Abstract
Introduction
Sublingual fentanyl tablets (SFTs) have been shown to be a safe and effective option in controlling breakthrough cancer pain (BTcP). However, further examination is required to investigate the use of SFTs among the elderly. The aim of this study was to examine the influence of age in BTcP management with SFTs in the elderly population.
Methods
We performed subgroup analyses of a recently completed trial in two subsets of individuals: patients aged 65–74 years (low age group) and patients ≥ 75 years (high age group). Pain intensity (PI), onset of pain relief, frequency and duration of BTcP episodes, and adverse events (AEs) were assessed at 3, 7, 15, and 30 days. Health status instruments used were the Hospital Anxiety and Depression Scale (HADS-A and HADS-D) and the Short Form 12, version 2 (SF-12v2) questionnaire.
Results
Levels of PI at the end of the study improved significantly as compared with baseline in both the low and the high age groups (30.0% and 27.7% reduction, respectively). The onset of analgesia at the end of the study began in < 10 min in 85.0% of young–old subjects and in 62.5% of patients ≥ 75 years, but no significant differences were found. BTcP episodes lasted < 15 min in 75.0% of patients in the low age group and 58.3% in the high age group (p = 0.24). Most of patients in both groups experienced one to five BTcP daily episodes, at all assessment points. HADS-D decreased from 10.78 (± 4.33) to 8.21 (± 3.57) in the low age group, and from 10.96 (± 4.26) to 9.36 (± 3.35) in the high age group (p = 0.02). Significant differences in HADS-A scores from baseline to the end of the study were also observed in both subgroups (p < 0.05). Patients in the low age group had less favorable mental component summary (MCS) and physical component summary (PCS) scores than patients in the high age group. At the end of the study, 10.0% of young–old patients and 29.2% of patients aged ≥ 75 years reported AEs related to their treatment. The most commonly reported AEs included nausea, vomiting, constipation, somnolence, and skin disorders and they were generally mild to moderate in severity.
Conclusions
The results of this study showed that SFTs provided safe and clinically meaningful pain relief in both elderly subgroups. Clinical implications of these findings await validation in large, confirmatory studies to identify age subgroup divergences among elderly cancer patients treated with SFTs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The aging of the population and the increase in cancer incidence among the elderly has become a major public health concern in Western countries. However, despite the rapid growth of the geriatric oncology population, older patients are underrepresented in cancer clinical trials and their pain is poorly managed [1,2,3,4].
Cancer pain prevalence rates are over 50% for patients subjected to treatment and about 75% for patients with advanced, metastatic, or terminal disease [5]. Optimal pain control requires the use of an analgesic (around-the-clock dosing) for persistent background pain and supplemental doses for transitory exacerbations of pain, commonly defined as breakthrough cancer pain (BTcP) [6, 7]. Usually, BTcP episodes are characterized as rapid in onset and being of moderate to severe intensity, but of brief duration [8]. Prevalence rates indicate that at least one of two patients with cancer pain also experiences BTcP [9], which is associated with poorer functional status and negative psychological consequences when uncontrolled [10]. Therefore, it is fundamental to choose pharmacological strategies with rapid onset of action, short-lasting effect, minimum side effects, and easy administration to control BTcP and its impact on patients’ quality of life (QoL) [11].
Sublingual fentanyl tablets (SFTs) are a fast-acting form of fentanyl, a potent and strongly lipophilic opioid, which provide a noninvasive mechanism for immediate drug absorption through the sublingual mucosa, avoiding the gastrointestinal tract and first pass hepatic metabolism [12,13,14]. SFTs have been shown to be a safe and effective option in controlling BTcP as well as improving QoL in cancer patients of all ages [15,16,17,18,19]. Nonetheless, further examination is required to investigate the within-group variation of the effect of SFTs in the aging population.
It is well known that geriatric patients can respond differently from younger patients to drug therapy, since age-related physiological changes can affect pharmacokinetics and pharmacodynamics of the drug [20]. Older adults also have a greater risk for drug-related toxicities and drug–drug interactions because of the use of multiple medications [21]. Polypharmacy is recognized as an increasingly serious problem in the management of elderly patients with cancer, and requires balancing the risks and benefits of multiple drug therapies. Moreover, as individuals age, they become more heterogeneous in terms of physiology and morbidity as a consequence of their previous lifestyle, environmental exposure, and genetic composition [22], resulting in a less predictable response to medications [23, 24,25,26,].
