Abstract
Purpose of Review
Commercially-available physical activity trackers (PAT) are promising tools for promoting physical activity (PA) in people with mental disorders. The present systematic review aims to examine the feasibility and acceptability of using PAT in people with substance use disorders (SUD), and how that can affect substance use, PA, and mental health.
Recent Findings
Previous review studies have noted the potential of using active and passive data collection methods in the context of SUDs. However, since then, novel technology has been developed and new studies on PAT have been published. There are no specific reviews about them in the context of treatment of SUD.
Summary
The current study included seven studies involving 178 participants. The findings provide preliminary evidence that using PAT as part of a broader behavioral intervention is feasible and promising in promoting healthy behavior and improving health-related outcomes, including reduction in substance use. However, considering the small number of studies and their limitations, there is a need for more systematic and rigorous research to determine the long-term effectiveness of incorporating PAT into existing treatments for SUD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Active and passive data collection methods have become increasingly popular in the treatment of mental disorders [1,2,3,4]. A wide range of methods are used to collect real-time reports of people’s daily life experiences in their natural environments, including self-reporting, observational measures, and psychophysiological procedures [5]. These methods allow professionals to gather ecological data in various ways (ecological momentary assessment (EMA), experience sampling, ecological momentary intervention, real-time data capture, continuous unified electronic diary method, or e-diary) [6].
Active and passive data collection methods are feasible thanks to wearable devices designed specifically for research purposes (precision accelerometers, temperature sensors, breathing sensors, etc.) [7], and commercial devices for daily use, such as activity trackers and smartphones [8, 9]. Physical activity trackers (PAT) have particularly come to researchers' and practitioners' attention. The characteristics of commercially-available PAT mean that people can wear these devices in their everyday lives comfortably and affordably [10, 11]. They provide users with acceptably accurate information about various (PA) variables (number of steps per day, exercise time or intensity, etc.) [12, 13]. Moreover, these devices may include some behavioral change techniques, so they can be used as an intervention to increase PA [14]. Recent literature also indicates that this technology seems to be feasible and acceptable across a range of demographics [15,16,17].
Studies examining the effects of PAT on health-related outcomes in various clinical and non-clinical populations have found an increase in steps per day and increased moderate-to-vigorous physical activity (MVPA) [16, 18••, 19••, 20], as well as lower levels of anxiety and depression [21••] in activity tracker users compared to non-users. In particular, PAT can help increase levels of PA, especially in combination with other strategies (text messages, personalization, support groups) [22, 23•], and the information from PAT can be used to reinforce healthy behaviors as part of a wider intervention [24, 25••].
PAT are a promising approach for assessing and treating substance use disorders (SUD) due to the clinical characteristics of this population. These include the presence of anxiety and depressive symptoms [26, 27], high levels of emotional dysregulation [28], low levels of daily PA [29, 30], and altered sleep patterns [31]. Recent studies have shown the potential of using wearable devices to collect ecological data related to SUD as well [32]. Furthermore, several clinical studies have concluded the feasibility of incorporating PAT into standard SUD treatments. For instance, in a sample of women receiving treatment for alcohol use disorder (AUD), combining 10 weeks of counseling and PAT for 12 weeks led to an increased number of daily steps and improvements in depression and anxiety symptoms [33]. Similarly, in people undergoing methadone maintenance treatment, wearing PAT as part of a 12-week peer-facilitatated physical activity intervention produced increased physical activity, positve affect, and decreased illicit opioid and cocaine use [34]. This is why PAT have received increasing attention in the field of addiction research.
Previous review studies have noted the potential of using active and passive data collection methods in the context of SUDs [35, 36]. However, since then, new technology has been developed, and new studies on PAT have been published. Despite this, there are no specific reviews about PAT in the context of treatment of SUD. Therefore, the present review aims to examine the feasibility and acceptability of using a physical activity tracker in people with SUD, and the effects on substance use, PA, and mental health.
Method
Literature Search Strategy
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [37], and the review protocol was registered in PROSPERO (CRD42023417229). The PRISMA checklist is provided in Supplementary Material 1. Studies published in English were identified through a comprehensive literature search with no restriction on the year of publication (up to March 2023) using MEDLINE (PubMed), WOS, and Scopus. The terms included in the search (through all databases) were related to drugs, substance use disorder (SUD), and wearable devices (Fitbit, activity tracker, smartband/watch). The Boolean terms for the electronic databases are provided in Supplementary Material 2. Titles and abstracts retrieved through the searches were exported to reference management software (Zotero) to remove duplicates.
