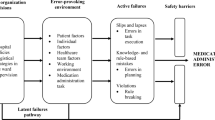
Abstract
Introduction
Children admitted to paediatric and neonatal intensive care units may be at high risk from medication errors and preventable adverse drug events.
Objective
The objective of this systematic review was to review empirical studies examining the prevalence and nature of medication errors and preventable adverse drug events in paediatric and neonatal intensive care units.
Data Sources
Seven electronic databases were searched between January 2000 and March 2019.
Study Selection
Quantitative studies that examined medication errors/preventable adverse drug events using direct observation, medication chart review, or a mixture of methods in children ≤ 18 years of age admitted to paediatric or neonatal intensive care units were included.
Data Extraction
Data on study design, detection method used, rates and types of medication errors/preventable adverse drug events, and medication classes involved were extracted.
Results
Thirty-five unique studies were identified for inclusion. In paediatric intensive care units, the median rate of medication errors was 14.6 per 100 medication orders (interquartile range 5.7–48.8%, n = 3) and between 6.4 and 9.1 per 1000 patient-days (n = 2). In neonatal intensive care units, medication error rates ranged from 4 to 35.1 per 1000 patient-days (n = 2) and from 5.5 to 77.9 per 100 medication orders (n = 2). In both settings, prescribing and medication administration errors were found to be the most common medication errors, with dosing errors the most frequently reported error subtype. Preventable adverse drug event rates were reported in three paediatric intensive care unit studies as 2.3 per 100 patients (n = 1) and 21–29 per 1000 patient-days (n = 2). In neonatal intensive care units, preventable adverse drug event rates from three studies were 0.86 per 1000 doses (n = 1) and 0.47–14.38 per 1000 patient-days (n = 2). Anti-infective agents were commonly involved with medication errors/preventable adverse drug events in both settings.
Conclusions
Medication errors occur frequently in critically ill children admitted to paediatric and neonatal intensive care units and may lead to patient harm. Important targets such as dosing errors and anti-infective medications were identified to guide the development of remedial interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Medication errors (MEs) are common and persistent problems that may pose significant risk to critically ill children admitted to paediatric and neonatal intensive care units. |
Prescribing and medication administration errors were the common types of MEs and dosing errors were the most frequent ME subtype in both paediatric and neonatal intensive care unit settings. |
Anti-infective medications were the commonly reported drug class associated with MEs/preventable adverse drug events across both intensive care unit types. |
Further research is needed to examine medication administration errors and preventable adverse drug events in children’s intensive care settings. |
1 Introduction
Patients admitted to intensive care units (ICUs) may be more likely to be affected by medication errors (MEs) and preventable adverse drug events (ADEs)/adverse drug reactions than those admitted to other wards [1]. This may be due to factors related to the ICU environment (e.g. differences in staff workload and pharmacological interventions) and the nature of patients admitted to ICUs (e.g. rapidly changing physiological functions, deranged drug metabolism) [2]. Medication errors in paediatric ICUs (PICUs) have been reported to occur seven times more frequently than other paediatric inpatient units in one UK study that examined 441 MEs in 682 hospitalised children over 2 years [3]. Elsewhere, infants in neonatal ICUs (NICUs) were found to be at higher risk of preventable ADEs than children in other wards using data from two hospitals in the USA [4].
Potential contributory factors behind these events may include: frequent use of unlicensed or off-label medicines in NICUs and PICUs [5, 6], which may be associated with one third of preventable ADEs in hospitalised children. Other related factors may include lack of adequate dosing information for children [7,8,9,10]; children in ICUs are also often sedated or may be pre-verbal and therefore unable to prevent errors themselves [11]. There is a need for the use of ‘high-risk’ medications and/or those with narrow therapeutic indices in these settings (including opioids, benzodiazepines and anticoagulants), which may also be a possible related factor [12].
The World Health Organization has made reducing patient harm due to MEs its current global patient safety challenge with the aim of reducing severe harm associated with MEs by 50% within 5 years [13]. Specifically, young children were identified as being at high risk of drug-related preventable harm. To support this global campaign, it is important to examine and understand the burden of MEs and associated preventable ADEs, particularly in patient populations at high risk such as critically ill children admitted to PICUs/NICUs. This provides an opportunity to inform the development and optimisation of safe policies and practices to prevent patient harm.