Traditionally, old age was defined as the chronological age of 65 years or more, but with increasing life expectancy, it has been necessary to categorize elderly adults according to age to properly investigate geriatric differences. Although there are various ways to classify this population, the elderly can be stratified into three life-stage subgroups: the young–old (aged 65–74), the middle–old (aged 75–84), and the old–old (or oldest–old) (aged over 85 years) [25,26,27,28].
The purpose of this investigation was to examine similarities and differences in pain relief, QoL, and treatment-related adverse effects in the older population of a recently completed trial contributing to the assessment of the effect of SFTs in patients with cancer pain [29]. We hypothesized that an appropriate analgesic effect of SFTs for BTcP control in the elderly might be subjected to the patients’ age. For this reason, we conducted subgroup analyses in two subsets of individuals: patients aged between 65 and 74 years (low age group) and patients aged 75 years and older (high age group), to investigate the influence of age in BTcP management with SFTs in the elderly population.
2 Methods
2.1 Study Design and Population
This was a multicenter, prospective observation post-authorization, open-label study conducted at nine pain units in Catalonia and the Balearic Islands in Spain between March and December 2013. Study design and eligibility criteria have been previously described in detail elsewhere [29]. Briefly, eligible patients were male and female adult patients with a confirmed diagnosis of cancer who were regularly experiencing episodes of BTcP that were partially relieved [scored ≥ 6 on an 11-point numerical rating scale (NRS)]. The procedure to diagnose BTcP was done following the algorithm of Davies et al. [7] and according to the consensus recommendations from the Spanish Pain Society. The algorithm indicates that baseline pain must be adequately controlled before a diagnosis of BTcP can be considered. Patients were receiving a fixed-dose schedule of opioids equivalent to oral morphine of at least 60 mg/day, or transdermal fentanyl 25 μg/h, oral oxycodone 30 mg/day, oral hydromorphone 8 mg/day, oral oxymorphone 25 mg/day or an equianalgesic dose of any other opioid. Participants were fully informed of the study and provided signed written informed consent. At the initial screening visit, patients provided data on their baseline health, treatment, pain, and QoL. All outcomes were assessed at 3 (visit 1), 7 (visit 2), 15 (visit 3), and 30 days (end of study) after starting the treatment. For each BTcP episode, patients self-administered SFT (Abstral®, Kyowa Kirin Farmacéutica SLU, Madrid, Spain). The initial dose of SFT was determined by the clinician on the basis of prior treatment for BTcP and with consideration of the opioid dose for background pain. The dose was then titrated up to a successful analgesia (100, 200, 300, 400, 600, 800 μg). Changes in the dose of SFT were recorded throughout the study.
2.2 Efficacy and Safety Assessments
The efficacy of SFTs on BTcP was mainly evaluated in terms of pain relief and QoL outcomes. Patients rated their pain intensity (PI) using an 11-point NRS, from 0 (no pain) to 10 (worst pain imaginable). Pain relief was assessed by asking patients to select from a list of time intervals, the ‘time to first effect’ and the ‘time to maximum effect’ following the administration of SFT. Additional endpoints were reported by each patient, including number of irruptive pain episodes and duration of each episode at each assessment point. The health status instruments used to evaluate the patients’ QoL included the Short Form 12 (version 2) questionnaire [SF-12v2], with a physical health composite score (PCS) and a mental health score (MCS) [12, 13]; and the Hospital Anxiety and Depression Scale (HADS), consisting of seven statements on the anxiety subscale (HADS-A) and seven on the depression subscale (HADS-D) [15]. The participants rated their levels on a 0–3 scoring system, with a range of 0–21 for each subscale (higher scores indicate more severe distress). Scores were classified as follows: 0–7, normal; 8–10, mild; 11–14, moderate; and 15–21, severe anxiety/depression [30, 31].
Safety and tolerability were assessed based on patients’ and clinicians’ reports of adverse events (AEs).
2.3 Statistical Analysis
Subgroup analyses were performed according to age (65–74 years versus ≥ 75 years). For each subgroup, demographics and disease-related features were analyzed descriptively using frequencies, means, and standard deviations (SDs), as appropriate. The SF-12 physical and mental health component summary scores (PCS and MCS, respectively) were computed as normalized scores (mean = 50, SD = 10). Statistical analyses were conducted using the Chi square test for categorical data, and the paired two-tailed t test or ANOVA for continuous data; p values < 0.05 were considered statistically significant, with no adjustments for multiplicity. All statistical analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC, USA).
