Abstract
Noninvasive neuromodulation refers to a family of device-based interventions that apply electrical or magnetic fields, either at convulsive or subconvulsive levels, to the brain through the intact skull to modulate neural function. This is a rapidly evolving field, with new research emerging regarding the various roles that these devices can play both in studying the neural mechanisms underlying mood and anxiety disorders, and in treating pharmacoresistant conditions either on their own or in combination with other therapies. Each neuromodulation modality has its pros and cons and should be carefully chosen after weighing the risks and benefits. This manuscript reviews some of the most exciting developments in this field over the past year and emphasizes themes that are emerging as being important for these tools to fulfill their potential to transform how we study and treat mood and anxiety disorders. Key among these themes is the concept of how we understand the “dose” of the stimulation, and how exogenously applied fields interact with endogenous brain activity. Refining the concept of dose will ultimately be important in allowing clinicians and researchers to apply the procedure with precision to engage the targeted network to achieve the desired effects in each individual. The large parameter space defining dose of neuromodulation makes interpreting the literature on safety and efficacy challenging and highlights the need for clear and accurate reporting of the spatial, temporal, and contextual features of dosage to make the emerging literature base as informative as possible. Ultimately, the impact of noninvasive neuromodulation devices is potentially transformational given their utility in providing mechanistic insight into the circuit-based and oscillatory origins of mood and anxiety disorders, as well as providing therapeutic interventions rationally designed to target disease-related processes.
Similar content being viewed by others
Introduction
Noninvasive neuromodulation refers to a family of device-based interventions that span our oldest somatic therapies in psychiatry (e.g., electroconvulsive therapy, ECT, which is nonfocal and convulsive) to our youngest and most recently approved therapeutic interventions (e.g. transcranial magnetic stimulation, TMS, which is relatively more focal and subconvulsive). These technologies have evolved over time to provide more precise control over the spatial distribution of the electric fields induced in the brain and the temporal components of the stimulation. TMS can induce intracerebral electrical currents with focal rapidly alternating magnetic fields applied to the scalp (1, 2) for both subconvulsive applications as well as for focal seizure induction (e.g., magnetic seizure therapy, MST). Transcranial direct current stimulation (tDCS) delivers electrical current to modulate cortical excitability by depolarizing or hyperpolarizing the cortical regions beneath the electrodes (3).
The use of noninvasive neuromodulation in depressive episodes, especially in major depressive disorder (MDD), has been the most well-studied clinical application. ECT remains the recommended treatment for severe depression, especially when a rapid response is warranted (4). A highly effective procedure, ECT, is associated with a risk of cognitive side effects, especially memory impairment (5). These risks have been lowered through improvements in ECT technique but have not been eliminated as of yet. In contrast, TMS paradigms approved for depression have not been found to be associated with cognitive side effects. In 2008, TMS was FDA-cleared in the USA for the treatment of “adult patients who have failed to achieve satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode” (6). More recently, the FDA labeling was expanded to include adults who have failed to respond to at least one antidepressant medication at or above the minimally effective dose. Three TMS devices are now on the market for depression in the United States. The European (7) and Canadian (8) guidelines also supported its tolerability and efficacy. Other subconvulsive interventions for depression are at various stages of investigation, including synchronized TMS (sTMS) and tDCS.
New literature in this emerging field has focused on examining new indications, identifying biomarkers of illness and response, and optimizing dosage. Although clinicians are familiar with the concept of dose when prescribing medications, this concept in neuromodulation is evolving (9, 10). Here we discuss three dimensions of neuromodulation dose, including spatial distribution (targeted brain region, electrode/coil placement, electrode/coil shape and orientation), temporal dynamics (pulse shape, frequency, train duration), and context (e.g., the state of the circuit at the time of stimulation, as influenced by concomitant cognitive task engagement, medication, or other contextual aspects of the treatment) (11). Various technologies have emerged to expand the dose parameter space in the interest of enhancing efficacy or expanding scientific value of the tool. For example, deeper penetrating coils are now FDA-approved for depression (dTMS)(12). Other relevant novel technologies include synchronized TMS (sTMS) (13), alpha TMS (αTMS) (14), theta-burst stimulation (TBS) (15), accelerated TMS (16), individualized low-amplitude seizure therapy (iLAST) (17), and magnetic seizure therapy (MST) (18). In the following sections, these technologies will be introduced and discussed.
Here we review interesting developments in this rapidly evolving field over the past year and place them into to the context of the larger field of neuromodulation, highlighting the importance of understanding dose-response relationships to fulfill the potential of neuromodulation to transform clinical neuroscience practice in psychiatry and neurology.
Methods
Databases, including Pubmed/Medline, Cochrane database for reviews, and PsycINFO, were searched using the following MeSH and non-MeSH terms: transcranial magnetic stimulation, transcranial direct current stimulation, cathodal stimulation, anodal stimulation, electroconvulsive therapy, mood disorder, affective disorders, depressive disorder, obsessive-compulsive disorder, post-traumatic stress disorder, panic disorder, Tourette syndrome, and generalized anxiety disorder. The inclusion criteria were publish date between January 2014 and March 2015, adult population, and published in English. Various types of study designs and article types were all included, such as randomized controlled trial, open-label trials, case reports, or case series. All studies were screened by title, abstract, and full text by two authors before decision to include or exclude (Fig. 1). Studies highlighted in this review were selected for their relative degree of innovation and potential impact on the field.
Electrical and Magnetic Seizure Therapies
ECT in Major Depressive Disorder
Still our most effective and rapidly acting treatment for depression, ECT has evolved over time with successive improvements in its risk/benefit ratio (19). This evolution nicely illustrates the importance of dosage in defining clinical outcomes. ECT technique has evolved from a “one size fits all” approach, to an individualized dosing approach where the number of pulses is titrated to individual seizure threshold. As well, refinements in electrode placement, which controls the spatial distribution of the field in the brain (20, 21), and pulse width, an aspect of the temporal dynamics of the stimulation, have each contributed to lowering of the risk of memory loss (9).
Evidence directly comparing clinical outcomes with different doses of ECT in adequately powered samples is still emerging. The efficacy of ultra-brief pulse right unilateral (RUL) ECT needs to be carefully evaluated given its more benign cognitive side effect profile than standard pulse width ECT and bilateral (BL) electrode placement (22). In the past year, studies have questioned the efficacy of ultra-brief pulse RUL in relapse prevention (23, 24) and failed to demonstrate a cognitive advantage relative to standard pulse width (25). However, issues of proper dosing, adequate study design, and optimal measurement sensitive enough to capture relative advantages in cognitive side effects are clearly important before making conclusions about the clinical value of ultra-brief RUL ECT (26). Phase 1 of the adequately powered Prolonging Remission in Depressed Elders (PRIDE) study is expected to shed light on this issue regarding the efficacy of ultra-brief pulse RUL ECT in a large cohort of seniors with unipolar depression (27, 28).