Earlier systematic reviews have examined the nature and burden of MEs in a specific country [14], investigated non-preventable ADEs (adverse drug reactions) in paediatrics [9, 15, 16], or involved database searches that are now more than 10 years old and are in need of updating [2, 17,18,19,20,21,22,23,24,25]. Other more recent systematic reviews were not designed for all age groups of critically ill children [7, 26,27,28], did not focus on both MEs (including different stages of medication use process) and preventable ADEs [27, 29], or assessed errors at the drug administration or prescribing phase only [30,31,32]. Therefore, this systematic review aims to identify and summarise the available evidence on the global prevalence and nature of both MEs and preventable ADEs in PICUs and NICUs.
2 Methods
2.1 Search Strategy
The search strategy followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [33]. This systematic review retrieved studies published from January 2000 through March 2019.
Seven electronic databases were searched without language restrictions including: MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature, International Pharmaceutical Abstracts, Web of Science, Maternity and Infant Care Database, and Scopus. The search terms used fell into four groups to describe related terms to ME/preventable ADE, target population, setting and study design [Table 1 of the Electronic Supplementary Material (ESM)]. We did not search non-English literature databases and the different terms we used in our search strategy were only in English and may not have covered terms in other languages.
To identify relevant studies, EndNote™ X8 was utilised as a reference manager to import citations and also to identify and exclude duplicate citations. The lead author (AA) screened titles followed by abstracts against the study inclusion criteria [34]. Subsequently, full-text papers were assessed by AA to identify potentially relevant studies. During screening, uncertain cases were discussed amongst the research team and agreed by consensus. A hand search was performed on the reference lists of all identified studies and relevant review articles to identify any additional eligible studies. Abstracts from conferences were also included where they provided rates of MEs or preventable ADEs in PICUs/NICUs. When additional data were required in relation to study methods and/or results, authors were contacted by e-mail to provide more detailed information.
2.2 Inclusion and Exclusion Criteria
To reflect the population of interest, included studies focused on children from birth to 18 years of age and reported data that were attributable specifically to ICU settings. The review considered all quantitative studies that assessed the prevalence of ME/preventable ADE at any phase of the medication use process, or contained sufficient information to calculate the prevalence rate. Studies examining the impact of interventions on ME/preventable ADE rates were also included if data on the baseline prevalence rates before implementation of the intervention for this systematic review could be extracted.
This systematic review excluded studies only focussing on subtypes of prescribing, dispensing or administration errors, for instance, focussing only on ‘wrong dose’. The review also excluded studies that used an estimated denominator to calculate the prevalence of MEs/preventable ADEs or reporting MEs/preventable ADEs rates for a single or specific class of medication, or specific treatment or patient group with a particular illness. Studies that relied on spontaneous error reporting systems for data collection were also excluded as these are widely known to underestimate the rate of MEs/preventable ADEs [25, 35,36,37,38,39]. However, studies that collected incident report data alongside other methods (e.g. chart review) were included. The review also excluded studies reporting data only on irrational/potentially inappropriate prescribing or non-preventable ADEs/adverse drug reactions.
2.3 Data Extraction
Relevant data were extracted from each included study independently by two authors (AA and RK, DMA or AS) using a standardised form. Any disagreements were resolved within the team by consensus. Data were collected on year of publication, country of origin, study type, setting, detection method, definitions of ME/preventable ADE, severity assessment criteria and any methods used for validation of the detected events. Data extraction also included rates of MEs/preventable ADEs and their types, severity and medication classes involved. We also collected data regarding the type of prescribing system (paper based or electronic medication chart) from each of the included studies to compare error rates between the two systems.
2.4 Quality Assessment
Assessment criteria established by Allan and Barker [40], which have been used frequently in previous systematic reviews examining MEs [41,42,43,44], were adapted to evaluate the quality of each study that met the inclusion criteria of this systematic review. The quality appraisal included the following ten criteria:
-
1.
Aims/objectives of the study clearly stated.
-
2.
Definition of what constitutes a ME/preventable ADE.
-
3.
ME/preventable ADE categories specified.
-
4.
ME/preventable ADE categories defined.
