Abstract
Purpose of Review
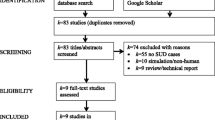
Co-occurring substance use disorders (SUDs) are highly common in individuals with psychiatric illnesses. Individuals with comorbid psychiatric illness and SUDs may experience poorer mental health and decreased treatment efficacy. However, there are no FDA-approved treatments for co-occurring substance use and psychiatric disorders. Repetitive transcranial magnetic stimulation (rTMS), a form of non-invasive brain stimulation that uses an electromagnetic field to change brain activity and behavior, may be a promising treatment for co-occurring disorders. A comprehensive literature search of PubMed, MEDLINE, and Google Scholar databases was conducted, and records were manually reviewed to include all studies testing the effects of rTMS for co-occurring SUDs.
Recent Findings
Eleven studies met our inclusion criteria. The majority (7/11) assessed rTMS for the treatment of schizophrenia and co-occurring substance use, and the remaining three studies assessed rTMS for the treatment of SUDs in mood and anxiety disorders. Potential neural circuitry targets for the treatment of co-occurring substance use and post-traumatic stress disorder, anxiety disorder, and bipolar disorder are discussed. We identify future directions and considerations for rTMS treatment and research. Namely, we recommend identification of novel treatment targets, the use of pragmatic treatment approaches, the evaluation of rTMS for substance withdrawal, the evaluation of state dependence as a predictor of treatment efficacy, the use of neurobiological measurements to identify underlying neural circuitry, and the assessment of individual predictors of rTMS treatment response.
Summary
There is preliminary evidence suggesting rTMS may be effective to treat co-occurring disorders, but additional research is needed.

Similar content being viewed by others
Data Availability
No datasets were generated or analyzed during the current study.
References
Cuffel BJ. Comorbid substance use disorder: prevalence, patterns of use, and course. New Dir Ment Health Serv. 1996;1996(70):93–105.
Key substance use and mental health indicators in the United States: results from the 2022 National Survey on Drug Use and Health [Internet]. Center for Behavioral Health Statistics and Quality: Substance Abuse and Mental Health Services Administration; 2023 Nov. (NSDUH Series H-58). Report No.: HHS Publication No. PEP23-07-01-006. Available from: https://www.samhsa.gov/data/report/2022-nsduh-annual-national-report
Drake RE, Mueser KT. Psychosocial approaches to dual diagnosis. Schizophr Bull. 2000;26(1):105–18.
Drake RE, McLaughlin P, Pepper B, Minkoff K. Dual diagnosis of major mental illness and substance disorder: an overview. New Dir Ment Health Serv. 1991;1991(50):3–12.
Harris MG, Bharat C, Glantz MD, Sampson NA, Al-Hamzawi A, Alonso J, et al. Cross-national patterns of substance use disorder treatment and associations with mental disorder comorbidity in the WHO World Mental Health Surveys. Addiction. 2019;114(8):1446–59.
Harris KM, Edlund MJ. Use of mental health care and substance abuse treatment among adults with co-occurring disorders. Psychiatr Serv. 2005;56(8):954–9.
Alsuhaibani R, Smith DC, Lowrie R, Aljhani S, Paudyal V. Scope, quality and inclusivity of international clinical guidelines on mental health and substance abuse in relation to dual diagnosis, social and community outcomes: a systematic review. BMC Psychiatry. 2021;21(1):209.
Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimulat. 2016;9(3):336–46.
Bart CP, Titone MK, Ng TH, Nusslock R, Alloy LB. Neural reward circuit dysfunction as a risk factor for bipolar spectrum disorders and substance use disorders: a review and integration. Clin Psychol Rev. 2021;87:102035.
Carlson HN, Weiner JL. The neural, behavioral, and epidemiological underpinnings of comorbid alcohol use disorder and post-traumatic stress disorder. In: International Review of Neurobiology [Internet]. Elsevier; 2021 [cited 2023 Nov 26]. p. 69–142. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0074774220301446
Michaels TI, Stone E, Singal S, Novakovic V, Barkin RL, Barkin S. Brain reward circuitry: the overlapping neurobiology of trauma and substance use disorders. World J Psychiatry. 2021;11(6):222–31.
Mellick WH, Tolliver BK, Brenner HM, Anton RF, Prisciandaro JJ. Alcohol cue processing in co-occurring bipolar disorder and alcohol use disorder. JAMA Psychiat. 2023;80(11):1150.
Stoychev KR. Neuroimaging studies in patients with mental disorder and co-occurring substance use disorder: summary of findings. Front Psychiatry. 2019;23(10):702.
Ryan JE, Veliz P, McCabe SE, Stoddard SA, Boyd CJ. Association of early onset of cannabis, cigarette, other drug use and schizophrenia or psychosis. Schizophr Res. 2020;215:482–4.