Relapse prevention following response to ECT is one of the leading unsolved problems in clinical ECT practice. A continued dose as maintenance treatment is recommended after acute ECT treatment, but the optimal means of delivering this maintenance treatment is still a matter of debate. Fixed schedule maintenance ECT was found to be no better than combination pharmacotherapy (29, 30). Psychopharmacology, psychotherapy, and neuromodulation were all examined; however, none of these approaches have yet achieved the degree of sustained benefit that would be optimal (30, 31). This clinically important issue will be addressed in Phase 2 of the PRIDE Trial (28), utilizing an individualized symptom-titrated algorithm-based longitudinal ECT (STABLE) intervention which tailors maintenance treatments according to symptom expression rather than a fixed schedule (32).
Given emerging evidence for rapid antidepressant efficacy of ketamine, there has been interest in whether combining ketamine with ECT may boost antidepressant response. The combination of ketamine and ECT did not seem to synergically improve the treatment outcomes according to a recent meta-analysis (33). Utilizing a different augmentation approach, a recent trial found that the combination of ECT and aerobic exercise training in depression resulted in better outcomes (34). Although blinding was not possible, this study raises the intriguing idea that these effects may be moderated by increases in brain-derived neurotropic factor (BDNF).
Biomarkers of Response to ECT
A growing number of studies have examined various genetic, molecular, and imaging biomarkers in relation to antidepressant response with ECT, with disparate results. Genetic polymorphism was examined, including serotonin transporter (5-HTTLPR), norepinephrine transporter (NET182C), COMT (Val158Met), DRD2 (C957T), and ApoE (35, 36). The association of plasma biomarker levels and treatment response was explored, such as brain-derived neurotropic factor (BDNF) levels (34, 37), nerve growth factor (NGF) (38), and plasma thioredoxin levels (39). A meta-analysis has indicated that serum BDNF increased after ECT but had no correlation with improvement in depressive symptoms (40). Imaging studies have revealed changes in hippocampus and amygdala following ECT. In depressed patients, a smaller hippocampus volume at baseline (41) and a larger amygdala volume (42) were associated with better treatment outcomes. After ECT treatment, the hippocampus and amygdala showed increased volumes (41, 43). Specifically, the right hippocampal connectivity increased and normalized after a course of RUL ECT (43). The interpretation of those changes are still pending. This correlation seemed to be a result of ECT, but it is unclear if those correlations were related to clinical response or to cognitive side effects.
ECT in Bipolar Depression and Tourette Syndrome
ECT was found to be effective in treating both unipolar and bipolar depression (44). A randomized controlled trial compared ECT to algorithm-based pharmacological treatment in bipolar depression. This study demonstrated a better response rate (73.9 vs. 35.0 %) with ECT (45) and without compromising general neurocognitive function (46). Developing effective treatment for bipolar depression continues to be an ongoing task for the future (47). Other studies examined the utility of ECT in other conditions, such as Tourette syndrome. A case report (48) showed successful treatment of Tourette syndrome with ECT.
Innovations in Seizure Therapy: MST and iLAST
Magnetic seizure therapy (MST), an investigational procedure, couples the spatial focality of magnetic induction with the powerful therapeutic efficacy of seizures in an attempt to improve the risk/benefit ratio of seizure therapy by minimizing risk of cognitive side effects (18, 49, 50). MST enables more precise control of the spatial extent of the induced electric field and resultant seizure than conventional ECT, presenting the opportunity to reduce involvement of medial temporal and frontal structures implicated in cognitive side effects (51). Computational modeling demonstrates that MST is less susceptible to variability introduced by anatomical differences across individuals than ECT (52).
In treatment-resistant depression (TRD), MST demonstrated efficacy comparable to ECT, with a response rate of 69 % and a remission rate of 46 % (53–55). Compared to ECT, MST has less cognitive side effects (56) and faster post-ictal recovery (57). As with ECT, MST appears to also carry a risk of treatment-induced mania as demonstrated by two case reports (58).
The observation that MST induces electric fields in the brain that are at much lower amplitude than conventional ECT, and nevertheless that they demonstrate antidepressant efficacy, suggests that the current amplitudes conventionally used in ECT may be higher than necessary to achieve therapeutic response (59). Computational modeling of the electric field induced in the brain by ECT and MST demonstrates that conventional ECT can be modified to approach the focality of MST by lowering the amplitude of the administered current and also by making the electrodes smaller and placing them closer together on the head (51). In addition to lowering the current amplitude to increase focality, current amplitude can also be individualized to compensate for individual differences in anatomy. This novel approach, termed individualized low-amplitude seizure therapy (iLAST), has been demonstrated to be feasible in computational and animal models and led to the discovery that the motor response to single pulses measured by EMG is a powerful predictor of seizure threshold (17, 51, 60, 61). The ability to predict seizure threshold without actually inducing a seizure as done in conventional seizure titration approaches opens the possibility of a less invasive means of dosage planning to personalize the treatment.
Transcranial Magnetic Stimulation
TMS in Major Depressive Disorder and Bipolar Disorder
Available evidence supports efficacy of two TMS protocols in moderate MDD when given daily for 4 to 6 weeks using a figure 8 coil: (1) high-frequency TMS (HF-TMS) over the left dorsolateral prefrontal cortex (DLPFC), 3000 pulses per session (7, 8), and (2) low-frequency TMS (LF-TMS) over the right DLPFC, more than 1200 pulses per session (7, 62). Limited literature compared those two protocols head-to-head. One study compared HF-TMS and LF-TMS in treatment-resistant unipolar and bipolar depression (62). No significant difference was reported between groups; however, the study was under-dosed and under-powered (N = 33) in its three-arm design. Studies with larger sample size are warranted to compare the effectiveness in these two protocols. This is especially clinically relevant since low-frequency TMS carries a lower risk of seizure compared with high-frequency TMS. Some studies have attempted to extend the success in unipolar depression to bipolar depression, although more work is needed to establish efficacy and safety in bipolar disorder (63–65).
As with ECT, relapse prevention following antidepressant response to TMS represents a clinically important topic. Studies suggest that antidepressant benefits of TMS can persist on the order of months (66, 67), but relatively few studies have addressed optimal relapse prevention strategies to sustain benefits. Available evidence suggests that antidepressant initiation is critical to maintain remission (68). One study reported reduced relapse rate with continued maintenance TMS two times weekly and then weekly. The results suggested that maintenance TMS might be helpful in preventing affective episode relapses in a 12-month follow-up period (69).