Eligibility and Exclusion Criteria
The primary eligibility criteria were peer-reviewed, published clinical or observational studies examining the effect of using a commercially-available PAT with people who use substances that met the following conditions: 1) The study involved adults who used substances (i.e., aged 18) from the community or enrolled in treatment/recovery for SUD; and 2) it provided at least one substance use measure (e.g., frequency, quantity, craving, withdrawal symptoms) during assessment or treatment. Studies that used non-commercially-available wearables (biosensors and research devices) or smartphone applications were not included due to the different characteristics of these devices (e.g., presence of behavioral change techniques, appearance, measuring instruments…). Case studies were also excluded.
Data Extraction
Two independent reviewers conducted the literature searches. Disagreements about whether to include an article or not were resolved with a third reviewer. Information about the sample size, participants’ sex, age, length of time the PAT were worn, and substance use-related measures were tabulated. Data on countries, study settings, PAT models, and results of the interventions were also provided.
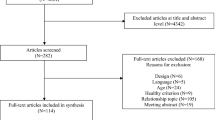
A flow chart depicting the literature search is shown in Fig. 1. A total of 297 articles were identified through the search and individually examined after duplicates were removed. Full-text screening of 12 articles was performed. Seven of those articles met the inclusion criteria and were therefore included in this review.
Risk of Bias and Quality Assessment
The risk of bias was assessed by two independent reviewers using the Critical Appraisal Checklist for Analytical Cross-Sectional Studies [38] and the Critical Appraisal Checklist for Quasi-Experimental Studies [39] from the Joanna Briggs Institute [JBI]. These scales assess the quality of studies based on eight and nine items respectively. The percentage of “yes” answers in the JBI was computed for each reviewed study, and an interrater reliability assessment using Cohen´s Kappa coefficients was provided. Disagreement on the assessed studies was solved by discussion between the two judges.
Outcomes
The outcomes were: 1) feasibility (adherence of using the PAT) and acceptability (satisfaction and perceived utility of the PAT), 2) substance use (alcohol, tobacco, and illicit substances, craving, and withdrawal) 3) PA (steps per day, moderate-to-vigorous PA) 4) emotion-related variables (depression, anxiety, and positive and negative affect).
Data Analysis
A narrative synthesis was performed to analyze the effects of using PAT on outcomes. Feasibility, acceptability, PA, and emotional variables were extracted and summarized using the quantitative and qualitative instruments reported in the studies. Cohen´s Kappa was used to quantify the level of agreement between judges when assessing the methodological quality of the studies included. The criteria used were: K < 0; no agreement, K = 0–0.20; insignificant, K = 0.21–0.40; low, 0.41–0.60 moderate, K = 0.61–0.80; good, K = 0.81–1; very good.
Results
Study Characteristics and Participants
The seven studies involved a total of 178 participants. The mean number of participants per study was 30 (SD = 15.93) and the sample sizes in each study ranged from 15 to 60 participants. The mean age of the participants was 33.67 (SD = 15.52) and 39.5% were male. Although all commercially-available PAT were considered, only Fitbit devices (i.e., Fitbit Flex, Charge, Alta, Alpha, and Charge HR) were used in the studies reviewed. The average time spent wearing the PAT ranged from 3 to 12 weeks. All the studies were conducted in the United States of America.
There were four (57.15%) observational studies that focused on the relationship between drug or alcohol use, PA, and mental health variables [40,41,42,43]. The remaining three (42.85%) were clinical trials that tested the effects of a PA promotion intervention—combined with a PAT—on substance use, physical activity habits, and emotion-related variables (anxiety, depression, positive affect, and negative affect) [33, 34, 44]. All clinical trials were single-armed and did not include follow-ups after the intervention. Incentives to comply with the study objectives were used in three studies (42.85%) [34, 40, 43].
In terms of target substances, the three clinical trials (42.85%) focused on alcohol [33, 43, 44], three of the observational studies (42.85%) targeted smoking [40,41,42], and one (14.28%) focused on opioids [34] (Table 1).
Feasibility and Acceptability Outcomes
Five of the seven studies (71.42%) provided adherence outcomes [33, 34, 41,42,43] and five (71.42%) reported results on the acceptability of PAT [33, 34, 41,42,43]. In terms of adherence outcomes, five studies (71.42%) [33, 34, 41,42,43] reported the number of valid data-gathering days, one study (14.28%) [43] reported the proportion of time wearing the PAT (including all 24 h/day) and for each of the 1 h prior to an EMA assessment, and one study (14.28%) [33] reported the number of times participants checked the Fitbit display per day (e.g. monitoring the steps taken or their active minutes that day). Five (71.42%) studies [33, 34, 41, 42, 44] reported data on participants’ perceived utility of the PAT, and two (28.5%) studies [33, 34] reported satisfaction with the intervention.
