]. Recently, the capacity of zeolite to collect and release ions, paired with its exceptional biocompatibility and long-lasting effectiveness, has increased the interest of dental researchers [2]. Several studies in dentistry have sought to combine zeolites with inorganic antibacterial ions such as silver and zinc for sustained release, in addition to providing zeolites alone to materials [2].
Ion-embedded zeolites act as potential antibacterial agents against pathogenic oral microbes. When ions released from zeolites encounter certain oral microbes, they can interfere with their metabolic activity by inactivating key enzymes, disrupting RNA replication, and blocking microbial respiration [3]. Therefore, cations incorporated zeolites can potentially inhibit oral bacterial growth when applied to dental materials.
When zeolites are used in dental materials for its antimicrobial qualities, it is vital to evaluate how they affect the material’s mechanical characteristics. Daily actions like chewing and speaking place stresses on the materials used to make tooth and dentures. As a result, the ability of the materials used to bear these stresses without losing their strength is critical for the efficacy of dental operations.
The present literature review concentrates on the origin and basic physical and chemical structure of zeolites highlighting its use for various aspects in dentistry.
Materials and Methods
A systematic search in PubMed, Ebscohost and Google Scholar with keywords “zeolite, dentin regeneration, antibacterial properties, and dentistry” was performed. All articles from 1980 to the present were searched for title-related data. Only articles in English language were considered for the review.
Results
In total 48 articles were selected. Zeolites are widely applied in various fields and are also attracting great attention in the dental field. Due to its antibacterial and regenerative properties, it is mainly used in dentistry [4••]. Zeolites are used in regenerative dentistry, root canal therapy, prosthetics, restorative dentistry, oral medicine, and implants, etc., as described in more detail below.
Origin of Zeolite
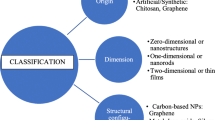
Zeolites are naturally formed by volcanic activity. In a volcanic eruption, magma along with various gases, dust particles, and thick ash breaks up the earth’s crust to form lava. Such incidents are caused by the convergence and divergence of tectonic plates. If such places are near water, lava often flows into seas and oceans. After contact with the sea, it undergoes a series of reactions with salt and water over thousands of years to produce crystalline structures called zeolites [5,6,7]. The word zeolite comes from two Greek words “zeo” = coke and “lithos” = stone, meaning boiling stone [8]. It was coined in 1756 by Swedish mineralogist Axel Fredrik Kronstaedt.
Chemically, zeolites are aluminosilicate agents with pore sizes between 3 and 10 Å. These pores lead to ion exchange between cations such as Ag and Zn, endowing zeolites with antibacterial activity [9, 10]. Main composition of zeolites is aluminum, silicon, oxygen, and phosphorus [11]. There are two types of zeolites, natural and synthetic, which have a wide range of applications in medicine. Zeolites are also used in dentistry for their antimicrobial properties and biocompatibility.
Chemical Composition
The basic structure of zeolites consists of a framework of aluminosilicate consisting of a tetrahedral array of cations such as Silicon (Si4 +) and Aluminum (Al3 +) surrounded by four oxygen anions (O2-). Each oxygen ion binds two cations within the Si–O and Al-O bonds shared by the two tetrahedra, forming the tetrahedral building blocks of the SiO2 and AlO2 three-dimensional polymeric frameworks (ratio of 1:2). Each tetrahedron is composed of four oxygen atoms surrounding a silicon atom and an aluminum atom [12]. Some silicon ions are replaced by aluminum ions, leaving the tectosilicate structure with a net negative charge. These negatively charged sites are primarily alkali or alkaline earth metal counter ions such as Na + , K + , or Ca2 + , as well as Li + , Mg2 + , Sr2 + , and Ba2 + [11]. These ions are bound to the aluminosilicate structure by weaker electrostatic bonds found on the outer surface of the zeolite [13, 14].