TMS in Anxiety Disorders
In addition to unipolar depression, TMS has been studied in post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), Tourette syndrome (TS), generalized anxiety disorder (GAD), and panic disorder (7). A recent review did not recommend TMS in anxiety disorders, except for PTSD (level C evidence) (7). HF-TMS over the right DLPFC was the recommended protocol per European guidelines (70–72). Unfortunately, no consensus could be made on other diagnoses due to various doses, heterogeneous protocols, and small sample size.
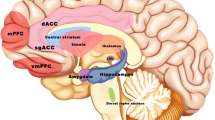
Right and left DLPFC were the most well-studied TMS targets in various psychiatric disorders. Other brain targets were based on imaging studies in the specific conditions, such as orbito-frontal cortex (OFC), supplementary motor area (SMA), and medial prefrontal cortex (mPFC).
A meta-analysis concluded that targeting OFC and SMA with LF-TMS shows promise in the treatment of OCD (73). A study stimulating LF-TMS over the right OFC with the double-cone coil for 1 week showed improvement in OCD symptoms (74). The normalization of OFC hyperactivity and an overall normalization of the brain circuitry hyperactivity (BA 9, 10, 11, 25, 47) were associated with TMS treatment response. In addition, LF-TMS over SMA has been reported in treating OCD and Tourette syndrome (75–77). Two case reports demonstrated TS symptom improvements with this target (78, 79), but a two-center randomized sham-controlled trial of 3 weeks of LF-TMS failed to find benefit (although there were suggestions that a longer treatment course could have value) (80). An open-label study used LF-TMS targeting mPFC (dACC, BA 24 and 32) in OCD for 10 sessions. The mean Yale Brown OCD Scale (Y-BOCS) improvement was 39 % (81). Studies targeting DLPFC in OCD had mixed findings. A case report applied 10-session cTBS with 1200 pulses over right DLPFC at 80 % motor threshold (82). The Y-BOCS score reduced from 19 to 8. fMRI acquired during a symptom provocation procedure before and after treatment demonstrated decreased activity in the right DLPFC. The study design was intriguing; however, the results were not impressive. More studies are warranted to explore or replicate brain targets to treat OCD and TS.
Biomarkers of Response to TMS
As with seizure therapy, there is considerable interest in identifying biomarkers of response and mechanisms of action. For example, normalization of subgenual anterior cingulate cortex (sgACC, BA 25) connectivity to the default mode network (DMN, medial prefrontal-medial parietal network) was reported after TMS treatment (83). In addition, baseline sgACC hyperconnectivity to DMN on fMRI (83–85), and baseline rostral ACC (rACC) glucose uptake on PET(14), predicated TMS antidepressant response. Current TMS devices are not able to reach sgACC directly, even with deep TMS (86–88). However, it is plausible to effect changes in sites remote from the coil via transsynaptic action.
Innovations in TMS Technique
Various TMS protocols have been examined in an attempt to enhance efficacy, such as by applying image-guided neuronavigation, modifying coil geometry, accelerating the delivery of the pulses, individually tailored stimulating frequency, and modulating the brain-activity-dependent neuroplasticity prior to or during the session. As summarized in Table 1, each of these studies explores different aspects of the infinitely large TMS parameter space, in terms of the spatial, temporal, and contextual aspects of dosing.
Spatial Aspects of TMS Dosing
Several therapeutic targets of interest are inaccessible to the conventional figure 8 coil, both the air core version and the iron-core version. Several approaches to extend the reach of TMS to access deeper structures have been explored through novel coil designs. The H-coil was designed to reach deeper brain regions (dTMS), such as the medial prefrontal cortex (mPFC) (87, 89). This device was approved by the FDA with a dose of HF-TMS over the left DLPFC (12, 90). The intention-to-treat remission rate was 36.6 % in active group compared to 16.7 in sham group (p = 0.032). The relative efficacy of dTMS versus figure 8 coil TMS has not been examined to date.
Computational modeling demonstrates that all of the approaches to deeper penetration involve a depth-focality trade-off (88). Focusing the TMS field in depth is not possible due to the physics inherent in electromagnetic induction (86, 87). It is important to note that the safety guidelines covering the selection of parameters of stimulation to reduce the risk of seizure with TMS were based on the figure 8 coil and do not directly apply to other coil geometries. Deeper and less focal coils may carry a higher risk of seizure due to the fact that they synchronously stimulate a larger brain volume. In a study of 19 bipolar patients treated with H-coil TMS, one had a TMS-induced seizure (91). In the pivotal trial leading to the FDA clearance of the H-coil for the treatment of depression (n = 212), one patient had a seizure, which was attributed to a protocol violation (12).
Low field magnetic stimulation (LFMS) penetrates deeply but with very low field strengths and an excellent safety profile. A recent controlled trial suggests that LFMS may exert rapid antidepressant effects (92), which are currently being evaluated in a large-scale multi-center trial (93).
Temporal Aspects of TMS Dosing
The temporal aspects of TMS dosing can be divided into the temporal aspects of each individual pulse (including its shape, width, and directionality), and the train of repeated pulses (including frequency, duration, and number of pulses per train). Two novel developments in the temporal aspects of individual pulses include controllable pulse shape TMS (cTMS) (94–96) and rotating field TMS (rfTMS) (97). Cortical response to TMS depends on the width of the pulse, and only recently have we had access to devices that allow independent user control of this aspect of temporal dosing (98). The availability of cTMS devices opens the door to the development of more efficient stimulation paradigms.
In addition to a distinctive pulse shape, each TMS pulse has a directionality of induced current flow. TMS pulses preferentially activate axons that are oriented parallel to the direction of induced current flow. In regions of the brain where neuronal orientation is not uniform, this means only a subset of neurons are likely to be stimulated. A workaround recently introduced is a cloverleaf coil that induces a pulse with directionality that rotates during the pulse itself. This rotation results in more efficient stimulation (97).
While the clinical utility of cTMS and rfTMS is yet to be explored, substantially more work has focused on the clinical utility of optimizing the temporal aspects of the pulse train (Table 1). For example, theta-burst stimulation (TBS) in which brief bursts of gamma frequency are given at a rate of five bursts per second, either continuously or intermittently, rapidly induces lasting inhibition or facilitation of cortical excitability, respectively (99). It was recently reported that continuous TBS (cTBS) over the right DLPFC followed by intermittent TBS (iTBS) over the left DLPFC was superior to either iTBS or cTBS alone (14, 15).