In Abrantes et al. (2017), at least 8 h of use of PAT was reported on 73% of days [33]. Participants reported checking their PAT display to monitor steps an average of 8.4 (SD = 6.8) times throughout the day. A similar study with methadone maintenance treatment seekers by Abrantes et al. (2021) reported 62.5% (SD = 27.6) of possible days (i.e., 8 h of data recording) using the activity tracker [34]. Sixty-nine percent of the participants wore the activity tracker for at least 6 weeks, 57.7% wore it for at least 9 weeks, and 26.9% wore it for the entire 12 weeks of the study. No data was reported about hours per day of PAT use.
In Stevenson et al. (2022), the mean percentage of days participants wore the Fitbit was 81.03% (SD = 20.38, range 24.02–98.53%) [43]. Participants had worn the PAT the previous hour up to 91.28% of the time when an EMA was completed (SD = 25.51, range 0–100%). 4445 data recordings from a possible 5040 (88.19% valid days) were obtained in another two observational studies by Shan et al. (2020) and Silverman-Lloyd et al. (2018) [41, 42]. Days with two or more 90-min non-wear periods (between 10 am and 10 pm) and wear times of less than 6 h in the target time window were considered not valid.
Concerning acceptability and perceived utility, two studies used the Participant Experience Questionnaire (PEQ), made up of 18 items using a 5-point Likert scale from 1 = strongly disagree to 5 = strongly agree, to measure overall satisfaction levels with the interventions tested [33, 34]. Abrantes et al. (2021) [34] indicated general satisfaction with the activity tracker of 4.38 out of 5, while the other study by Abrantes et al. (2017) [33] study reported a total mean score of 74.9 in the PEQ (range 18–90), with higher scores indicating greater satisfaction. Five studies did not report quantitative measures of satisfaction with activity tracker use [40,41,42,43,44].
Four studies used either semi-structured or qualitative interviews [33, 34, 41, 42]. In the study by Abrantes et al. (2017) [33], participants reported “good opinions” on the activity tracker used in combination with the physical activity intervention (e.g., “It just brought a lot of awareness. I wasn’t paying attention to it before. Now it’s made it so easy to pay attention. And it makes me accountable"). Another study by Abrantes et al. (2021) [34] reported that the activity tracker helped SUD treatment seekers improve their physical activity levels and make positive changes in their lives. This information was extracted from qualitative interviews but no specific examples or quotes were included in the study. The participants of the other two studies by Shan et al. (2020) and Silverman-Lloyd et al. (2018) (i.e. [41, 42].) reported being more aware of their physical activity thanks to the activity tracker from yes/no questions included in the end-of-study survey (i.e. Did wearing the Fitbit increase your awareness about daily physical activity?). Three studies did not report qualitative measures of satisfaction with activity tracker use [40, 43, 44].
Substance Use Outcomes
The three clinical trials [33, 34, 44] used the timeline follow-back (TLFB) method to measure alcohol-use outcomes and reported a reduction in daily alcohol use after 12 weeks (see Table 2). A significant pre-post decrease in alcohol use was found in two studies [33, 44] (see Table 2). In addition, Linke et al. (2019) [44] reported a significant reduction in the number of drinks consumed per day. However, no statistically significant differences were found in alcohol cravings and drinks per day in Abrantes et al. (2017) [33]. Additionally, Abrantes et al. (2021) [34] reported a reduction in days of drug use in the previous 3 months, with small-to-medium effect sizes (cannabis reduction d = 0.18, cocaine d = 0.37, illicit opioids d = 0.41), while Linke et al. (2019) [44] found statistically significant differences in days of daily drug use per month.
The four observational studies [40,41,42,43] explored the relationship between health-related behaviors such as sleep habits, PA, and substance use outcomes. Notably, craving was considered the principal outcome in all of the non-clinical studies included in this review. In Purani et al. (2019) [40], poor sleep quality was associated with increased tobacco craving and withdrawal. Shan et al. (2020) and Silverman-Lloyd et al. (2018) [41, 42] found that smoking urges were significantly related to recent steps (steps taken in the 30, 60, and 120 min prior to a reported urge) but not to daily steps and MVPA. However, the relationship between steps taken and smoking urges was mixed, and was strong and consistently inverse in some participants. Similarly, Stevenson et al. (2022) [43] used EMA to study the relationships between alcohol urges and steps per day and found a small inverse effect. No association was found between alcohol urges and steps in previous hours.