Structure of Zeolites
Zeolites are very difficult to classify because they cannot be defined simply as a family of crystalline solids [15]. In 1997, a Mineralogical Association subcommittee, the Commission on New Minerals and Mineral Names, found intrinsic zeolites with topologically identical structures independent of the Si and Al composition of the tetrahedral layers which was later classified as Zeolites [8, 16]. In a subsequent revision Zeolitic minerals described as crystalline substances with a structure characterized by a linked tetrahedral framework composed of four oxygen atoms surrounding cations. This structure consists of open voids in the shape of channels and cages which are often filled by H2O molecules and extra-framework cations. Guest species can flow through channels that are large enough. Dehydration in the hydrated phase is highly reversible and arises at temperatures below 400 °C. OH, F groups may interrupt the framework, which results in tetrahedral vertices that are not occupied by other tetrahedra [8].
Building Units: Primary and Secondary
Zeolites are often classified as having primary building units (PBU) and secondary building units (SBU). The PBU is composed of (SiO4)4+ and (AlO4)5+ tetrahedra. They connect to adjacent tetrahedra through shared oxygen atoms to form simple geometrically shaped spatial arrays (SBUs). SBUs can be solitary rings, polyhedrons, dual rings, or complicated units that construct unique systems of interconnecting channels and cages. The number of SBUs in a zeolite unit cell is fixed. Currently, there are 23 distinct SBUs [17].
Because of their crystalline structure, zeolites are naturally porous, constituted of a 3D tetrahedral network of silicon and aluminum linked together by shared oxygen atoms. These pores arise due to interconnected cages formed because of the tetrahedral structural arrangement of atoms. Zeolites are microporous materials because their pore sizes are typically less than 2 nm [18]. Microporous materials, according to IUPAC definition [19], are those with pore diameters smaller than 2 nm.
Uses in Dentistry
Glass Ionomer Cements (GIC)
When ion-embedded zeolites were combined with GIC, in vitro ion release rate or agar diffusion assays were commonly used to assess antibacterial activity. As the silver-incorporated zeolite (AgZ) weight ratio increases, so does the inhibitory activity against oral bacteria such as S. mutans [20]. It is important to note that the AgZ GIC can sustainably release silver ions over an extended period, whereas the GIC alone can rapidly release fluoride for only two days [20]. Antibacterial properties similar to AgZ have been discovered in zinc-containing zeolites (ZnZ) versus E. coli, S. aureus, P. aureginosa, B. subtilis, and C. albicans [21]. Furthermore, when loaded with chlorhexidine, GIC zeolites may exhibit excellent antibacterial activity against S. mutans [22].
The antibacterial efficiency of GICs used for root canal sealants against E. faecalis has been variable [23, 24•]. ZUT (modified, experimental root canal sealer), a blend of 0.2% AgZ and the GIC sealer KT-308, reduced E. faecalis development more effectively than KT-308 alone, independent of concentration or duration [23]. Padachie et al. and McDougal et al., on the other hand, both determined that ZUT was less efficacious than other GICs [25]. Current results indicate that GICs can have enhanced and prolong antibacterial properties, depending on the concentration of incorporated zeolite. Nevertheless, the outcomes may be influenced by the use and kind of GIC, which might be a future study issue [26•].
Although the antibacterial capabilities of GICs are directly connected to zeolite concentration, the quantity of zeolite that GICs may successfully integrate is restricted by the mechanical qualities that arise. The shear bond strength of GICs containing zeolite varied depending on the kind and application of the GIC [27, 28]. ZUT demonstrated greater shear bond strength than GIC Ketac-Endo alone and was unaltered by conditioning with calcium hydroxide, chlorhexidine, formocresol, or deionized water [27].
The compressive strength of zeolite GICs varied according on the zeolite type and application [20, 22]. Lee et al. AgZ GIC had greater compressive strength at 1% He but reduced compressive strength over 3% He [20]. When a small amount of chlorhexidine-containing zeolite nanoparticles (~ 1 wt%) was added to GICs, there was no discernible change in compressive or adhesive strength [22].