3 Results
Details on patient demographics and baseline characteristics of the main study have been previously published [29]. A total of 54 patients were included in the age subgroup analyses performed on the elderly population (mean age 76.7 years; range 65.6–91.7). There were 24 individuals in the low age group (mean age 70.6 years; range 65.6–74.8), and 30 subjects in the high age group (mean age 81.5 years; range 77.7–91.7). Patient demographics for the two subgroups are summarized in Table 1.
Cancer type and cancer stage according to the two age categories are shown in Table 2. Breast, prostate, and gastrointestinal cancer were the most common types of cancer. The majority of patients in both groups were diagnosed with locally advanced cancer (58.3% of patients in the low age group and 73.3% of patients in the high age group). The most common BTcP was incidental pain, present in 78.3% of young–old individuals, and 61.5% of subjects aged ≥ 75 years.
3.1 Efficacy Endpoints
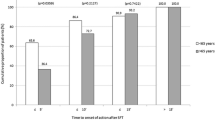
Mean pain intensity outcomes based on an 11-point numerical scale for both subgroups of patients are shown in Fig. 1. Levels of PI improved significantly compared with baseline for all assessment points and the two age subgroups (p < 0.05). Differences in PI-start scores at the end of the study versus baseline values were statistically significant in both the low age group and the high age group (30.0% and 27.7% reduction, respectively). Patients even reported a greater level of relief at the end of the BTcP episode (PI-end: 54.6% reduction for the young–old patients and 36.5% for the older individuals).
Pain intensity (PI) outcomes based on an 11-point numerical rating scale in two subgroups of elderly patients. PI-start pain intensity at the start of the breakthrough cancer pain (BTcP) episode, PI-end pain intensity at the end of the BTcP episode. Error bars represent standard deviations above and below the mean (error bars have only been depicted in one direction for greater clarity)
The onset of analgesia at the end of the study began in < 10 min in 85.0% of young–old subjects and in 62.5% of patients aged ≥ 75 years (Fig. 2), but there were no statistically significant differences between groups.
At baseline, the low age group of patients experienced BTcP episodes that lasted < 15 min in 73.9% of cases, as compared with 44.4% of patients belonging to the high age group (p = 0.03). At the end of the study, a higher number of young–old subjects also experienced BTcP events of shorter duration as compared with older patients (BTcP episodes lasted < 15 min in 75.0% and 58.3% of patients, respectively; p = 0.24).
Overall, the number of BTcP daily episodes remained relatively low throughout the study. Most patients in both groups experienced one to five BTcP daily episodes, at all assessment points. At all visits, 22 patients aged 65–74 years (95.7%) and 24 patients ≥ 75 years (88.9%) reported experiencing one to five pain episodes per day. Thus, a lower number of individuals of the low age group had more than five episodes of BTcP per day as compared with the high age group, although differences were not statistically significant (4.3% vs 11.1%, respectively; p = 0.37).
Table 3 shows the mean number of BTcP reported at each visit, that corresponded to the number of irruptive pain episodes experienced by patients since the previous visit. Patients ≥ 75 years old presented a higher mean number of irruptive pain episodes than younger–old patients, at all visit points. However, differences between groups were not statistically significant except for those found at visit 2: 9.83 (± 8.92) versus 19.52 (± 17.69); p = 0.02.
BTcP management with SFT was examined at each visit based on patients’ and clinicians’ reports. The mean dose of treatment was 450.0 (± 261.12) in the low age group and 352.4 (± 258.11) in the high age group. Same doses of SFT were maintained in most patients throughout the study, although a slightly higher percentage of young–old patients needed either a dose increase or a change of treatment more frequently than older patients. However, differences were not statistically significant between groups (Table 4).
3.2 Quality-of-Life Outcomes
The results of the HADS-D and HADS-A scores and the mental and physical SF-12v2 scores registered at baseline and at the end of the study are presented in Table 5. Differences between age subgroups were not statistically significant. Scores on HADS-D and HADS-A revealed significant reductions in the levels of depression and anxiety at the end of the study as compared with baseline, in both groups. HADS-D decreased from 10.78 (± 4.33) to 8.21 (± 3.57) in the low age group, and from 10.96 (± 4.26) to 9.36 (± 3.35) in the high age group (p = 0.02). Significant differences in HADS-A scores from baseline to the end of the study were also observed in both subgroups (p < 0.05).