Physical Activity Outcomes
All three clinical trials reported an increase in PA [34, 34, 44] (see Table 3). Abrantes et al. (2017) [33] reported a statistically significant increase in the number of steps per day and general PA duration between baseline and the end-of-treatment after 12 weeks. Non-significant differences were found in the average weekly MVPA duration (minutes) between baseline (M = 48.49 SD = 71.62) and end-of-treatment (M = 77.21 SD = 57.43). In addition, there was an increase in days when participants exercised to cope with SUD between baseline (M = 2.90 SD = 5.98) and end-of-treatment (M = 15.67 SD = 16.96). In Abrantes et al. (2021) [34], participants were recommended to take 10,000 steps/day and to spend 150 min a week doing MVPA. The mean number of steps per day during the intervention was 10,572 (SD = 4409). Linke et al. (2019) [44] reported a positive small effect size (d = 0.23) in the increase in number of steps per day from Week 1 to Week 12, despite these differences not being statistically significant. Nevertheless, it is important to note that the baseline in week 1 was above 10,000 steps/day in participants completing the study (n = 11), above the WHO recommendations [45].
The observational studies described their participants’ PA habits. Shan et al. (2020) and Silverman-Lloyd et al. (2018) [41, 42] reported a median of 7807 steps per day, with no differences over the study. The median total MVPA minutes per week was 80 (IQR 31–162). The median number of minutes spent on MVPA per day was significantly higher in men (12 min, IQR 3–20) than in women (3.5 min, IQR 1–9). The median number of minutes spent on lighter activity per day was also significantly higher in men (34 min, IQR 26–52) than in women (18 min, IQR 15–23).
Stevenson et al. (2022) [43] found that step count did not vary throughout the study (M = 8,183 SD = 5,560). Fifty-six percent (14/25) of the participants engaged in bouts of moderate-intensity exercise lasting at least 10 min. The percentage of time wearing the Fitbit was not significantly related to the number of bouts of exercise.
Mental Health Outcomes
The three clinical trials examined changes in mental health [33, 34, 44] (see Table 4). Abrantes et al. (2017) [33] found significant decreases in levels of depression, anxiety, and negative affect and an increase in levels of positive affect between baseline and end-of-treatment.
The other two studies showed that wearing an activity tracker may have had a positive, but not statistically significant effect on mental health outcomes. Abrantes et al. (2021) [34] found no difference between baseline and end-of-treatment for depression, anxiety, or negative affect. However, they reported a medium effect size for an increase in positive affect (d = 0.63). Similarly, Linke et al. (2019) [44] found no statistically significant differences in positive affect, negative affect, or depression between baseline and end-of-treatment, although descriptive statistics showed differences in levels of depression.
Methodological Quality Assessment
The Cohen`s Kappa for the assessed studies ranged between 0.41 (moderate) and 1 (very good). Cohen`s Kappa for quasi-experimental and cross-sectional studies were K = 0.83 (very good) and K = 0.645 (good) respectively. After discussion, the two independent reviewers agreed on 100% of the items.
Two-thirds of the quasi-experimental studies, 66.6% (n = 2) complied with 4/8 items and 33.3% (n = 1) complied with 5/8 items (see Table 5). The principal limitations of the studies were mainly due to exposure to different conditions other than the activity tracker that can confound the main outcomes (e.g., steps/day, improvements in mental health); the lack of control groups, and limitations in statistical analysis.
On the other hand, 25% (n = 1) of the cross-sectional studies complied with 4/7 items on the JBI checklist; 25% (n = 1) complied with 5/7 items and 50% (n = 2) complied with 7/7 items. Studies that had the lowest scores in the risk assessment did not include a clear description of the study subject or settings and lacked strategies to deal with confounding factors (Table 6).
Discussion
PAT are a promising approach for the investigation and treatment of substance use. To the best of our knowledge, this is the first systematic review to summarize the evidence for the feasibility of using PAT in people with SUD and its effects on substance use, PA, and mental health.
Feasibility and acceptability outcomes indicated high adherence to using activity trackers in terms of time of use and satisfaction. The percentage of time using the activity tracker was very high, over 62% of days in all the studies. Additionally, satisfaction and perceived utility were high when reported. Overall, using activity trackers seems feasible for various purposes, such as assessing health-related variables or complementing interventions to promote PA in the SUD context. This is consistent with the previous literature about using activity trackers with other populations, such as people with serious mental illness, elderly adults, and young people and adults from the general population [16, 46, 47]. The high levels of compliance suggest that incorporating activity trackers into treatment may be acceptable with SUD patients. Moreover, adherence and acceptability may be enhanced through several methods such as offering financial incentives [33, 34, 44] or adding personalized feedback or sessions to address possible problems (e.g. difficulties in synchronizing or operating the tracker) using the trackers [41].