Wing VC, Wass CE, Soh DW, George TP. A review of neurobiological vulnerability factors and treatment implications for comorbid tobacco dependence in schizophrenia. Ann N Y Acad Sci. 2012;1248(1):89–106.
Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104(4):653–60.
Wing VC, Bacher I, Wu BS, Daskalakis ZJ, George TP. High frequency repetitive transcranial magnetic stimulation reduces tobacco craving in schizophrenia. Schizophr Res. 2012;139(1–3):264–6.
Prikryl R, Ustohal L, Kucerova HP, Kasparek T, Jarkovsky J, Hublova V, et al. Repetitive transcranial magnetic stimulation reduces cigarette consumption in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2014;49:30–5.
Huang W, Shen F, Zhang J, Xing B. Effect of repetitive transcranial magnetic stimulation on cigarette smoking in patients with schizophrenia. Shanghai Arch Psychiatry. 2016;28(6):309–17.
Cordes J, Falkai P, Guse B, Hasan A, Schneider-Axmann T, Arends M, et al. Repetitive transcranial magnetic stimulation for the treatment of negative symptoms in residual schizophrenia: rationale and design of a sham-controlled, randomized multicenter study. Eur Arch Psychiatry Clin Neurosci. 2009;259(S2):189–97.
Kamp D, Engelke C, Wobrock T, Kunze B, Wölwer W, Winterer G, et al. Letter to the Editor: Influence of rTMS on smoking in patients with schizophrenia. Schizophr Res. 2018;192:481–4.
Kozak K, Sharif-Razi M, Morozova M, Gaudette EV, Barr MS, Daskalakis ZJ, et al. Effects of short-term, high-frequency repetitive transcranial magnetic stimulation to bilateral dorsolateral prefrontal cortex on smoking behavior and cognition in patients with schizophrenia and non-psychiatric controls. Schizophr Res. 2018;197:441–3.
Pallanti S, Di Ponzio M, Makris N, Kubicki M. Theta-burst stimulation over the pre-supplementary motor area in schizophrenia and comorbid substance use disorder: preliminary clinical data. Ann Psychiatry Treat. 2022;6(1):028–32.
Bidzinski KK, Lowe DJE, Sanches M, Sorkhou M, Boileau I, Kiang M, et al. Investigating repetitive transcranial magnetic stimulation on cannabis use and cognition in people with schizophrenia. Schizophrenia. 2022;8(1):2.
Johnstone S, Lowe DJE, Kozak-Bidzinski K, Sanches M, Castle DJ, Rabin JS, et al. Neurocognitive moderation of repetitive transcranial magnetic stimulation (rTMS) effects on cannabis use in schizophrenia: a preliminary analysis. Schizophrenia. 2022;8(1):99.
Girardi P, Rapinesi C, Chiarotti F, Kotzalidis GD, Piacentino D, Serata D, et al. Add-on deep transcranial magnetic stimulation (dTMS) in patients with dysthymic disorder comorbid with alcohol use disorder: a comparison with standard treatment. World J Biol Psychiatry. 2015;16(1):66–73.
Rapinesi C, Curto M, Kotzalidis GD, Del Casale A, Serata D, Ferri VR, et al. Antidepressant effectiveness of deep transcranial magnetic stimulation (dTMS) in patients with major depressive disorder (MDD) with or without alcohol use disorders (AUDs): a 6-month, open label, follow-up study. J Affect Disord. 2015;174:57–63.
Janes AC, Zegel M, Ohashi K, Betts J, Molokotos E, Olson D, et al. Nicotine normalizes cortico-striatal connectivity in non-smoking individuals with major depressive disorder. Neuropsychopharmacology. 2018;43(12):2445–51.
Vieyra-Reyes P, Mineur YS, Picciotto MR, Túnez I, Vidaltamayo R, Drucker-Colín R. Antidepressant-like effects of nicotine and transcranial magnetic stimulation in the olfactory bulbectomy rat model of depression. Brain Res Bull. 2008;77(1):13–8.
Abdelrahman AA, Noaman M, Fawzy M, Moheb A, Karim AA, Khedr EM. A double-blind randomized clinical trial of high frequency rTMS over the DLPFC on nicotine dependence, anxiety and depression. Sci Rep. 2021;11(1):1640.
UzduYaşar A, Ci̇Nemre B, Erdoğan A. Effects of repetitive transcranial magnetic stimulation (rTMS) treatment in comorbid nicotine addiction with major depressive disorder and obsessive-compulsive disorder. Bağımlılık Derg. 2022;23(3):275–82.