The perceived health status questionnaire SF-12v2 revealed that scores of the mental component summary (MCS) were higher than scores of the physical component summary (PCS) in both subgroups. Patients in the low age group had less favorable MCS and PCS scores than patients in the high age group at the end of the study. However, although no significant differences were found, the two SF-12v2 components improved from baseline in both groups.
3.3 Safety Endpoints
At baseline, 34.8% of young–old patients and 28.6% of patients ≥ 75 years reported AEs related to their treatment. At the end of the study, these percentages were lower in the low age group (10.0% of patients), and remained almost the same in the high age group (29.2% of patients). Differences in number of treatment-related AEs between groups were not statistically significant at any visit time (p > 0.05) nor at the end of the study as compared with baseline (p = 0.11). The most commonly reported AEs included nausea, vomiting, constipation, somnolence, and skin disorders, and they were generally mild to moderate in severity.
4 Discussion
The prevalence of BTcP in patients with cancer still remains a primary challenge in the integral management of cancer patients, particularly in the elderly [12, 32]. However, it is generally agreed that there is a need for more information regarding the use of opioids for BTcP in older individuals [33].
In this study, we performed a subgroup analysis of a multicenter, prospective study to evaluate possible differences in efficacy and safety of SFTs for BTcP among the elderly population, according to two age categories: patients aged between 65 and 74 years (low age group) and patients aged 75 years and over (high age group). Given the potential variations in health status with age, we expected responses to BTcP treatment were likely to be heterogeneous among older patients.
The results showed that SFTs provided a clinically meaningful pain relief in both age subgroups. Consequently, we can conclude that treatment with SFTs was significantly effective in reducing pain intensity in elderly patients. Although differences were not statistically significant, young–old patients experienced greater pain improvement. In addition, a short time to onset of pain relief (< 10 min) was observed in the majority of patients. Again, although differences were not significant, the low age group underwent more rapid pain relief in a higher percentage of patients. These outcomes are in accordance with previous investigations that suggested that aging has an influence on the response to the opioid used to treat BTcP [34, 35].
With regard to the number of daily pain episodes, around 96% and 89% of patients aged 65–74 years and ≥ 75 years, respectively, reported experiencing fewer than five pain flares per day. The frequency of BTcP episodes has implications for treatment since the products available should only be used for up to four daily episodes. Usually, the adequacy of the background medication for persistent pain needs to be reevaluated if the patient consistently experiences more than four episodes a day [36]. Our results indicate that the dose of SFTs was successfully titrated to achieve adequate relief from pain in the vast majority of patients. Interestingly, despite young–old subjects experiencing a smaller number of irruptive pain episodes than patients aged ≥ 75 years, they needed either a dose increase or a change of treatment more frequently than older patients. Although differences were not significant, these results outline one of the biggest challenges in cancer pain management, which is that most older patients usually perceive pain as a normal part of aging and they tend to be more satisfied with their pain treatment as they grow older [21]. These perceptions may have interfered with both the assessment and management of pain and could explain the differences observed between age groups in terms of treatment adjustment.
In addition to the positive results observed for the pain decrease, patients achieved meaningful improvements in QoL according to HADS-D and HADS-A scores, which revealed significant reductions in the levels of depression and anxiety at the end of the study compared with baseline in both age groups. Scores obtained with the SF-12v2 questionnaire did reflect the same tendency on both mental and physical health components, although the results were less conclusive.
The safety outcomes showed that SFTs were generally well tolerated in both groups, as the number of AEs was reasonable and AEs were mostly mild and moderate in intensity. The incidence of AEs was lower for patients in the low age group than for older patients (10.0% vs 29.2%, p > 0.05). These results suggest that AEs increase as aging progresses, probably due to multimorbidity, polypharmacy, and pathophysiologic changes [37]. Nonetheless, more evidence is needed to confirm these conclusions and also on how to best make treatment decisions for older cancer patients whose health status is often complicated not only by the presence of comorbidities but also by factors such as frailty, which should also be taken into account to overcome the issue of under-treatment due to health perception based on age alone [38, 39].
There are some limitations to our analysis that should be considered when interpreting these results. First, the small sample size included in the analysis implies a low statistical power to detect meaningful differences between age subgroups. Another weakness of this study is the patients’ subjective self-report of pain and well-being, which can be influenced by a variety of psychosocial factors. Therefore, outcomes should be interpreted with caution unless there is strong supporting evidence. Indeed, the clinical implications of these findings await validation in large, confirmatory studies to identify age subgroup divergences among elderly cancer patients treated with SFTs, and to obtain accurate drug safety data to enable more rational therapeutic decisions. The present results are best viewed as a hypothesis-generation investigation. Despite these limitations, the findings of this study provide valuable information for the management of BTcP with SFTs in the elderly. To the best of our knowledge, this is the first analysis that specifically investigated the age-based disparities among the elderly in the treatment of BTcP with SFTs in terms of pain relief, QoL, and safety.