The combination of activity trackers and other interventions (peer-facilitated intervention, programs to promote PA, or counseling) may promote PA and consequently, improve substance use outcomes (days of alcohol and drug use) in clinical settings. All the reviewed studies found a reduction in days of alcohol and drug use [33, 34, 44]. Nevertheless, the evidence of these devices’ effectiveness is very weak due to the lack of studies testing solely the effect of the PAT. Various factors may contribute to the positive effect of activity trackers on substance use outcomes. PAT incorporate behavioral change techniques [14] that have demonstrated that they can increase PA. This may have an impact on reducing substance craving and negative affect leading to better treatment results. Using wearable PAT may increase patients’ motivation and adherence to treatment as a whole, not only improving engagement with PA but also enhancing adherence to SUD-specific treatment as some studies in other populations have suggested [48]. However, this specific effect should be explored in future research on using PAT in treating SUD. All three clinical trials reported an increase in PA, which in turn has a positive effect on reducing anxiety, drug craving, and withdrawal [49,50,51, 52•]. Nevertheless, the results of PA in observational studies were mixed [41,42,43]. These inconsistent results might be a consequence of the complex relationship between PA and substance use [53]. Variables such as intensity, frequency, and exercise time are determinants in understanding the relationship between PA and substance use. It is important to bear in mind that the maximum benefit of PA on craving and withdrawal symptom reduction is achieved in medium or high amounts, and the benefits are smaller when the exercise level is low [52•].
In addition to increases in PA, Abrantes et al. (2017) [33] found improvements in depression, anxiety, and levels of negative affect, as well as an increase in levels of positive affect. Small changes in mental health variables were also found in other studies [34, 44]. Physical activity is known to have a positive effect on emotional regulation [52•, 54] and depression symptoms [46], and these devices may be able to improve mental health outcomes mediated by PA increase [18••].
The results from this systematic review are of clinical importance, however, there are several limitations intrinsic to the reviewed studies that should be addressed. Firstly, there was substantial variety in study designs, which may have affected comparability. Most of the studies in this review used designs (e.g., single-armed) that were not designed to isolate the effect of PAT, and small sample sizes that meant we could not extract firm conclusions about the effects of the activity trackers. In additon, the heterogeneity of the participants' characteristics limits the comparison of the results. Nevertheless, including such populations can be considered as one of the review’s strengths, as it is in line with the most current transdiagnostic conceptualizations of addictive behaviors. Another limitation was that the reviewed studies were rated as moderate in terms of methodological quality, with the cross-sectional studies having lower scores than the quasi-experimental studies. Finally, some of the studies did not report any specific instructions given to the participants, so we do not know the number of hours per day a participant should be using the activity tracker and therefore cannot calculate the actual time of use from the total. Although some studies reported the criteria for excluding a day of use or for excluding a participant from the analysis, this is not a precise way of reporting adherence. Beyond studying adherence, missing data plays an important role in making conclusions from the data. Not considering missing data can bias the final results of the studies.
Conclusions and Future Directions
Despite the limitations, the current study provides preliminary evidence that using PAT as part of a broader behavioral intervention is feasible and promising in promoting physical activity and improving health-related outcomes, including a reduction in substance use. Considering the small number of studies and their limitations, future randomized clinical trials should be designed to isolate the effect of PAT and to test the long-term effectiveness of incorporating these devices into existing interventions for SUD.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Burgess-Hull A, Epstein DH. Ambulatory assessment methods to examine momentary state-based predictors of opioid use behaviors. Curr Addict Rep. 2021;8:122–35. https://doi.org/10.1007/s40429-020-00351-7.
Bell IH, Lim MH, Rossell SL, Thomas N. Ecological momentary assessment and intervention in the treatment of psychotic disorders: a systematic review. Psychiatr Serv Wash DC. 2017;68:1172–81. https://doi.org/10.1176/appi.ps.201600523.
Smith TO, McKenna MC, Salter C, Hardeman W, Richardson K, Hillsdon M, et al. A systematic review of the physical activity assessment tools used in primary care. Fam Pract. 2017;34:384–91. https://doi.org/10.1093/fampra/cmx011.
Hall M, Scherner PV, Kreidel Y, Rubel JA. A systematic review of momentary assessment designs for mood and anxiety symptoms. Front Psychol. 2021;12. https://doi.org/10.3389/fpsyg.2021.642044.
Trull TJ, Ebner-Priemer U. Ambulatory assessment. Annu Rev Clin Psychol. 2013;9:151–76. https://doi.org/10.1146/annurev-clinpsy-050212-185510.