-
5.
Presence of a clearly defined denominator.
-
6.
Data collection method described clearly.
-
7.
Setting in which study conducted described.
-
8.
Validity measure in place to confirm the occurrence of ME/preventable ADE.
-
9.
Reliability measures.
-
10.
Limitations of study listed.
Two authors (AA and RK, DMA or AS) calculated the quality of each included study independently and consensus was achieved through discussion for any inconsistencies in scoring items.
2.5 Data Analysis
Data were summarised descriptively in tables, including prevalence rates for overall ME/preventable ADEs as well as prevalence rates of ME types including prescribing error, and dispensing, transcription and administration errors. Where appropriate, studies were grouped using common denominators (e.g. medication orders or patient-days) and rates presented using medians with interquartile ranges (IQRs). Rates of events were calculated where sufficient information was provided by dividing the total number of MEs/preventable ADEs that occurred by the relevant denominator such as patients, medication orders or administrations and then multiplying by 100.
The most commonly observed drug classes involved with MEs/preventable ADEs in PICUs or NICUs were extracted. Common drugs presented in this systematic review were the frequently reported top three drug classes across studies. The most common ME subtype(s) (e.g. common subtypes of prescribing errors) reported in this systematic review were the most commonly reported error categories reported in each of included study. The median rates with IQRs of prescribing errors in PICU and NICU were calculated based on the type of prescribing system (electronic or paper based) in each ICU type where possible.
3 Results
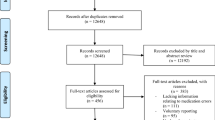
Thirty-seven publications were eligible for inclusion in this systematic review including seven conference abstracts, as shown in Fig. 1. All eligible studies were published in English. Two of the included studies that focused on NICUs [45, 46] and two others that included data for both units [4, 47] reported on the same population of patients and were considered as single studies (using the reports that include more relevant information for this systematic review), resulting in a total of 35 unique studies being included.
As the included studies were heterogeneous in setting and design, a meta-analysis could not be performed. For instance, sources of heterogeneity were in event measures such as error type and subtype (e.g. including/excluding dosing error in assessing prescribing errors) examined and denominators used in reporting event rates (e.g. per 100 patients, per 100 medication orders, per 100 or 1000 patient-days, or per 1000 occupied bed day). Clinical heterogeneity was also noted in included studies in specifying different age ranges (neonates/children) for patients admitted to these ICU types or not reporting this basic demographic data.
3.1 Summary of Study Characteristics
More than half of the included studies (20/35, 57.1%) were published from January 2010 onwards and the remaining 15 studies (15/35, 42.9%) between January 2000 and December 2009. Eighteen studies (17/35, 48.6%) were conducted in PICUs, and 13 studies (13/35, 37.1%) were conducted in NICUs. Five studies (5/35, 14.3%) were conducted across both ICU types [4, 18, 48,49,50]. The included PICU studies are summarised in Table 2 of the ESM, NICU studies in Table 3 of the ESM and studies in both ICU types in Table 4 of the ESM. These tables present extracted data from each study regarding country of origin, publication date, study design, detection method(s), setting(s), patient’s age, rates of MEs and preventable ADEs, and severity data.
Six (6/17, 35.3%) PICU studies were conducted in the USA [37, 51,52,53,54,55], and five studies (5/17, 29.4%) were undertaken in the UK [56,57,58,59,60]. The remainder included one study each from the Netherlands [61], Switzerland [20], Egypt [62], Israel [63], Iran [21] and Hong Kong [19]. The NICU studies included three studies (3/13, 23.1%) from the USA [64,65,66] and two studies from India (2/13, 15.4%) [22, 36]. The remainder included one study each from the UK [67], Spain [68], the Netherlands [69], New Zealand [45], Malaysia [70], Switzerland [25], Brazil [23] and South Africa [24]. The five studies involving both ICU settings were from the USA [4], Japan [50], Morocco [48], Argentina [18] and Malaysia [49].