Resin Cements
Incorporating zeolite into the resin, enhanced its antimicrobial properties against some micro-organisms. S. mutans and S. mitis were suppressed by different AgZ and ZnZ ratios, but not S. salivarius or colony of S. sanguis. In contrast to GIC, greater concentrations of Ag-Zn zeolite did not raise the resin’s antibacterial activity level [10]. The compressive and flexural strengths of modified resin-based composites enhanced or remained the same after the zeolites were changed with active diazonium [29]. However, this aspect of zeolite modification has been subject to limited research and may require further investigation.
Mineral Trioxide Aggregate (MTA)
The addition of AgZ to MTA demonstrated considerable antibacterial activity against selected oral microorganisms. Most oral bacteria, including E. faecalis, S. aureus, and C. albicans, were inhibited by AgZ in MTA. However, it had no effect on P. intermedia and A. israelii [30, 31]. Although there was no substantial difference in bacterial reduction amount by 0.2% AgZ MTA and 2% AgZ MTA after 72 h, 2% AgZ MTA had a considerably stronger inhibitory effect than 0.2% AgZ MTA.
Notably, 2% AgZ released the greatest quantity of silver ion after 24 h [30]. Furthermore, compared to MTA comprising 2% chlorhexidine, 2% AgZ was shown to have strongest antibacterial effect [31]. As a result, 2% AgZ might be used as a possible addition to boost MTA's antibacterial characteristics. Zeolites boosted MTA’s antibacterial characteristics greatly. However, they have a negative impact on physical parameters such as curing period, water permeability, injection bond strength, and compressive strength of MTA [32•, 33, 34].
Curing time was reduced as the quantity of 2% AgZ increased, and water absorption was at its lowest in 2% AgZ integrated into MTA compared to MTA-only controls [32•]. Furthermore, the inclusion of Ag-Zn-Ze composites had a detrimental impact on MTA’s tensile bond strength and compressive strength [33, 34]. The very porous nature of zeolites might explain the lower tensile bond strength. If water molecules are present in the pores, they can interfere with the hydration and crystallization of MTA [33].
In summary, zeolites can boost MTA’s antibacterial activities while decreasing its mechanical qualities. However, more studies are required to identify the precise concentration of zeolites which may effect mechanical and physical properties of MTA.
Root Canal Irrigation Solutions
When compared to saline, 2% AgZ had significantly more antibacterial efficacy as a root canal irrigation solution. However, compared to 5% sodium hypochlorite, 2% chlorhexidine, and 0.10% octenidine (OCT) 2% AgZ showed significantly less antibacterial efficacy [35]. One probable explanation is that AgZ was not as efficient against E. faecalis, S. aureus and C. albicans as other root canal irrigation solution [35]. Yet further studies are needed on mechanical and chemical characteristics of AgZ as a root canal irrigation solution to make an evidence-based decision.
Non-Acrylic Resins
Soft denture liners to all-ceramic dentures were among the acrylic-free materials evaluated with zeolite. AgZ added to soft liners improved antibacterial capabilities against Candida albicans and gram-negative bacteria while retaining viscoelastic qualities [36]. In terms of mechanical properties, sodalite zeolites were the most frequently used zeolites in ceramic prosthesis [37,38,39,40]. They are a zeolite subtype with high selectivity and catalytic activity that can easily permeate other materials [37].
In addition, all specimens impregnated with sodalite zeolites exhibited flexural strengths exceeding the tolerances considered by ISO standards [39, 40]. Furthermore, multiple investigations have revealed that zeolite-infiltrated materials had much greater flexural hardness and strength when heated to 1600 °C than glass-infiltrated control samples [39]. Finally, compared to its glass-infiltrated equivalent, sodalite zeolite-infiltrated Zirconia Toughened Alumina (ZTA) provides one of the highest fracture toughness and modulus values [37]. Therefore, sodalite zeolite-infiltrated specimens are potential substitutes for glass-infiltrated ZTA due to their superior properties in terms of bond strength, flexural strength, Vickers hardness, fracture toughness, and Young’s modulus [37,38,39,40].