Fonseca-Pedrero E, Ródenas-Perea G, Pérez-Albéniz A, Al-Halabí S, Pérez M, Muñiz J. The time of ambulatory assessment. Papeles Psicol. 2022;2022:21–8. https://doi.org/10.23923/pap.psicol.2983.
Goldfine C, Lai JT, Lucey E, Newcomb M, Carreiro S. Wearable and wireless mhealth technologies for substance use disorder. Curr Addict Rep. 2020;7:291–300. https://doi.org/10.3390/ijerph182111357.
Chu AHY, Ng SHX, Paknezhad M, Gauterin A, Koh D, Brown MS, et al. Comparison of wrist-worn Fitbit Flex and waist-worn ActiGraph for measuring steps in free-living adults. PLoS ONE. 2017;12:e0172535. https://doi.org/10.1371/journal.pone.0172535.
Vaghefi I, Tulu B. The continued use of mobile health apps: insights from a longitudinal study. JMIR MHealth UHealth. 2019;7:e12983. https://doi.org/10.2196/12983.
Fritz T, Huang EM, Murphy GC, Zimmermann T. Persuasive technology in the real world: a study of long-term use of activity sensing devices for fitness. Proc SIGCHI Conf Hum Factors Comput Syst. 2014;487–96. https://doi.org/10.1145/2556288.2557383.
Uthman OA, Nduka CU, Abba M, Enriquez R, Nordenstedt H, Nalugoda F, et al. Comparison of mHealth and face-to-face interventions for smoking cessation among people living with HIV: meta-analysis. JMIR MHealth UHealth. 2019;7:e9329. https://doi.org/10.2196/mhealth.9329.
Shin G, Jarrahi MH, Fei Y, Karami A, Gafinowitz N, Byun A, et al. Wearable activity trackers, accuracy, adoption, acceptance and health impact: A systematic literature review. J Biomed Inform. 2019;93:103153. https://doi.org/10.1016/j.jbi.2019.103153.
Witt DR, Kellogg RA, Snyder MP, Dunn J. Windows into human health through wearables data analytics. Curr Opin Biomed Eng. 2019;9:28–46. https://doi.org/10.1016/j.cobme.2019.01.001.
Mercer K, Li M, Giangregorio L, Burns C, Grindrod K. Behavior change techniques present in wearable activity trackers: a critical analysis. JMIR MHealth UHealth. 2016;4:e40. https://doi.org/10.2196/mhealth.4461.
Mercer K, Giangregorio L, Schneider E, Chilana P, Li M, Grindrod K. Acceptance of commercially available wearable activity trackers among adults aged over 50 and with chronic illness: a mixed-methods evaluation. JMIR MHealth UHealth. 2016;4(1):e7. https://doi.org/10.2196/mhealth.4225.
Naslund JA, Aschbrenner KA, Bartels SJ. Wearable devices and smartphones for activity tracking among people with serious mental illness. Ment Health Phys Act. 2016;10:10–7. https://doi.org/10.1016/j.mhpa.2016.02.001.
Ridgers ND, McNarry MA, Mackintosh KA. Feasibility and effectiveness of using wearable activity trackers in youth: a systematic review. JMIR MHealth UHealth. 2016;4:e6540. https://doi.org/10.2196/mhealth.6540.
•• Brickwood K-J, Watson G, O’Brien J, Williams AD. Consumer-based wearable activity trackers increase physical activity participation: systematic review and meta-analysis. JMIR MHealth UHealth. 2019;7:e11819. https://doi.org/10.2196/11819. A meta-analytic study summarizing the randomized controlled trial of commercially available PAT in adults.
•• Ferguson T, Olds T, Curtis R, Blake H, Crozier AJ, Dankiw K, et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digit Health. 2022;4:e615–26. https://doi.org/10.1016/S2589-7500(22)00111-X. An umbrella review that includes 39 reviews about the impact of using PAT on health outcomes.
Oliveira JS, Sherrington C, Zheng ERY, Franco MR, Tiedemann A. Effect of interventions using PAT on physical activity in people aged 60 years and over: a systematic review and meta-analysis. Br J Sports Med. 2020;54:1188–94. https://doi.org/10.1136/bjsports-2018-100324.
•• Blount DS, McDonough DJ, Gao Z. Effect of wearable technology-based physical activity interventions on breast cancer survivors’ physiological, cognitive, and emotional outcomes: a systematic review. J Clin Med. 2021;10:2015. https://doi.org/10.3390/jcm10092015. A systematic review examining the effects of physical activity interventions assisted with wearables on physiological, cognitive, and emotional outcomes in breast cancer survivors.