Recent work has also examined the utility of individualizing the stimulation frequency, tuning it to the individual alpha frequency. One set of studies has taken this approach with a conventional figure 8 TMS coil (alpha TMS, αTMS), while another utilized a novel device with three static magnets that are rotated at the designated frequency to induce an alternating magnetic field (sTMS).
In both instances, it was hypothesized that individually tailored alpha frequency, rather than standard low or high frequency, was more efficient in manipulating thalamo-cortical oscillations. In both protocols, individual alpha frequencies (IAFs) were calculated by the average of alpha band on scalp EEG (14, 100). The sTMS device was developed to diffusely stimulated three regions (frontal polar region, superior frontal gyrus, and parietal region) with a low-intensity sinusoidal magnetic field. The treatment course in depression was 20 sessions over 4 weeks. No additional benefit was found with the use of individualized frequency over a standard frequency (14). A recently published multi-site randomized sham-controlled trial demonstrated an advantage of sTMS over sham in the subset of the sample that was treated per protocol, but this failed to reach statistical significance in the intent to treat analysis. To date, the hypothesis that individualization of frequency enhances outcome has not been convincingly demonstrated.
Accelerated TMS refers to giving the total number of pulses of a full 4–6-week TMS course over the span of 1–2 days. The remission rate of accelerated TMS (37 %) was comparable to that of conventional TMS, and the changes in sgACC connectivity were found in responders (16, 85, 101). However, the sample size was small and the studies lacked randomization and sham control.
Contextual Aspects of TMS Dosing
The contextual aspects of TMS dosing refer to the brain state at the time of stimulation, and the interaction of this brain state with the applied exogenous stimulation. Brain state at the time of stimulation may be manipulated via pharmacological or cognitive/behavioral means. For example, one study examined if manipulating pre-TMS EEG frontal theta power with computerized rACC-engaging cognitive task (RECT) was associated with augmented antidepressant effect in TMS (14). The remission rates in the three-arm groups were 41.6 % (pre-TMS RECT and TMS), 16.6 % (TMS with sham RECT), and 0 % (sham TMS and sham RECT), respectively, on HAMD-17.
In many clinical trials, and in routine clinical practice, it is commonplace for the patient to be on psychopharmacological agents at the time of TMS, and typically, these are conventional antidepressant, anxiolytic, and/or mood stabilizing medications. Less well examined but extremely intriguing is the prospect of using pharmacological agents that modulate the acquisition of plasticity induced by TMS, or that alter neural oscillations in ways that may be synergistic with the stimulation. An intriguing case report administered accelerated TMS treatment and then combined ketamine infusion during weekly TMS sessions in refractory unipolar depression (102). The BDI II score reduced from 17 to 0 in this previously treatment-resistant case. The same protocol was applied to a case with bipolar depression (65). The patient had partial remission with partial functional improvement. The prospect of combining pharmacological enhancement of TMS effects on plasticity is at a relatively early phase of investigation but potentially promising.
Cognitive behavioral therapy (CBT), such as prolonged exposure therapy (PE), and exposure and response prevention therapy (ERP) are evidence-based treatments for anxiety disorders (103–105). The purpose of psychotherapy is to create a new experience in a conditioned context. In the process, it changes the perception about the past, fosters resilience at the present moment, and transforms lived experience in the future. Behaviorally, those changes are meant to “unlearn” pre-existing maladaptive responses and enable new forms of learning. Modulating activity-dependent neuroplasticity via TMS could theoretically facilitate the learning process involved in CBT (106). Alternatively, the brain state induced by the CBT intervention could potentate the cortical response to TMS. An example of this approach concerns the use of TMS targeting mPFC to facilitate fear extinction learning in PTSD (107). A script-driven imaginary “ultra-brief exposure” procedure was performed prior to the HF-TMS session. The combination of consecutive treatments significantly improved the PTSD symptoms on clinician-administered PTSD scale (108). The same hypothesis was tested in an OCD case. In this case report, in vivo ERP exercises were preceded by HF-TMS over the left DLPFC (109). Further work is needed to fully explore the potential impact of optimizing the contextual aspects of TMS dosing, and the meaningful integration of pharmacological and cognitive behavioral interventions with noninvasive neuromodulation.
Other Forms of Noninvasive Neuromodulation on the Horizon
There are several novel forms of noninvasive neuromodulation at various stages of development, including transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), transcranial random noise stimulation, trigeminal nerve stimulation (TNS); and low-intensity focused ultrasound (LIFU). Of these, the most work on depression to date has been with tDCS. A recent systematic review and meta-analysis found active tDCS to be superior to sham in the treatment of depression (110). Further investigations were warranted to explore effectiveness of tDCS in treating depression with larger sample size. Less well studied in anxiety disorders, a case report demonstrated tDCS efficacy in OCD with a Y-BOCS reduction of 40 % and normalization of pre-SMA/SMA hyperactivity (111, 112). Preliminary studies have started to combine tDCS with either medication or psychotherapy (113, 114). Given its relatively safety and portability, further work to refine the dosimetry of tDCS to optimize efficacy seems warranted. In contact to TMS where the temporal aspects of dosimetry are complex, in the case of direct current polarization, the only element of time to consider is the number of minutes of stimulation since there are no frequency, pulse shape, or pulse train parameters to specify.
Conclusions
The field of noninvasive neuromodulation is rapidly evolving, propelled by collaborations across psychiatry, engineering, neurobiology, and neuroscience. Developments in the field have led to newly FDA-approved clinical treatments, as well as new tools to study mechanisms of action and to better understand the pathophysiology underlying mood and anxiety disorders. Beyond electrical and magnetic stimulation, novel approaches involving acoustic and photic stimulation are areas on the horizon to watch as the technology continues to evolve.
ECT continues to have unparalleled efficacy in severe depression, and recent advances in the spatial aspects of the induced electric field (as determined by electrode placement) and the temporal aspects of the pulses (e.g., pulse width) have improved its tolerability. Leading challenges for the future include further reductions in the cognitive side effects, which may be facilitated by MST and iLAST, and relapse prevention strategies to sustain benefits in the long term. A critical knowledge gap that will need to be addressed to optimize efficacy in the short and long term is a deep understanding of the mechanisms of action of seizures, which at present have efficacy that far outstrips the subconvulsive interventions for mood disorders.
TMS has demonstrated an excellent safety profile, and significant antidepressant efficacy with three FDA-cleared devices and more in the pipeline. Leading challenges for the future include optimizing efficacy, through better understanding of dose-response relationships across the spatial, temporal, and contextual aspects of dosimetry. New technologies have enabled the exploration of an ever expanding parameter space, making systematic study of all parameter combinations in clinical trials not feasible. This highlights the need for computational modeling and pre-clinical studies to narrow the parameter space and to enable the rational design of stimulation paradigms targeting specific disease-related aspects of circuit dysfunction.