Acrylic Resin
Acrylic resins with AgZ have better antibacterial activity against oral pathogens as S. mutans, F. nucleatum, and C. albicans. Chemically polymerized acrylic resin absorbs water and is difficult to polymerize, making it easier for the bacteria that cause periodontal disease to propagate. Acrylic resins incorporating AgZ can solve this problem by effectively reducing the adhesion of S. mutans, F. nucleatum, and C. albicans to polymethyl methacrylate (PMMA) for up to 45–60 days [41, 42•, 43].
When 2.5% Ag-Zn-Ze was added to PMMA, it worked effectively in suppressing C. albicans and S. mutans [44]. As a result, both AgZ and Ag-Zn-Ze may be feasible choices for improving PMMA’s antibacterial characteristics. However, depending on the proportion of AgZ used, it might have a negative impact on the mechanical qualities of acrylic resins [42•, 43,44,45]. Depending on the type of acrylic resin, the addition of AgZ at concentrations above 2.5% significantly reduces impact strength and flexural strength [42•, 45]. However, some thermosetting acrylic resins such as QC20 and Lucitone 550 can meet the criteria for denture resins requiring a flexural strength greater than 65 MPa [42•, 43, 44]. Both the average tensile strength and flexural strength decreased depending on the concentration of added zeolite. To retain acceptable mechanical strength, less than 4% by weight of zeolite is advised, and 2% by weight can be added if both structural and antimicrobial benefits are considered recommended [43].
As a result, a modest proportion of antimicrobial silver-zinc zeolite added to polymethyl methacrylate might be a beneficial antibacterial effectiveness option to assist avoid frequent mouth infections such as denture stomatitis [42•].
Implants
Many zeolite applications include antibacterial coatings for implants. Despite a paucity of research, covering titanium implants with AgZ proved successful in suppressing the development of methicillin-resistant Staphylococcus aureus (MRSA) [46]. This favorable discovery, along with zeolites’ outstanding biocompatibility, suggests that they might be prospective novel materials for use in orthopaedic implants.
Oral Medicine
Oral cancer is the sixth most common cancer in the world, most of which are oral squamous cell carcinomas. Recently, volatile organic compounds (VOCs) emitted by the human body have been considered to detect medical conditions. Several studies have shown that VOCs are produced by in vitro cancer cell lines as molecular cancer markers [47,48,49]. Shigeyama et al. evaluated VOCs in oral squamous cell carcinoma patients by a combined method of thin-film microextraction and gas chromatography–mass spectrometry based on Zeolite Socony Mobil–5 (ZSM) zeolites/Polydimethylsiloxane (PBMS) hybrid films. This study demonstrated that ZSM-5/PBMS hybrid films can be used to identify tumor-specific candidate biomarkers in a more cost-effective manner than taking blood samples [50, 51•].
Concluding Remarks
This review gives a brief overview on the origin of zeolites and on their physical and chemical properties as dental materials. The natural characteristics of zeolite materials can be modified and manipulated to prepare zeolitic frameworks which are application-driven. It is a microporous material, where different molecules, including cations and antibacterial compounds, can be sieved in. They are stable in oral environment, especially in contact with saliva, making them an efficient additive to various dental materials. Incorporation of zeolites in restorative materials and denture resins increases antimicrobial activity and improves the mechanical properties of restorative materials, especially in GIC and MTA. However, information on AgZ incorporation in different dental materials is still limited, as well as its use as a root canal irrigation solution which requires further investigations. This review could improve our understanding of the use of zeolites as a novel material in the various aspects of dentistry.
Data Availability
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
KraljevićPavelić S, SimovićMedica J, Gumbarević D, et al. Critical review on zeolite clinoptilolite safety and medical applications in vivo. Front Pharmacol. 2018;9:1350.