Jo A, Coronel BD, Coakes CE, Mainous AG. Is there a benefit to patients using wearable devices such as fitbit or health apps on mobiles? A systematic review. Am J Med. 2019;132:1394-1400.e1. https://doi.org/10.1016/j.amjmed.2019.06.018.
• Laranjo L, Ding D, Heleno B, Kocaballi B, Quiroz JC, Tong HL, et al. Do smartphone applications and activity trackers increase physical activity in adults? Systematic review, meta-analysis and metaregression. Br J Sports Med. 2021;55:422–32. https://doi.org/10.1136/bjsports-2020-102892. This article summarizes the evidence of the effect of smartphone applications and activity trackers on physical activity in the general adult population.
Farnell G, Barkley J. The effect of a wearable physical activity monitor (Fitbit One) on physical activity behaviour in women: a pilot study. J Hum Sport Exerc. 2017;4:1230–7. https://doi.org/10.14198/jhse.2017.124.09.
•• Lynch C, Bird S, Lythgo N, Selva-Raj I. Changing the physical activity behavior of adults with fitness trackers: a systematic review and meta-analysis. Am J Health Promot AJHP. 2020;34:418–30. https://doi.org/10.1177/089011711989520. A systematic review focusing on the evidence of PAT to change physical activity in adults.
Aas CF, Vold JH, Gjestad R, Skurtveit S, Lim AG, Gjerde KV, et al. Substance use and symptoms of mental health disorders: a prospective cohort of patients with severe substance use disorders in Norway. Subst Abuse Treat Prev Policy. 2021;16:20. https://doi.org/10.1186/s13011-021-00354-1.
McHugh R. Alcohol use disorder and depressive disorders. Alcohol Res Curr Rev. 2019;40. https://doi.org/10.35946/arcr.v40.1.01.
Weiss NH, Kiefer R, Goncharenko S, Raudales AM, Forkus SR, Schick MR, et al. Emotion regulation and substance use: a meta-analysis. Drug Alcohol Depend. 2022;230:109131. https://doi.org/10.1016/j.drugalcdep.2021.109131.
Kopp M, Burtscher M, Kopp-Wilfling P, Ruedl G, Kumnig M, Ledochowski L, et al. Is there a link between physical activity and alcohol use? Subst Use Misuse. 2015;50:546–51. https://doi.org/10.3109/10826084.2014.980957.
Rosen D, Smith ML, Reynolds CF. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2008;16:488–97. https://doi.org/10.1097/JGP.0b013e31816ff35a.
Conroy DA, Arnedt JT. Sleep and substance use disorders: an update. Curr Psychiatry Rep. 2014;16:487. https://doi.org/10.1007/s11920-014-0487-3.
Takano A, Ono K, Nozawa K, Sato M, Onuki M, Sese J, et al. Wearable sensor and mobile app-based mhealth approach for investigating substance use and related factors in daily life: protocol for an ecological momentary assessment study. JMIR Res Protoc. 2023;12:e44275. https://doi.org/10.2196/44275.
Abrantes AM, Blevins CE, Battle CL, Read JP, Gordon AL, Stein MD. Developing a Fitbit-supported lifestyle physical activity intervention for depressed alcohol dependent women. J Subst Abuse Treat. 2017;80:88–97. https://doi.org/10.1016/j.jsat.2017.07.006.
Abrantes AM, Van Noppen D, Bailey G, Uebelacker LA, Buman M, Stein MD. A feasibility study of a peer-facilitated physical activity intervention in methadone maintenance. Ment Health Phys Act. 2021;21:100419. https://doi.org/10.1016/j.mhpa.2021.100419.
Franssen WMA, Franssen GHLM, Spaas J, Solmi F, Eijnde BO. Can consumer wearable activity tracker-based interventions improve physical activity and cardiometabolic health in patients with chronic diseases? A systematic review and meta-analysis of randomised controlled trials. Int J Behav Nutr Phys Act. 2020;17:57. https://doi.org/10.1186/2Fs12966-020-00955-2.
Shiffman S. Ecological Momentary Assessment (EMA) in studies of substance use. Psychol Assess. 2009;21:486–97. https://doi.org/10.1037/a0017074.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu P-F. Chapter 7: Systematic reviews of etiology and risk . In: Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. JBI, 2020. Available from https://synthesismanual.jbi.global
Barker TH, Habibi N, Aromataris E, Stone JC, Leonardi-Bee J, Sears K, et al. The revised JBI critical appraisal tool for the assessment of risk of bias quasi-experimental studies. JBI Evid Synth. 2024;22(3):378–88.