5 Conclusions
The results of this study indicate that SFTs are well tolerated and effective in controlling BTcP in elderly patients of all ages. Nevertheless, findings underscore the need for additional research on BTcP treatment in geriatric oncology patients, since the treatment of older adults with pain is complex and is affected by age-related changes.
References
Yee KW, Pater JL, Pho L, et al. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol. 2003;21:1618–23.
Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–9.
Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7.
Mercadante S, Arcuri E. Pharmacological management of cancer pain in the elderly. Drugs Aging. 2007;24(9):761–76.
van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manag. 2016;51(6):1070–90. https://doi.org/10.1016/j.jpainsymman.
Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41(3):273–81.
Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain. 2009;13:331–8. https://doi.org/10.1016/j.ejpain.2008.06.014.
Caraceni A, Martini C, Zecca E, Working Group of an IASP Task Force on Cancer Pain, et al. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliat Med. 2004;18(3):177–83.
Deandrea S, Corli O, Consonni D, Villani W, Greco MT, Apolone G. Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manag. 2014;47(1):57–76. https://doi.org/10.1016/j.jpainsymman.2013.02.015 (Epub 2013 Jun 21).
Payne R. Recognition and diagnosis of breakthrough pain. Pin Med. 2007;8(suppl 1):S3–7.
Margarit C, Juliá J, López R, et al. Breakthrough cancer pain—still a challenge. J Pain Res. 2012;5:559–66.
Pautex S, Vogt-Ferrier N, Zulian GB. Breakthrough pain in elderly patients with cancer: treatment options. Drugs Aging. 2014;31(6):405–11. https://doi.org/10.1007/s40266-014-0181-5 (Review).
Lennernäs B, Frank-Lissbrant I, Lennernäs H, Kälkner KM, Derrick R, Howell J. Sublingual administration of fentanyl to cancer patients is an effective treatment for breakthrough pain: results from a randomized phase II study. Palliat Med. 2010;24(3):286–93. https://doi.org/10.1177/0269216309356138 (Epub 2009 Dec 16).
American Academy of Pediatrics, Committee on Drugs. Alternative routes of drug administration-advantages and disadvantages (subject review). Pediatrics. 1997;100(1):143–52.
Chwieduk CM, McKeage K. Fentanyl sublingual: in breakthrough pain in opioid-tolerant adults with cancer. Drugs. 2010;70(17):2281–8. https://doi.org/10.2165/11200910-000000000-00000.
Rauck RL, Tark M, Reyes E, Hayes TG, Bartkowiak AJ, Hassman D, Nalamachu S, Derrick R, Howell J. Efficacy and long-term tolerability of sublingual fentanyl orally disintegrating tablet in the treatment of breakthrough cancer pain. Curr Med Res Opin. 2009;25(12):2877–85.
Überall MA, Müller-Schwefe GH. Sublingual fentanyl orally disintegrating tablet in daily practice: efficacy, safety and tolerability in patients with breakthrough cancer pain. Curr Med Res Opin. 2011;27(7):1385–94. https://doi.org/10.1185/03007995.2011.583231 (Epub 2011 May 11).
Guitart J, Vargas MI, De Sanctis V, Folch J, Salazar R, Fuentes J, Coma J, Ferreras J, Moya J, Tomás A, Estivill P, Rodelas F, Jiménez AJ, Sanz A. Breakthrough pain management with sublingual fentanyl tablets in patients with cancer: age subgroup analysis of a multicenter prospective study. Drugs R D. 2017;17(3):419–25. https://doi.org/10.1007/s40268-017-0198-4.
Guitart J, Vargas MI, De Sanctis V, Folch J, Salazar R, Fuentes J, Coma J, Ferreras J, Moya J, Tomás A, Estivill P, Rodelas F, Jiménez AJ, Sanz A. Efficacy and safety of sublingual fentanyl tablets in breakthrough cancer pain management according to cancer stage and background opioid medication. Drugs R D. 2018;18(2):119–28. https://doi.org/10.1007/s40268-018-0231-2.