Foad W. Repetitive transcranial magnetic stimulation (rTMS) is associated with increased abstinence in substance use disorder and comorbid depression. Ann Clin Psychiatry [Internet]. 2023 Feb [cited 2023 Nov 26];35(1). Available from: https://www.aacp.com/article/buy_now/?id=793
Young JR, Galla JT, Polick CS, Deng ZD, Dannhauer M, Kirby A, et al. Multimodal smoking cessation treatment combining transcranial magnetic stimulation, cognitive behavioral therapy, and nicotine replacement therapy in veterans with posttraumatic stress disorder: a feasibility randomized controlled trial protocol [Internet]. Addiction Medicine; 2023 Sep [cited 2023 Nov 26]. Available from: http://medrxiv.org/lookup/doi/10.1101/2023.09.06.23294958
Grunze H, Schaefer M, Scherk H, Born C, Preuss UW. Comorbid bipolar and alcohol use disorder-a therapeutic challenge. Front Psychiatry. 2021;12:660432.
Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, et al. What goes up, can come down: novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res. 2015;1628:199–209.
Ward HB, Brady RO, Halko MA, Lizano P. Noninvasive brain stimulation for nicotine dependence in schizophrenia: a mini review. Front Psychiatry. 2022;9(13):824878.
Diefenbach GJ, Bragdon LB, Zertuche L, Hyatt CJ, Hallion LS, Tolin DF, et al. Repetitive transcranial magnetic stimulation for generalised anxiety disorder: a pilot randomised, double-blind, sham-controlled trial. Br J Psychiatry. 2016;209(3):222–8.
Boggio PS, Rocha M, Oliveira MO, Fecteau S, Cohen RB, Campanhã C, et al. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J Clin Psychiatry. 2010;71(08):992–9.
Bystritsky A, Kerwin LE, Feusner JD. A preliminary study of fMRI-guided rTMS in the treatment of generalized anxiety disorder: 6-month follow-up. J Clin Psychiatry. 2009;70(3):431–2.
Cohen H, Kaplan Z, Kotler M, Kouperman I, Moisa R, Grisaru N. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2004;161(3):515–24.
Ibrahim C, Rubin-Kahana DS, Pushparaj A, Musiol M, Blumberger DM, Daskalakis ZJ, et al. The insula: a brain stimulation target for the treatment of addiction. Front Pharmacol. 2019;2(10):720.
Jung J, Lambon Ralph MA, Jackson RL. Subregions of DLPFC display graded yet distinct structural and functional connectivity. J Neurosci. 2022;42(15):3241–52.
Philip NS, Barredo J, Van ’T Wout-Frank M, AR Tyrka M, Price LH, Carpenter LL. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 2018;83(3):263–72.
Harmelech T, Hanlon CA, Tendler A. Transcranial magnetic stimulation as a tool to promote smoking cessation and decrease drug and alcohol use. Brain Sci. 2023;13(7):1072.
Moraga-Amaro R, Muñoz P, Villalobos T, Linsambarth S, Maldonado F, Meirone V, et al. Real-world data of non-invasive stimulation of the human insula-prefrontal cortices using deep TMS to treat anxiety for occupational stress and generalized anxiety disorder. Psychiatry Res. 2023;320:115036.
Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacol. 2010;35(1):169–91.
Adams ZW, McCauley JL, Back SE, Flanagan JC, Hanson RF, Killeen TK, et al. Clinician perspectives on treating adolescents with co-occurring post-traumatic stress disorder, substance use, and other problems. J Child Adolesc Subst Abuse. 2016;25(6):575–83.
Nguyen TD, Hieronymus F, Lorentzen R, McGirr A, Østergaard SD. The efficacy of repetitive transcranial magnetic stimulation (rTMS) for bipolar depression: a systematic review and meta-analysis. J Affect Disord. 2021;15(279):250–5.
Bitter SM, Adler CM, Eliassen JC, Weber WA, Welge JA, Burciaga J, et al. Neurofunctional changes in adolescent cannabis users with and without bipolar disorder. Addiction. 2014;109(11):1901–9.
Martyn FM, McPhilemy G, Nabulsi L, Quirke J, Hallahan B, McDonald C, et al. Alcohol use is associated with affective and interoceptive network alterations in bipolar disorder. Brain Behav. 2023;13(1):e2832.
Upton S, Brown AA, Ithman M, Newman-Norlund R, Sahlem G, Prisciandaro JJ, et al. Effects of hyperdirect pathway theta burst transcranial magnetic stimulation on inhibitory control, craving, and smoking in adults with nicotine dependence: a double-blind, randomized crossover trial. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023;8(11):1156–65.