Purani H, Friedrichsen S, Allen AM. Sleep quality in cigarette smokers: associations with smoking-related outcomes and exercise. Addict Behav. 2019;90:71–6. https://doi.org/10.1016/j.addbeh.2018.10.023.
Shan R, Yanek LR, Silverman-Lloyd LG, Kianoush S, Blaha MJ, German CA, et al. Using mobile health tools to assess physical activity guideline adherence and smoking urges: secondary analysis of mactive-smoke. JMIR Cardio. 2020;4:e14963. https://doi.org/10.2196/14963.
Silverman-Lloyd LG, Kianoush S, Blaha MJ, Sabina AB, Graham GN, Martin SS. mActive-smoke: a prospective observational study using mobile health tools to assess the association of physical activity with smoking urges. JMIR MHealth UHealth. 2018;6:e121. https://doi.org/10.2196/mhealth.9292.
Stevenson BL, Kunicki ZJ, Brick L, Blevins CE, Stein M, Abrantes AM. Using ecological momentary assessments and fitbit data to examine daily associations between physical activity, affect and alcohol cravings in patients with alcohol use disorder. Int J Behav Med. 2022;29:543–52. https://doi.org/10.1007/s12529-021-10039-5.
Linke SE, Hovsepians R, Schnebly B, Godfrey K, Noble M, Strong DR, et al. The Go-Var (Veterans Active Recovery): An adjunctive, exercise-based intervention for veterans recovering from substance use disorders. J Psychoactive Drugs. 2019;51:68–77. https://doi.org/10.1080/02791072.2018.1560518.
World Health Organization. Global status report on physical activity. Geneva: World Health Organization; 2022.
Zhang T, Wang K, Li N, Hurr C, Luo J. The relationship between different amounts of physical exercise, internal inhibition, and drug craving in individuals with substance-use disorders. Int J Environ Res Public Health. 2021;18:12436. https://doi.org/10.1186/s12877-022-02931-w.
Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act. 2015;12:159. https://doi.org/10.1186/s12966-015-0314-1.
Janevic MR, Shute V, Murphy SL, Piette JD. Acceptability and effects of commercially available activity trackers for chronic pain management among older African American adults. Pain Med. 2020;21:e68-78. https://doi.org/10.1093/pm/pnz215.
Zhang Z, Giordani B, Margulis A, Chen W. Efficacy and acceptability of using wearable activity trackers in older adults living in retirement communities: a mixed method study. BMC Geriatr. 2022;22:231. https://doi.org/10.1186/s12877-022-02931-w.
Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, et al. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS ONE. 2011;6:e17465. https://doi.org/10.1371/journal.pone.0017465.
Ellingsen MM, Clausen T, Johannesen SL, Martinsen EW, Hallgren M. Effects of acute exercise on drug craving in adults with poly-substance use disorder. A randomized controlled trial. Ment Health Phys Act. 2021;21:1–9. https://doi.org/10.2196/18553.
• Ye X, Liu R. Intervention effect of aerobic exercise on physical fitness, emotional state and mental health of drug addicts: a systematic review and meta-analysis. Int J Environ Res Public Health. 2023;20:2272. https://doi.org/10.3390/2Fijerph20032272. This research aimed to analyze the effects of aerobic exercise-based intervention on multiple outcomes related to physical and mental health in people who use substances.
Martinez-Calderon J, Villar-Alises O, García-Muñoz C, Matias-Soto J. Evidence level of physical exercise in the treatment of substance abuse/dependence: an overview of systematic reviews including 53 meta-analyses that comprised 103 distinct clinical trials. Ment Health Phys Act. 2023;24:100519. https://doi.org/10.1016/j.mhpa.2023.100519.
Singh B, Olds T, Curtis R, Dumuid D, Virgara R, Watson A, et al. Effectiveness of physical activity interventions for improving depression, anxiety and distress: an overview of systematic reviews. Br J Sports Med. 2023;57:1203–9. https://doi.org/10.1136/bjsports-2022-106195.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was funded by the Foundation for the Promotion of Applied Scientific Research and Technology (FICYT) -Principality of Asturias- (Spain) (SV-PA-21-AYUD/2021/50884). The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. I.C-L. and A.G-R. managed the literature searches, conducted the statistical analyses and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have nothing to disclose.
Human and Animal Rights and Informed Consent
No human subjects by the authors were used in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Digital Health Technology
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cuesta-López, I., Secades-Villa, R. & González-Roz, A. Feasibility and Effects of Using Physical Activity Trackers With People Who Use Substances: A Systematic Review. Curr Addict Rep 11, 713–723 (2024). https://doi.org/10.1007/s40429-024-00573-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40429-024-00573-z