Shenoy P, Harugeri A. Elderly patients’ participation in clinical trials. Perspect Clin Res. 2015;6(4):184–9.
Brant JM. Assessment and management of cancer pain in older adults: strategies for success. Asia Pac J Oncol Nurs. 2018;5(3):248–53.
Bellizzi KM, Mustian KM, Bowen DJ, Resnick B, Miller SM. Aging in the context of cancer prevention and control: perspectives from behavioral medicine. Cancer. 2008;113(12 Suppl):3479–83.
Hilmer SN, McLachlan AJ, Le Couteur DG. Clinical pharmacology in the geriatric patient. Fundam Clin Pharmacol. 2007;21:217–30.
McLachlan AJ, Hilmer SN, Le Couteur DG. Variability in response to medicines in older people—phenotypic and genotypic factors. Clin Pharmacol Ther. 2009;85:431–3.
Cohen-Mansfield J, Shmotkin D, Blumstein Z, Shorek A, Eyal N, Hazan H, et al. The old, old–old, and the oldest old: continuation or distinct categories? An examination of the relationship between age and changes in health, function, and wellbeing. Int J Aging Hum Dev. 2013;77(1):37–57 (PMID: 23986979).
Alterovitz SS, Mendelsohn GA. Relationship goals of middle-aged, young–old, and old–old internet daters: an analysis of online personal ads. J Aging Stud. 2013;27:159–65.
Valasek DL. Retirement satisfaction: Is there a young/old, old/old difference? In: Joint Annual Meeting of the Scientific Gerontological Society (34th) and the Scientific & Educational Canadian Association on Gerontology (10th); Toronto, Ontario, Canada; 1981.
Balducci L. Management of cancer in the elderly. Oncology. 2006;20(2):135–52 (PubMed: 16562648).
Guitart J, Vargas MI, De Sanctis V, et al. Sublingual fentanyl tablets for relief of breakthrough pain in cancer patients and association with quality-of-life outcomes. Clin Drug Investig. 2015;35(12):815–22 (Erratum in: Clin Drug Investig 2016).
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Snaith RP. The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes. 2003;1(1):29.
Pergolizzi JV, Gharibo C, Ho KY. Treatment considerations for cancer pain: a global perspective. Pain Pract. 2015;15(8):778–92. https://doi.org/10.1111/papr.12253 (Epub 2014 Dec 3).
Mercadante S, Giarratano A. Assessing age and gender in studies of breakthrough pain medications. Curr Med Res Opin. 2014;30(7):1353–6. https://doi.org/10.1185/03007995.2014.901942.
Mercadante S, Casuccio A, Pumo S, Fulfaro F. Factors influencing the opioid response in advanced cancer patients with pain followed at home: the effects of age and gender. Support Care Cancer. 2000;8(2):123–30.
Green CR, Hart-Johnson T. Cancer pain: an age-based analysis. Pain Med. 2010;11(10):1525–36. https://doi.org/10.1111/j.1526-4637.2010.00957.x.
Dickman A. Basics of managing breakthrough cancer pain. Pharm J. 2009;283:213–6.
Mercadante S, Aielli F, Masedu F, Valenti M, Ficorella C, Porzio G. Pain characteristics and analgesic treatment in an aged adult population: a 4-week retrospective analysis of advanced cancer patients followed at home. Drugs Aging. 2015;32(4):315–20. https://doi.org/10.1007/s40266-015-0253-1.
Swaminathan D, Swaminathan V. Geriatric oncology: problems with under-treatment within this population. Cancer Biol Med. 2015;12(4):275–83.
Swaminathan V, Audisio RA. Cancer in older patients: an analysis of elderly oncology. Ecancermedicalscience. 2012;6:243.
Acknowledgements
The authors thank Blanca Martínez-Garriga, who provided medical writing assistance on behalf of Trialance (www.trialance.com).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Kyowa Kirin Farmacéutica SLU, Spain.
Financial disclosure
The authors received research funding from Kyowa Kirin Farmacéutica SLU for this study.
Conflict of interest
Antonio Javier Jiménez and Almudena Sanz are employees of Kyowa Kirin Farmacéutica SLU. The authors declared they have no further competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Guitart, J., Vargas, M.I., De Sanctis, V. et al. Effects of Age Among Elderly Cancer Patients on Breakthrough Pain Management with Sublingual Fentanyl Tablets. Drugs R D 19, 247–254 (2019). https://doi.org/10.1007/s40268-019-0276-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-019-0276-x