Derakhshankhah H, Jafari S, Sarvari S, Barzegari E, et al. Biomedical applications of zeolitic nanoparticles, with an emphasis on medical interventions. Int J Nanomedicine. 2020;15:363.
Sivakumar I, Arunachalam KS, Sajjan S, et al. Incorporation of antimicrobial macromolecules in acrylic denture base resins: a research composition and update. J Prosthodont. 2014;23(4):284–90.
•• Hao J, Lang S, Mante F, et al. Antimicrobial and mechanical effects of zeolite use in dental materials: a systematic review. Acta Stomatol Croat. 2021;55(1):76–89. This systematic review comprehensively covers the spectrum of zeolitic materials and its applications in dentistry with a focus on zeolite incorporation with different dental materials.
de’Gennaro M, Cappelletti P, Langella A, et al. Genesis of zeolites in the Neapolitan Yellow Tuff: geological, volcanological and mineralogical evidence. Contrib Mineral Petrol. 2000;139(1):17–35.
Harpel CJ, Kyle PR, Dunbar NW. Englacial tephrostratigraphy of Erebus volcano. Antarctica J Volcanol Geotherm Res. 2008;177(3):549–68.
Weckhuysen BM, Yu J. Recent advances in zeolite chemistry and catalysis. Chem Soc Rev. 2015;44(20):7022–4.
Coombs DS, Alberti A, Armbruster T, et al. Recommended nomenclature for zeolite minerals: report of the subcommittee on zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Mineral Mag. 1998;62(4):533–71.
Nikawa H, Yamamoto T, Hamada T, et al. Antifungal effect of zeolite-incorporated tissue conditioner against Candida albicans growth and/or acid production. J Oral Rehabil. 1997;24(5):350–7.
Hotta M, Nakajima H, Yamamoto K, et al. Antibacterial temporary filling materials: the effect of adding various ratios of Ag-Zn-Zeolite. J Oral Rehabil. 1998;25(7):485–9.
Van Koningsveld H, Jansen JC, Van Bekkum H. The monoclinic framework structure of zeolite H-ZSM-5. Comparison with the orthorhombic framework of as-synthesized ZSM-5. Zeolites. 1990;10(4):235–42.
Armbruster T, Gunter ME. Crystal structures of natural zeolites. Rev Mineral Geochem. 2001;45(1):1–67.
Farkaš A, Rožić M, Barbarić-Mikočević Ž. Ammonium exchange in leakage waters of waste dumps using natural zeolite from the Krapina region. Croatia J Hazard Mater. 2005;117(1):25–33.
Widiastuti N, Wu H, Ang HM, et al. Removal of ammonium from greywater using natural zeolite. Desalination. 2011;277(1–3):15–23.
Baerlocher C, McCusker LB, Olson DH. Atlas of zeolite framework types. Sixth Revised ed. Elsevier; 2007.
Eleroğlu H, Yalçın H. Use of natural zeolite-supplemented litter increased broiler production. S Afr J Ani Sci. 2005;35(2):90–7.
Moshoeshoe M, Nadiye-Tabbiruka MS, Obuseng V. A review of chemistry, structure, properties and applications of zeolites. Am J Mater Sci. 2017;7(5):196–221.
Castaldi P, Santona L, Enzo S, et al. Sorption processes and XRD analysis of a natural zeolite exchanged with Pb2+, Cd2+ and Zn2+ cations. J Hazard Mater. 2008;156(1–3):428–34.
Rouquerol J, Avnir D, Fairbridge CW, et al. Recommendations for the characterization of porous solids (Technical Report). Pure Applchem. 1994;66(8):1739–58.
Lee JH, Lee SB, Kim KN, et al. Antibacterial effect of silver-zeolites in glass-ionomer cements. Key Eng Mater. 2007;330:831–4.