Several of these approaches, such as theta-burst stimulation (TBS) and delivery of neurocognitive or psychotherapeutic interventions peri-TMS, seek to leverage enhanced plasticity induced by the TMS and optimize the resonance between the temporal pattern of exogenously applied pulses with respect to endogenous brain state at the time of stimulation. Ultimately, a detailed understanding of how endogenously generated oscillations interact with exogenously applied fields to induce lasting changes in circuit function will be essential to optimize the clinical utility of noninvasive neuromodulation in the treatment of mood and anxiety disorders, as well as other potential applications in psychiatry and neurology.
References
Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–7.
Pascual-Leone A, Houser CM, Reese K, Shotland LI, Grafman J, Sato S, et al. Safety of rapid-rate transcranial magnetic stimulation in normal volunteers. Electroencephalogr Clin Neurophysiol. 1993;89(2):120–30.
Paulus W. Transcranial direct current stimulation (tDCS). Suppl Clin Neurophysiol. 2003;56:249–54.
Husain MM, Rush AJ, Fink M, Knapp R, Petrides G, Rummans T, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry. 2004;65(4):485–91.
Lisanby SH, Maddox JH, Prudic J, Devanand DP, Sackeim HA. The effects of electroconvulsive therapy on memory of autobiographical and public events. Arch Gen Psychiatry. 2000;57(6):581–90.
M. M. “Special premarket 510(k) notification for NeuroStar TMS therapy system for major depressive disorder”. Food Drug Adm. 2008.
Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125(11):2150–206.
Kennedy SH, Milev R, Giacobbe P, Ramasubbu R, Lam RW, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) Clinical guidelines for the management of major depressive disorder in adults. IV. Neurostimulation therapies. J Affect Disord. 2009;117 Suppl 1:S44–53.
Deng ZD, McClintock SM, Oey NE, Luber B, Lisanby SH. Neuromodulation for mood and memory: from the engineering bench to the patient bedside. Curr Opin Neurobiol. 2015;30:38–43.
Peterchev AV, Rosa MA, Deng ZD, Prudic J, Lisanby SH. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT. 2010;26(3):159–74.
Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul. 2012;5(4):435–53.
Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14(1):64–73.
Leuchter AF, Cook I, Feifel D, Goethe JW, Husain MM, Carpenter LL, et al. Synchronized Transcranial Magnetic Stimulation (sTMS): Efficacy and safety of low-field synchronized transcranial magnetic stimulation (sTMS) for treatment of major depression. Brain Stimul. 2015;In Press.
Jin Y, Phillips B. A pilot study of the use of EEG-based synchronized Transcranial Magnetic Stimulation (sTMS) for treatment of Major Depression. BMC Psychiatry. 2014;14:13.
Plewnia C, Pasqualetti P, Grosse S, Schlipf S, Wasserka B, Zwissler B, et al. Treatment of major depression with bilateral theta burst stimulation: a randomized controlled pilot trial. J Affect Disord. 2014;156:219–23.
Baeken C, Marinazzo D, Wu GR, Van Schuerbeek P, De Mey J, Marchetti I, et al. Accelerated HF-rTMS in treatment-resistant unipolar depression: Insights from subgenual anterior cingulate functional connectivity. World J Biol Psychiatry. 2014;15(4):286–97.
Peterchev AV, Krystal AD, Rosa MA, Lisanby SH. Individualized Low-Amplitude Seizure Therapy: Minimizing Current for Electroconvulsive Therapy and Magnetic Seizure Therapy. Neuropsychopharmacology. 2015. doi:10.1038/npp.2015.122.
Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA. Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology. 2003;28(10):1852–65.
Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939–45.
Lee W, Lisanby S, Laine A, Peterchev A. Electric Field Model of Transcranial Electric Stimulation in Nonhuman Primates: Correspondence to Individual Motor Threshold. IEEE Trans Biomed Eng. 2015. doi:10.1109/TBME.2015.2425406.
Lee WH, Deng ZD, Kim TS, Laine AF, Lisanby SH, Peterchev AV. Regional electric field induced by electroconvulsive therapy in a realistic finite element head model: influence of white matter anisotropic conductivity. Neuroimage. 2012;59(3):2110–23.
Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71–83.
Youssef NA, McCall WV. Continuation antidepressant strategies after electroconvulsive therapy: ultrabrief pulse versus cognitive-behavioral therapy. Biol Psychiatry. 2015;77(3), e7.
Brakemeier EL, Merkl A, Wilbertz G, Quante A, Regen F, Buhrsch N, et al. Cognitive-behavioral therapy as continuation treatment to sustain response after electroconvulsive therapy in depression: a randomized controlled trial. Biol Psychiatry. 2014;76(3):194–202.
Spaans HP, Verwijk E, Comijs HC, Kok RM, Sienaert P, Bouckaert F, et al. Efficacy and cognitive side effects after brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy for major depression: a randomized, double-blind, controlled study. J Clin Psychiatry. 2013;74(11):e1029–36.
Kellner CH, McClintock SM, McCall WV, Petrides G, Knapp RG, Weiner RD, et al. Brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy (ECT) for major depression: efficacy, effectiveness, and cognitive effects. J Clin Psychiatry. 2014;75(7):777.
Lisanby SH, Husain MM, Knapp R, Young RC, Weiner RD, McClintock S, et al. Prolonging Remission in Depressed Elderly (PRIDE) Group: Ultrabrief right unilateral ECT in geriatric depression: Antidepressant and cognitive outcomes. Biol Psychiatry 2014;75:21S SOBP Annual Meeting Abstract #61.
ClinicalTrials.gov. Prolonging Remission in Depressed Elderly (PRIDE). Available from: https://clinicaltrials.gov/ct2/show/NCT01028508?term=NCT01028508&rank=1.
Kellner CH, Knapp RG, Petrides G, Rummans TA, Husain MM, Rasmussen K, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Arch Gen Psychiatry. 2006;63(12):1337–44.
Kellner C. Review: maintenance antidepressants reduce risk of relapse in the 6 months following ECT in people with major depression. Evid Based Ment Health. 2014;17(1):8.
Elias A, Chathanchirayil SJ, Bhat R, Prudic J. Maintenance electroconvulsive therapy up to 12 years. J Affect Disord. 2014;156:228–31.
Lisanby SH, Sampson S, Husain MM, Petrides G, Knapp RG, McCall V, et al. Toward individualized post-electroconvulsive therapy care: piloting the Symptom-Titrated, Algorithm-Based Longitudinal ECT (STABLE) intervention. J ECT. 2008;24(3):179–82.