The majority of studies that focused on one type of ICU were single-center studies [15/17 (88.2%) PICU, 13/13 (100%) NICU] while the combined PICU and NICU studies were almost all multi-centre (4/5, 80%). Many of the included studies were interventional [8/17 (47.1%) PICU, 7/13 (54%) NICU]; however, there was only one interventional study across both units [18]. There was variation in study design, with 11/18 (61.1%) PICU studies, 8/13 (61.5%) NICU and 3/5 (60%) combined settings collecting data prospectively, with the remainder being retrospective or cross-sectional studies.
Medication chart review was the most common method used for ME/preventable ADE detection in PICUs (15/17, 88.2%), in NICUs (10/13, 76.9%) and across both units (4/5, 80%). Pharmacists were the only data collectors in 12/35 (34.3%) studies, with a mixture of healthcare professionals used across the remaining studies. A total of five studies (5/35, 14.3%) did not provide any details on those involved in data collection [24, 53, 57, 59, 61].
The definition of ME/preventable ADE varied across studies (as reported in Tables 5–7 of the ESM). Almost half of the included studies (17/35, 48.6%) used locally developed definitions of ME/preventable ADE, while the remainder used a range of other definitions used previously (e.g. three studies [18, 23, 70] used the standard definition of MEs developed by the American Society of Health-System Pharmacists [71]). Nine studies (9/35, 25.7%) did not provide any operational ME/ADE definitions [22, 37, 57,58,59, 61, 66,67,68].
There was variation in the methods used for ensuring the validity (confirming causation) of identified MEs/preventable ADEs. For PICU studies, 11/17 (64.7%) reported a method for reassessment of some or all of the identified cases along with 3/5 (60%) joint studies, while only 4/13 (30.8%) NICU studies reported some mechanism to assure validity. The most common method to validate data across all studies involved a panel of healthcare professionals reassessing some or all detected outcome events (18/35 studies reporting validation method, 51.4%).
Some element of severity or impact assessment was described in 8/17 (47.1%) of PICU studies [19,20,21, 52,53,54, 62, 63], 4/5 (80%) of the joint studies [4, 48,49,50] and 4/13 (30.8%) of NICU studies [36, 45, 64, 69]. Severity assessment methods were variable, with some reporting expert panels convened to assign severity using a scale the researchers either developed internally, adapted from existing definitions used internally (n = 10) or from existing definitions (National Coordinating Council for Medication Error Reporting and Prevention (n = 5) [72], American Society of Health-System Pharmacists criteria (n = 1) [73]) being reported.
3.2 Quality Assessment
After applying the quality assessment criteria as shown in Supplemental Table 8, only six studies of the 35 included studies (17.1%) fulfilled all ten criteria and were considered as high-quality studies (Table 8 of the ESM) [48, 51, 53,54,55, 59]. Six studies met nine criteria, ten met eight criteria [4, 45, 49, 50, 52, 69], four met seven criteria [19, 60, 62, 70], two met six criteria [58, 68] and three met five criteria [57, 61, 67]. The remaining studies met less than five criteria [22, 37, 59, 66].
The data collection method and denominator used were described in all included studies. Nine of the 35 included studies (25.7%) did not provided any definition for MEs/preventable ADEs. Six studies did not specify categories of MEs/preventable ADEs and more than half of the studies (18/35, 51.4%) did not define ME categories. Many of the included studies did not describe any validity measures to confirm the occurrence of ME/preventable ADE (18/35, 51.4%) and did not assess inter-rater reliability (25/35, 71.4%).
3.3 Prevalence and Nature of Medication Errors and Preventable Adverse Drug Events in Paediatric Intensive Care Units
Data regarding rates and common types of MEs/preventable ADE rates in PICUs were provided in 21 (21/35, 60%) of the included studies (Table 1). The overall ME rate in PICUs was reported in five studies (5/21, 23.8%); three used medication orders as a denominator with a median prevalence of 14.6 per 100 medication orders (IQR 5.7–48.8) [4, 21, 52] and the remaining two studies, one which was rated as high quality [48], used patient-days as a denominator with the ME rate ranging from 6.4 to 9.1 per 1000 patient-days [48, 50]. Errors in prescribing and drug administration were the most commonly reported types of MEs in PICUs across all five studies.