Mabrouk M, Selim MM, Beherei H, El-Gohary MI. Incorporation effect of silver and zinc-zeolites into commercial glass ionomer cement. Interceram. 2013;62(1):50–4.
Kim HJ, Son JS, Kim KH, et al. Antimicrobial activity of glass ionomer cement incorporated with chlorhexidine-loaded zeolite nanoparticles. J Nanosci Nanotechnol. 2016;16(2):1450–3.
Patel V, Santerre JP, Friedman S. Suppression of bacterial adherence by experimental root canal sealers. J Endod. 2000;26(1):20–4.
• Padachey N, Patel V, Santerre P, et al. Resistance of a novel root canal sealer to bacterial ingress in vitro. J Endod. 2000;26(11):656–9. The novel experimental ZUT material developed at the University of Toronto, Canada was evaluated in this study which is a dentine bonding root canal sealer containing antimicrobial silver containing zeolite (0.2% by weight).
McDougall IG, Patel V, Santerre P, et al. Resistance of experimental glass ionomer cement sealers to bacterial penetration in vitro. J Endod. 1999;25(11):739–42.
• Li W, Qi M, Sun X, et al. Novel dental adhesive containing silver exchanged EMT zeolites against cariogenic biofilms to combat dental caries. Microporous Mesoporous Mater. 2020;299:110113. This study used the adhesives containing Ag++ exchanged zeolites, having remarkable antibacterial properties to serve as “bioactive” adhesive materials for clinical applications.
Chung HA, Titley K, Torneck CD, et al. Adhesion of glass-ionomer cement sealers to bovine dentin conditioned with intracanal medications. J Endod. 2001;27(2):85–8.
Can-Karabulut DC, Akincioglu A, Özyegin SL, et al. Influence of experimental provisional cements containing zeolite, bone hydroxyapatite and linoleic acid on bond strength of composite to dentin in vitro. J Adhes Dent. 2010;12(6):469–75.
Sandomierski M, Okulus Z, Voelkel A. Active diazonium-modified zeolite fillers for methacrylate-based composites. Compos Interfaces. 2019;26(7):643–57.
Odabaş ME, Çinar Ç, Akça G, et al. Short-term antimicrobial properties of mineral trioxide aggregate with incorporated silver-zeolite. Dent Traumato. 2011;27(3):189–94.
Ghatole K, Patil A, Giriyappa RH, et al. Evaluation of antibacterial efficacy of MTA with and without additives like silver zeolite and chlorhexidine. J Clin Diagn Res. 2016;10(6):ZC11.
• Çinar Ç, Odabaş M, Gürel MA, et al. The effects of incorporation of silver-zeolite on selected properties of mineral trioxide aggregate. Dent Mater J. 2013;32(6):872–6. This in vitro study showed increased calcium release and reduced setting time for antibacterial silver zeolites when incorporated with MTA.
Ghasemi N, Rahimi S, Samiei M, et al. Effect of the of zeolite containing silver-zinc nanoparticles on the push out bond strength of mineral trioxide aggregate in simulated furcation perforation. J Dent. 2019;20(2):102–6.
Samiei M, Ghasemi N, Asl-Aminabadi N, et al. Zeolite-silver-zinc nanoparticles: biocompatibility and their effect on the compressive strength of mineral trioxide aggregate. J Clin Exp Dent. 2017;9(3):e356.
Ghivari SB, Bhattacharya H, Bhat KG, et al. Antimicrobial activity of root canal irrigants against biofilm forming pathogens-an in vitro study. J Conserv Dent. 2017;20(3):147–51.
Saravanan M, Kumar VA, Padmanabhan TV, et al. Viscoelastic properties and antimicrobial effects of soft liners with silver zeolite in complete dental prosthesis wearers: an in vivo study. Int J Prosthodont. 2015;28(3):265–9.
Naji GA, Omar RA, Yahya R. The effect of sodalite zeolite infiltrated material on the fracture toughness, elastic modulus and optical properties of all-ceramic dental prostheses. Ceram Int. 2016;42(16):18737–46.