McGirr A, Berlim MT, Bond DJ, Neufeld NH, Chan PY, Yatham LN, et al. A systematic review and meta-analysis of randomized controlled trials of adjunctive ketamine in electroconvulsive therapy: Efficacy and tolerability. J Psychiatr Res. 2015;62C:23–30.
Salehi I, Hosseini SM, Haghighi M, Jahangard L, Bajoghli H, Gerber M, et al. Electroconvulsive therapy and aerobic exercise training increased BDNF and ameliorated depressive symptoms in patients suffering from treatment-resistant major depressive disorder. J Psychiatr Res. 2014;57:117–24.
Bousman CA, Katalinic N, Martin DM, Smith DJ, Ingram A, Dowling N, et al. Effects of COMT, DRD2, BDNF, and APOE Genotypic Variation on Treatment Efficacy and Cognitive Side Effects of Electroconvulsive Therapy. J ECT. 2015;31(2):129–35.
Kautto M, Kampman O, Mononen N, Lehtimaki T, Haraldsson S, Koivisto PA, et al. Serotonin transporter (5-HTTLPR) and norepinephrine transporter (NET) gene polymorphisms: Susceptibility and treatment response of electroconvulsive therapy in treatment resistant depression. Neurosci Lett. 2015;590:116–20.
Sartorius A, Bumb JM, Aksay SS, Gass P, Hellweg R, Kranaster L. ECT seizure quality and serum BDNF, revisited. Eur Arch Psychiatry Clin Neurosci. 2015;265(4):359–60.
Bilgen AE, Bozkurt Zincir S, Zincir S, Ozdemir B, Ak M, Aydemir E, et al. Effects of electroconvulsive therapy on serum levels of brain-derived neurotrophic factor and nerve growth factor in treatment resistant major depression. Brain Res Bull. 2014;104:82–7.
Genc A, Kalelioglu T, Karamustafalioglu N, Tasdemir A, Gungor FC, Genc ES, et al. Level of plasma thioredoxin in male patients with manic episode at initial and post-electroconvulsive or antipsychotic treatment. Psychiatry Clin Neurosci. 2015;69(6):344–50.
Brunoni AR, Baeken C, Machado-Vieira R, Gattaz WF, Vanderhasselt MA. BDNF blood levels after electroconvulsive therapy in patients with mood disorders: a systematic review and meta-analysis. World J Biol Psychiatry. 2014;15(5):411–8.
Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, et al. Structural Plasticity of the Hippocampus and Amygdala Induced by Electroconvulsive Therapy in Major Depression. Biol Psychiatry. 2015. doi:10.1016/j.biopsych.2015.02.029.
Ten Doesschate F, van Eijndhoven P, Tendolkar I, van Wingen GA, van Waarde JA. Pre-treatment amygdala volume predicts electroconvulsive therapy response. Front Psychiatry. 2014;5:169.
Abbott CC, Jones T, Lemke NT, Gallegos P, McClintock SM, Mayer AR, et al. Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry. 2014;4, e483.
Fink M. What was learned: studies by the consortium for research in ECT (CORE) 1997-2011. Acta Psychiatr Scand. 2014;129(6):417–26.
Schoeyen HK, Kessler U, Andreassen OA, Auestad BH, Bergsholm P, Malt UF, et al. Treatment-resistant bipolar depression: a randomized controlled trial of electroconvulsive therapy versus algorithm-based pharmacological treatment. Am J Psychiatry. 2015;172(1):41–51.
Kessler U, Schoeyen HK, Andreassen OA, Eide GE, Malt UF, Oedegaard KJ, et al. The effect of electroconvulsive therapy on neurocognitive function in treatment-resistant bipolar disorder depression. J Clin Psychiatry. 2014;75(11):e1306–13.
Tohen M, Abbott CC. Use of electroconvulsive therapy in bipolar depression. Am J Psychiatry. 2015;172(1):3–5.
Guo JN, Kothari JS, Leckman JF, Ostroff RB. Successful Treatment of Tourette Syndrome with Electroconvulsive Therapy: A Case Report. Biol Psychiatry. 2014. doi:10.1016/j.biopsych.2014.09.020.
Lisanby SH, Luber B, Finck AD, Schroeder C, Sackeim HA. Deliberate seizure induction with repetitive transcranial magnetic stimulation in nonhuman primates. Arch Gen Psychiatry. 2001;58(2):199–200.
Lisanby SH, Schlaepfer TE, Fisch HU, Sackeim HA. Magnetic seizure therapy of major depression. Arch Gen Psychiatry. 2001;58(3):303–5.
Deng ZD, Lisanby SH, Peterchev AV. Controlling stimulation strength and focality in electroconvulsive therapy via current amplitude and electrode size and spacing: comparison with magnetic seizure therapy. J ECT. 2013;29(4):325–35.
Deng ZD, Lisanby SH, Peterchev AV. Effect of anatomical variability on electric field characteristics of electroconvulsive therapy and magnetic seizure therapy: a parametric modeling study. IEEE Trans Neural Syst Rehabil Eng. 2015;23(1):22–31.
Kayser S, Bewernick BH, Matusch A, Hurlemann R, Soehle M, Schlaepfer TE. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol Med. 2015;45(5):1073–92.
Kayser S, Bewernick BH, Hurlemann R, Soehle M, Schlaepfer TE. Comparable seizure characteristics in magnetic seizure therapy and electroconvulsive therapy for major depression. Eur Neuropsychopharmacol. 2013;23(11):1541–50.
Fitzgerald PB, Hoy KE, Herring SE, Clinton AM, Downey G, Daskalakis ZJ. Pilot study of the clinical and cognitive effects of high-frequency magnetic seizure therapy in major depressive disorder. Depress Anxiety. 2013;30(2):129–36.
Polster JD, Kayser S, Bewernick BH, Hurlemann R, Schlaepfer TE. Effects of electroconvulsive therapy and magnetic seizure therapy on acute memory retrieval. J ECT. 2015;31(1):13–9.
Soehle M, Kayser S, Ellerkmann RK, Schlaepfer TE. Bilateral bispectral index monitoring during and after electroconvulsive therapy compared with magnetic seizure therapy for treatment-resistant depression. Br J Anaesth. 2014;112(4):695–702.
Noda Y, Daskalakis ZJ, Fitzgerald PB, Downar J, Rajji TK, Blumberger DM. Magnetic seizure therapy-induced mania: a report of 2 cases. J ECT. 2015;31(1):e4–6.
Rosa MA, Abdo GL, Lisanby SH, Peterchev A. Seizure induction with low-amplitude-current (0.5 A) electroconvulsive therapy. J ECT. 2011;27(4):341–2.