Prescribing errors were the most common ME type examined in PICUs. Sixteen studies (15/21, 71.4%) presented rates of prescribing errors; three used different denominators and further analysis could not be performed [56, 58, 62] with the remaining 12 studies (two of which were rated as high quality [51, 53]) using medication orders as the denominator yielding a median rate of 13.25 (IQR 9.5–29.35) per 100 medication orders. Six studies out of these 12 studies were conducted in PICUs that utilised paper-based medication chart systems with a median rate of prescribing errors calculated as 13 per 100 medication orders (IQR 10.9–37.4) [18,19,20, 51, 57, 60]. Two studies (2/12) assessed prescribing errors in PICUs using electronic prescribing systems and the rate of error ranged from 8.3 to 27.1 per 100 medication orders [49, 53]. The remaining four studies did not describe how their prescribing systems functioned [21, 59, 61, 63]. Dosing and documentation errors were the most frequently reported prescribing error subtypes.
One study presented rates of dispensing and transcription errors (0.78 and 4.88 per 100 orders, respectively) [21]. Two studies reported rates of medication administration errors using two different denominators [18, 21]; these rates were 28.9 per 100 orders and 8.2 per 100 drug administrations, with wrong time or wrong route errors being commonly reported medication administration errors.
A total of ten out of the 21 studies reported ME severity data in PICUs using different scales [19,20,21, 49, 50, 52, 53, 57, 62, 63], which could not be grouped into main categories to allow comparison between studies. Therefore, severity data of each study were summarised in Tables 2–4 of the ESM.
Three studies originating from the USA presented overall preventable ADE rates [37, 54, 55]. In two of these studies, which were rated to be of high quality, preventable ADEs ranged from 21 to 29 per 1000 patient-days [54, 55]. One of these studies reported a 4% increase in the risk of preventable ADE for each additional 1-year increase in age [54]. The rate of preventable ADEs in the remaining study was 2.3 per 100 patients [37].
The severity of harm from preventable ADEs was assessed in two studies [37, 54]. The majority of events in one study by Agarwal et al. were assigned a low level of severity using the National Coordinating Council for Medication Error Reporting and Prevention Scale [72]. Larsen et al. [37] in the other study categorised all detected harms as minor, but did not describe the assessment scale used. The most common drug classes associated with MEs/preventable ADEs in PICUs were reported in four studies (4/21, 19.04%) (Table 2) and involved anti-infective agents (n = 4), cardiovascular agents (n = 3), nervous system agents such as sedatives (n = 2), intravenous fluids (n = 1), respiratory agents (n = 1) and diuretics (n = 1) [19, 49, 52, 62].
3.4 Prevalence and Nature of Medication Errors and Preventable Adverse Drug Events in Neonatal Intensive Care Units
Seventeen studies (17/35, 48.6%) provided data from NICUs to calculate the rates of MEs and preventable ADEs. Table 3 shows the rates and frequently occurring ME types as well as the rates of preventable ADEs.
The overall rates of MEs in NICUs ranged from 4 to 35.1 per 1000 patient-days (two studies [48, 50], one was rated as high quality [48]) and from 5.5 to 77.9 per 100 medication orders (two studies) [4, 24]. A further two studies, one was rated to be of high quality [64], reported ME rates using different denominators; namely, 69.5 MEs per 1000 doses [64] and 26.4 per 100 case records [22]. Prescribing errors and medication administration errors were the most commonly reported ME types in NICUs. The severity of detected MEs in these studies was either not addressed [22, 24, 48] or addressed through preventable/potential ADEs; two preventable ADEs out of 148 MEs [50], 46 potential ADEs per 100 admissions [4] and 0.86 preventable ADEs per 1000 doses [64].
Six out of eight studies reporting prescribing error rates provided rates per medication orders with a median of 14.9% (IQR 4.25–29.9). Three of these six studies examined NICUs using paper-based prescribing systems and the median error rate was calculated as 28.9 per 100 medication orders (IQR 22.5–32.8) [18, 25, 68]. Neonatal ICUs with electronic medication chart systems were examined only in one study where the prescribing error rate was 7.3 per 100 medication orders [49]. The remaining two studies (2/6) did not describe their NICU prescribing systems [66, 67]. Dosing errors were the prevalent prescribing errors subtype [18, 25, 49, 66,67,68]. Only one of these studies reported prescribing error severity data finding that most errors were not significant. One study reported a combined rate of prescribing errors and dispensing errors, which was 0.7 per patient with no significant harm and dosing errors representing 48.1% of errors [36].