Sahlem GL, Kim B, Baker NL, Wong BL, Caruso MA, Campbell LA, et al. A preliminary investigation of repetitive transcranial magnetic stimulation applied to the left dorsolateral prefrontal cortex in treatment seeking participants with cannabis use disorder [Internet]. Addiction Medicine; 2023 Jul [cited 2023 Nov 26]. Available from: http://medrxiv.org/lookup/doi/10.1101/2023.07.10.23292461
Chen T, Su H, Li R, Jiang H, Li X, Wu Q, et al. The exploration of optimized protocol for repetitive transcranial magnetic stimulation in the treatment of methamphetamine use disorder: a randomized sham-controlled study. EBioMedicine. 2020;60:103027.
Jin L, Yuan M, Zhang W, Wang L, Chen J, Wang F, et al. Default mode network mechanisms of repeated transcranial magnetic stimulation in heroin addiction. Brain Imaging Behav. 2023;17(1):54–65.
Li X, Hartwell KJ, Henderson S, Badran BW, Brady KT, George MS. Two weeks of image-guided left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation improves smoking cessation: a double-blind, sham-controlled, randomized clinical trial. Brain Stimulat. 2020;13(5):1271–9.
Liang Y, Wang L, Yuan TF. Targeting withdrawal symptoms in men addicted to methamphetamine with transcranial magnetic stimulation: a randomized clinical trial. JAMA Psychiat. 2018;75(11):1199.
Martinotti G, Pettorruso M, Montemitro C, Spagnolo PA, AcutiMartellucci C, Di Carlo F, et al. Repetitive transcranial magnetic stimulation in treatment-seeking subjects with cocaine use disorder: a randomized, double-blind, sham-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2022;116:110513.
Mahoney JJ, Hanlon CA, Marshalek PJ, Rezai AR, Krinke L. Transcranial magnetic stimulation, deep brain stimulation, and other forms of neuromodulation for substance use disorders: review of modalities and implications for treatment. J Neurol Sci. 2020;418:117149.
McCalley DM, Kaur N, Wolf JP, Contreras IE, Book SW, Smith JP, et al. Medial prefrontal cortex theta burst stimulation improves treatment outcomes in alcohol use disorder: a double-blind, sham-controlled neuroimaging study. Biol Psychiatry Glob Open Sci. 2023;3(2):301–10.
Sack AT, Paneva J, Küthe T, Dijkstra E, Zwienenberg L, Arns M, et al. Target engagement and brain state dependence of transcranial magnetic stimulation: implications for clinical practice. Biol Psychiatry. 2023 Sep;S0006322323015718.
Kearney-Ramos TE, Dowdle LT, Mithoefer OJ, Devries W, George MS, Hanlon CA. State-dependent effects of ventromedial prefrontal cortex continuous thetaburst stimulation on cocaine cue reactivity in chronic cocaine users. Front Psychiatry. 2019;8(10):317.
Zangen A, Moshe H, Martinez D, Barnea-Ygael N, Vapnik T, Bystritsky A, et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry. 2021;20(3):397–404.
Petersen N, London ED. Addiction and dopamine: sex differences and insights from studies of smoking. Curr Opin Behav Sci. 2018;23:150–9.
Perez Diaz M, Pochon JB, Ghahremani DG, Dean AC, Faulkner P, Petersen N, et al. Sex Differences in the association of cigarette craving with insula structure. Int J Neuropsychopharmacol. 2021;24(8):624–33.
Okita K, Petersen N, Robertson CL, Dean AC, Mandelkern MA, London ED. Sex differences in midbrain dopamine D2-type receptor availability and association with nicotine dependence. Neuropsychopharmacology. 2016;41(12):2913–9.
Peltier MR, Roberts W, Verplaetse TL, Zakiniaeiz Y, Burke C, Moore KE, et al. Sex differences across retrospective transitions in posttraumatic stress and substance use disorders. J Dual Diagn. 2022;18(1):11–20.
Hanlon CA, McCalley DM. Sex/gender as a factor that influences transcranial magnetic stimulation treatment outcome: three potential biological explanations. Front Psychiatry. 2022;29(13):869070.
Funding
This work was supported by the National Institute of Mental Health K24 AA030788 and R01 DA054275 to JP and the National Center for Advancing Translational Sciences KL2TR002245 to HBW.
Author information
Authors and Affiliations
Contributions
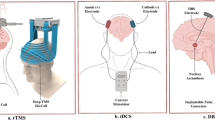
SB, NZ, AK, JP, and HBW wrote the main manuscript text. SB and HBW prepared Fig. 1. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
This is a literature review, so this is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Blyth, S.H., Zabik, N.L., Krosche, A. et al. rTMS for Co-occurring Psychiatric and Substance Use Disorders: Narrative Review and Future Directions. Curr Addict Rep 11, 342–351 (2024). https://doi.org/10.1007/s40429-024-00542-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40429-024-00542-6