Lee WH, Lisanby SH, Laine AF, Peterchev AV. Electric field characteristics of electroconvulsive therapy with individualized current amplitude: a preclinical study. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:3082–5.
Lee WH, Lisanby SH, Laine AF, Peterchev AV. Stimulation strength and focality of electroconvulsive therapy with individualized current amplitude: a preclinical study. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:6430–3.
Dell'Osso B, Oldani L, Camuri G, Dobrea C, Cremaschi L, Benatti B, et al. Augmentative repetitive Transcranial Magnetic Stimulation (rTMS) in the acute treatment of poor responder depressed patients: a comparison study between high and low frequency stimulation. Eur Psychiatry. 2015;30(2):271–6.
Zendjidjian XY, Lodovighi MA, Richieri R, Guedj E, Boyer L, Dassa D, et al. Resistant bipolar depressive disorder: case analysis of adjunctive transcranial magnetic stimulation efficiency in medical comorbid conditions. Bipolar Disord. 2014;16(2):211–3.
Pallanti S, Grassi G, Antonini S, Quercioli L, Salvadori E, Hollander E. rTMS in resistant mixed states: an exploratory study. J Affect Disord. 2014;157:66–71.
Best SR. Combined ketamine/transcranial magnetic stimulation treatment of severe depression in bipolar I disorder. J ECT. 2014;30(4):e50–1.
Mantovani A, Pavlicova M, Avery D, Nahas Z, McDonald WM, Wajdik CD, et al. Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (TMS) in treatment-resistant depression. Depress Anxiety. 2012;29(10):883–90.
Dunner DL, Aaronson ST, Sackeim HA, Janicak PG, Carpenter LL, Boyadjis T, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75(12):1394–401.
Kedzior KK, Reitz SK, Azorina V, Loo C. Durability of the Antidepressant Effect of the High-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) in the Absence of Maintenance Treatment in Major Depression: a systematic Review and Meta-Analysis of 16 Double-Blinded, Randomized. Sham-Controlled Trials Depress Anxiety. 2015;32(3):193–203.
Rapinesi C, Bersani FS, Kotzalidis GD, Imperatori C, Del Casale A, Di Pietro S, et al. Maintenance Deep Transcranial Magnetic Stimulation Sessions are Associated with Reduced Depressive Relapses in Patients with Unipolar or Bipolar Depression. Front Neurol. 2015;6:16.
Karsen EF, Watts BV, Holtzheimer PE. Review of the effectiveness of transcranial magnetic stimulation for post-traumatic stress disorder. Brain Stimul. 2014;7(2):151–7.
Wahbeh H, Senders A, Neuendorf R, Cayton J. Complementary and Alternative Medicine for Posttraumatic Stress Disorder Symptoms: A Systematic Review. J Evid Based Complementary Altern Med. 2014;19(3):161–75.
Berlim MT, Van Den Eynde F. Repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex for treating posttraumatic stress disorder: an exploratory meta-analysis of randomized, double-blind and sham-controlled trials. Can J Psychiatry. 2014;59(9):487–96.
Berlim MT, Neufeld NH, Van den Eynde F. Repetitive transcranial magnetic stimulation (rTMS) for obsessive-compulsive disorder (OCD): an exploratory meta-analysis of randomized and sham-controlled trials. J Psychiatr Res. 2013;47(8):999–1006.
Nauczyciel C, Le Jeune F, Naudet F, Douabin S, Esquevin A, Verin M, et al. Repetitive transcranial magnetic stimulation over the orbitofrontal cortex for obsessive-compulsive disorder: a double-blind, crossover study. Transl Psychiatry. 2014;4, e436.
Mantovani A, Lisanby SH, Pieraccini F, Ulivelli M, Castrogiovanni P, Rossi S. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette's syndrome (TS). Int J Neuropsychopharmacol. 2006;9(1):95–100.
Mantovani A, Simpson HB, Fallon BA, Rossi S, Lisanby SH. Randomized sham-controlled trial of repetitive transcranial magnetic stimulation in treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2010;13(2):217–27.
Gomes PV, Brasil-Neto JP, Allam N, Rodrigues de Souza E. A randomized, double-blind trial of repetitive transcranial magnetic stimulation in obsessive-compulsive disorder with three-month follow-up. J Neuropsychiatry Clin Neurosci. 2012;24(4):437–43.
Salatino A, Momo E, Nobili M, Berti A, Ricci R. Awareness of symptoms amelioration following low-frequency repetitive transcranial magnetic stimulation in a patient with Tourette syndrome and comorbid obsessive-compulsive disorder. Brain Stimul. 2014;7(2):341–3.
Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: A feasibility study. World J Biol Psychiatry. 2014:1-5.
Landeros-Weisenberger A, Mantovani A, Motlagh MG, de Alvarenga PG, Katsovich L, Leckman JF, et al. Randomized Sham Controlled Double-blind Trial of Repetitive Transcranial Magnetic Stimulation for Adults With Severe Tourette Syndrome. Brain Stimul. 2015;8(3):574–81.
Modirrousta M, Shams E, Katz C, Mansouri B, Moussavi Z, Sareen J, et al. The Efficacy of Deep Repetitive Transcranial Magnetic Stimulation Over the Medial Prefrontal Cortex in Obsessive Compulsive Disorder: Results from an Open-Label Study. Depress Anxiety. 2015;32(6):445–50.
Wu CC, Tsai CH, Lu MK, Chen CM, Shen WC, Su KP. Theta-burst repetitive transcranial magnetic stimulation for treatment-resistant obsessive-compulsive disorder with concomitant depression. J Clin Psychiatry. 2010;71(4):504–6.
Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76(7):517–26.
Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, et al. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacol : Off Publ Am College Neuropsychopharmacol. 2015;39(2):488–98.
Baeken C, Marinazzo D, Everaert H, Wu GR, Van Hove C, Audenaert K, et al. The Impact of Accelerated HF-rTMS on the Subgenual Anterior Cingulate Cortex in Refractory Unipolar Major Depression: Insights From FDG PET Brain Imaging. Brain Stimul. 2015;8(4):808–15.
Deng ZD, Lisanby SH, Peterchev AV. On the characterization of coils for deep transcranial magnetic stimulation. Clin Neurophysiol. 2015;126(7):1456–7.
Deng ZD, Lisanby SH, Peterchev AV. Coil design considerations for deep transcranial magnetic stimulation. Clin Neurophysiol. 2014;125(6):1202–12.
Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013;6(1):1–13.
Deng ZD, Peterchev AV, Lisanby SH. Coil design considerations for deep-brain transcranial magnetic stimulation (dTMS). Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5675–9.