Naji GA, Omar RA, Yahya R. Influence of sodalite zeolite infiltration on the coefficient of thermal expansion and bond strength of all-ceramic dental prostheses. J Mech Behav Biomed Mater. 2017;67:135–43.
Naji GA, Omar RA, Dabbagh A, et al. Effect of sintering temperature on the microstructures and mechanical properties of sodalite infiltrate all-ceramic material for dental restorations. Adv Appl Ceram. 2018;117(5):291–302.
Naji GA, Omar RA, Yahya R, et al. Sodalite zeolite as an alternative all-ceramic infiltrating material for alumina and zirconia toughened alumina frameworks. Ceram Int. 2016;42(10):12253–61.
Kuroki K, Hayashi T, Sato K, et al. Effect of self-cured acrylic resin added with an inorganic antibacterial agent on Streptococcus mutans. Dent Mater J. 2010;29(3):277–85.
• Malic S, Rai S, Redfern J, et al. Zeolite-embedded silver extends antimicrobial activity of dental acrylics. Colloids Surf B. 2019;173:52–7. This study showed that the silver in zeolite-loaded dental acrylic (DAZ) resins does not influence the mechanical or optical properties of the dental acrylic resins, providing a long-term antimicrobial activity.
Nakanoda S, Nikawa H, Hamada T, et al. The material and antifungal properties of antibiotic zeolite incorporated acrylic resin. J Jpn Prosthodont Soc. 1995;39:919–26.
Casemiro LA, Martins CH, Pires-de-Souza FD, et al. Antimicrobial and mechanical properties of acrylic resins with incorporated silver–zinc zeolite–part I. Gerodontology. 2008;25(3):187–94.
Yadav NS, Saraf S, Mishra SK, et al. Effects of fluconazole, chlorhexidine gluconate, and silver-zinc zeolite on flexural strength of heat-cured polymethyl methacrylate resin. J Nat Sci Biol. 2015;6(2):340.
Wang J, Wang Z, Guo S, et al. Antibacterial and anti-adhesive zeolite coatings on titanium alloy surface. Microporous Mesoporous Mater. 2011;146(1–3):216–22.
Sponring A, Filipiak W, Mikoviny T, et al. Release of volatile organic compounds from the lung cancer cell line NCI-H2087 in vitro. Anticancer Res. 2009;29(1):419–26.
Mochalski P, Sponring A, King J, et al. Release and uptake of volatile organic compounds by human hepatocellular carcinoma cells (HepG2) in vitro. Cancer Cell Int. 2013;13(1):1–9.
Nozoe T, Goda S, Selyanchyn R, et al. In vitro detection of small molecule metabolites excreted from cancer cells using a Tenax TA thin-film microextraction device. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;991:99–107.
Shigeyama H, Wang T, Ichinose M, et al. Identification of volatile metabolites in human saliva from patients with oral squamous cell carcinoma via zeolite-based thin-film microextraction coupled with GC–MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1104:49–58.
• Hao, J.; Stavljeni´c Milašin, I.; Batu Eken, Z.; Mravak-Stipetic, M.; Paveli´c, K.; Ozer, F. Effects of zeolite as a drug delivery system on cancer therapy: a systematic review. Molecules. 2021;26:6196. This systematic review suggested that anticancer drug-incorporated zeolites/ZIFs can be used as alternative treatment options to enhance the efficacy of cancer treatment.
Funding
Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Section Editors for the topical collection Dental Restorative Materials are Mutlu Özcan and Paulo Francisco Cesar. Please note that Dr. Özcan was not involved in the editorial process of this article as she is a co-author.
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deshpande, S., Kheur, S., Kheur, M. et al. A Review on Zeolites and Their Applications in Dentistry. Curr Oral Health Rep 10, 36–42 (2023). https://doi.org/10.1007/s40496-023-00330-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40496-023-00330-7