The median prevalence of medication administration errors was 31.4 per 100 administrations (IQR 8.2–84.8) across three studies; with severity addressed in only one study, which found that most observed errors were of moderate severity [18, 69, 70]. Commonly reported medication administration errors were dosing errors, omitted doses, wrong time and wrong administration rate errors.
Three studies (3/17, 17.6%) reported rates of preventable ADEs in NICUs across different countries [45, 50, 64]. Two of these studies used the same denominator (per 1000 patient-days) and preventable ADE rates ranged from 0.47 to 14.38 [45, 50]. These studies classified preventable ADE severity using different scales. One study [50] categorised all preventable ADEs as serious and another [45] found 14.3% of preventable ADEs resulted in persistent disability. The third study reported a preventable ADE rate of 0.86 per 1000 administered doses and no data were provided regarding the severity of these events [64]. Only three studies (3/17, 17.6%) reported the commonly observed drug classes associated with MEs/preventable ADEs in NICUs (Table 2) including anti-infective agents (n = 3), nervous system agents (n = 1), intravenous fluids (n = 1), cardiovascular agents (n = 1), respiratory agents (n = 1), folates (n = 1) and multi-vitamins (n = 1) [23, 25, 49].
4 Discussion
We present the findings from a comprehensive systematic review examining the frequency and nature of both MEs and preventable ADEs in critically ill neonates and children. We found that MEs are a common problem in PICUs (14.6 per 100 medication orders and 6.4–9.1 per 1000 patient-days) and NICUs (ranging from 4 to 35.1 per 1000 patient-days and from 5.5 to 77.9 per 100 medication orders). Medication error rates were reported in few studies that examined all stages of medication use process in PICUs (five studies) and NICUs (four studies). Anti-infective agents were found to be commonly associated with MEs/preventable ADEs across both PICUs and NICUs. Errors in prescribing and drug administration were found to be frequent types of MEs and dosing errors were a frequently reported error subtype in both ICU settings.
There are limited published data concerning the frequency and nature of harms due to MEs (preventable ADEs) in PICUs and NICUs and restricted mostly to one country [37, 52, 54, 64], which constitutes an important area for further research as this type of harm contributes to an extended length of hospital stay and additional healthcare costs [74]. In the UK, it was recently estimated that preventable ADEs cost the National Health Service an additional £14.8 million per year [75]. Understanding preventable harm has greater significance for identifying key challenges to improve patient care. This would enable the development of successful remedial interventions to reduce harmful MEs and associated costs, ultimately supporting the current World Health Organization’s safety global campaign to improve patient safety [13].
In most of the included studies in our review, dosing errors were reported as being a common subtype of MEs in PICUs and NICUs. This is similar to the findings of other systematic reviews conducted in the paediatric population [7, 14, 44, 76] and our findings therefore reinforce recommendations that have previously been made to prioritise interventions designed to reduce dosing errors in clinical practice [44, 77]. A previous systematic review published in 2004 demonstrated that some healthcare professionals were not competent in calculating correct doses (e.g. weight based) in paediatric patients, which could result in ten-fold errors [78]. Prevalent dosing errors also imply the use of off-label and unlicensed drugs in children as a common associated factor [79]. Safe and effective dosing of these medications has been reported to be difficult because of variable scientific recommendations and a lack of appropriate formulations for children [80, 81] and may thus lead to errors in prescribed/administered doses and ultimately harmful events [82, 83].
There are systematic reviews that have evaluated the effectiveness of existing interventions to reduce MEs in children; one focusing on PICUs published in 2014 [84], two on paediatrics including ICUs published in 2014 [85] and 2015 [86] and one on neonatal settings involving NICUs published in 2018 [87]. These reviews found that implementing technical innovations (e.g. computerised physician order entry or clinical decision support systems) might help in reducing MEs. However, all these reviews concluded that the evidence remains limited, with methodological variations identified across the reviewed studies, which prevented a more thorough assessment.