Harvey PO, Van den Eynde F, Zangen A, Berlim MT. Neural correlates of clinical improvement after deep transcranial magnetic stimulation (DTMS) for treatment-resistant depression: a case report using functional magnetic resonance imaging. Neurocase. 2015;21(1):16–22.
Harel EV, Zangen A, Roth Y, Reti I, Braw Y, Levkovitz Y. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011;12(2):119–26.
Rohan ML, Yamamoto RT, Ravichandran CT, Cayetano KR, Morales OG, Olson DP, et al. Rapid mood-elevating effects of low field magnetic stimulation in depression. Biol Psychiatry. 2014;76(3):186–93.
ClinicalTrials.gov. Trial of Low Field Magnetic Stimulation Augmentation of Antidepressant Therapy in Treatment-Resistant Depression (RAPID).
Peterchev AV, Murphy DL, Lisanby SH. Repetitive transcranial magnetic stimulator with controllable pulse parameters (cTMS). Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2922–6.
Peterchev AV, Jalinous R, Lisanby SH. A transcranial magnetic stimulator inducing near-rectangular pulses with controllable pulse width (cTMS). IEEE Trans Biomed Eng. 2008;55(1):257–66.
Peterchev AV, Murphy DL, Lisanby SH. Repetitive transcranial magnetic stimulator with controllable pulse parameters. J Neural Eng. 2011;8(3):036016.
Rotem A, Neef A, Neef NE, Agudelo-Toro A, Rakhmilevitch D, Paulus W, et al. Solving the orientation specific constraints in transcranial magnetic stimulation by rotating fields. PLoS One. 2014;9(2), e86794.
Peterchev AV, Goetz SM, Westin GG, Luber B, Lisanby SH. Pulse width dependence of motor threshold and input-output curve characterized with controllable pulse parameter transcranial magnetic stimulation. Clin Neurophysiol. 2013;124(7):1364–72.
Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–6.
Ma W, Huang Y, Liao L, Jin Y. A randomized double-blinded sham-controlled trial of α electroencephalogram-guided transcranial magnetic stimulation for obsessive-compulsive disorder. Chin Med J. 2014;127(4):601–6.
McGirr A, Van den Eynde F, Tovar-Perdomo S, Fleck MP, Berlim MT. Effectiveness and acceptability of accelerated repetitive transcranial magnetic stimulation (rTMS) for treatment-resistant major depressive disorder: an open label trial. J Affect Disord. 2015;173:216–20.
Best SR, Griffin B. Combination therapy utilizing ketamine and transcranial magnetic stimulation for treatment-resistant depression: a case report. Int J Neurosci. 2014.
Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 Suppl):5–53.
Practice guideline for the treatment of patients with panic disorder. Work Group on Panic Disorder. American Psychiatric Association. Am J Psychiatry. 1998;155(5 Suppl):1-34.
Ursano RJ, Bell C, Eth S, Friedman M, Norwood A, Pfefferbaum B, et al. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am J Psychiatry. 2004;161(11 Suppl):3–31.
Ahmed Z, Wieraszko A. Modulation of learning and hippocampal, neuronal plasticity by repetitive transcranial magnetic stimulation (rTMS). Bioelectromagnetics. 2006;27(4):288–94.
Marin MF, Camprodon JA, Dougherty DD, Milad MR. Device-based brain stimulation to augment fear extinction: implications for PTSD treatment and beyond. Depress Anxiety. 2014;31(4):269–78.
Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder--a pilot study. Brain Stimul. 2013;6(3):377–83.
Grassi G, Godini L, Grippo A, Piccagliani D, Pallanti S. Enhancing cognitive-behavioral therapy with repetitive transcranial magnetic stimulation in refractory obsessive-compulsive-disorder: a case report. Brain Stimul. 2015;8(1):160–1.
Shiozawa P, Fregni F, Bensenor IM, Lotufo PA, Berlim MT, Daskalakis JZ, et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17(9):1443–52.
Narayanaswamy JC, Jose D, Chhabra H, Agarwal SM, Shrinivasa B, Hegde A, et al. Successful Application of Add-on Transcranial Direct Current Stimulation (tDCS) for Treatment of SSRI Resistant OCD. Brain stimulation. 2015;In Press.
Mondino M, Haesebaert F, Poulet E, Saoud M, Brunelin J. Efficacy of Cathodal Transcranial Direct Current Stimulation Over the Left Orbitofrontal Cortex in a Patient With Treatment-Resistant Obsessive-Compulsive Disorder. J ECT. 2015;March (ePub).
Brunoni AR, Boggio PS, De Raedt R, Bensenor IM, Lotufo PA, Namur V, et al. Cognitive control therapy and transcranial direct current stimulation for depression: a randomized, double-blinded, controlled trial. J Affect Disord. 2014;162:43–9.
Brunoni AR, Junior RF, Kemp AH, Lotufo PA, Bensenor IM, Fregni F. Differential improvement in depressive symptoms for tDCS alone and combined with pharmacotherapy: an exploratory analysis from the Sertraline vs. Electrical Current Therapy for Treating Depression Clinical Study. Int J Neuropsychopharmacol. 2014;17(1):53–61.
Acknowledgments
Dr. Lisanby is supported by grants from NIH (U01 MH084241, R01 MH091083, R01 NS088674), foundations (Brain & Behavior Foundation, Stanley Foundation), and industry (Neosync, Brainsway, NexStim).
Compliance with Ethics Guidelines
ᅟ
Conflicts of Interest
Yupei P. Hu and Gopalkumar Rakesh have no conflict of interest to declare. Sarah H. Lisanby is PI or co-investigator on research contracts to the University from Neosync, Brainsway, NexStim. Sarah Lisanby’s lab has received equipment loans from Magstim and Magventure. Sarah Lisanby is listed as a co-inventor on patent applications on TMS technology and receives no royalties or fees.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Mood and Anxiety Disorders
Rights and permissions
About this article
Cite this article
Hu, Y.P., Rakesh, G. & Lisanby, S.H. Recent Developments in Noninvasive Neuromodulation for Mood and Anxiety Disorders. Curr Behav Neurosci Rep 2, 173–185 (2015). https://doi.org/10.1007/s40473-015-0043-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40473-015-0043-4
Keywords
- Noninvasive neuromodulation
- Transcranial magnetic stimulation
- Electroconvulsive therapy
- Magnetic seizure therapy
- Transcranial direct current therapy
- Major depressive disorder
- Bipolar depression
- Treatment-resistant depression
- Post-traumatic stress disorder
- Obsessive-compulsive disorder
- Combination therapy
- Anterior cingulate cortex
- Dorsolateral prefrontal cortex
- Supplementary motor area
- Medial prefrontal cortex
- Dose