We compared error rates between ICUs using paper-based and electronic prescribing systems in PICUs and NICUs; however, available data on ICUs that use electronic prescribing systems were provided only by small number of studies (only two studies in PICUs and one in a NICU). We identified four studies with a pre-post-intervention design [51, 60, 63, 65], one of which was rated as high quality [51], that assessed the impact of introducing electronic medication charts on prescribing errors in paper-based ICUs. All these studies found a significant reduction in prescribing error rates (e.g. from 30.1 to 0.2 [51] and from 8.24 to 1.4 [63] per 100 medication orders) following the introduction of electronic prescribing systems.
Issues with study heterogeneity were also identified in our systematic review. There were marked differences in definitions and research methodologies used across studies in detecting MEs/preventable ADEs, which likely contributed to a wide variability in reported rates and made direct comparisons between different studies challenging. For instance, the highest overall ME rate (77.9% of medication orders) was reported in a NICU study [24], which employed a chart review for prescribing errors and direct observation of nurses preparing and administering medications for medication administration errors. This detailed two-stage screening could have resulted in an overall high rate of MEs. In addition, direct observation of nursing practice is known to identify more medication administration errors than other approaches such as reviewing medication administration records [88]. Similar issues in ME research have been noted in other systematic reviews [2, 7, 32].
Furthermore, the definitional variation may have an important impact on effect estimates. One study defined prescribing error according to the Institute of Medicine definition including any errors during the prescribing or transcribing phases in physicians’ orders containing pharmacological (e.g. medications) and non-pharmacological (e.g. nutritional supplements) items [19]. The broad definition used in this study that encapsulated transcription could have had influenced the rate of prescribing error (59.4%), which was higher than the rate reported in another study (8.24%) using a more specific prescribing error definition “incomplete or illegible prescription that required additional clarification to be executed” [63].
Lower prescribing error rates were also found in retrospective studies [36, 59, 63, 65, 66]. The limitations of this approach (such as risk of poor-quality documentation and missing data) are acknowledged [89]. This may lead to low accuracy of detection and underestimation of the prevailing error rate. Prospectively designed studies with pharmacists collecting data are associated with higher rates of prescribing errors [19, 25, 51, 61, 68]. Subsequently, this approach has been found to be sensitive in detecting MEs [2, 90].
Therefore, we highlight a need for researchers in the field to work towards greater standardisation to help ensure future ME and preventable ADE studies in critically ill children utilise more consistent study designs and definitions. This would facilitate greater comparability of studies and aligns with a call for greater standardisation that have been made in other ME/ADE systematic reviews [42, 91, 92].
Another possible reason for the variability of outcome rates may be related to differences in healthcare systems between countries, hospitals or even medical teams on hospital wards [93, 94]. For example, two studies conducted in Iran and the USA using the same study design, non-electronic prescribing systems, over a similar period time with a similar sample size reported notable differences in ME rates of 48.8% and 14.6%, respectively [21, 52]. Medication safety issues may require nationally coordinated interventions in which policymakers aim to create safer environments for patient care rather than efforts by individual teams [95].
Few studies included a robust assessment of severity of MEs and preventable ADEs in PICU and NICU settings. In the small number of studies that considered severity, the variation in scales used (e.g. using a panel of medical experts [62], criteria set out by the National Coordinating Council for Medication Error Reporting and Prevention [64] or the American Society of Health-System Pharmacists criteria [36]) made comparisons across these studies impractical. This also hindered judgement as to which patient group(s), stage(s) in the medication process or particular medication class(es) may be more vulnerable to preventable ADEs. Consequently, we recommend that future studies use a standardised approach to assess the severity of preventable medication-related events to help identify targets for serious adverse events.
Data regarding medications commonly associated with MEs/preventable ADEs were reported in four PICU and three NICU studies. The commonly reported prevalent drugs implicated in MEs/preventable ADEs in both settings were medicines used to treat infections followed by cardiovascular and nervous system agents as less frequently reported drug classes. This may be because anti-infective agents are more frequently prescribed in critically ill patients. Previous systematic reviews on paediatric patients have also reported that antibiotics were the most common drug class associated with MEs [44] and antimicrobials with medication administration errors [32], which suggests that future efforts in reducing MEs/preventable ADEs in critically ill paediatric patients could target this group of medications.
Among all phases of the medication use process, prescribing was examined more frequently in both settings with comparatively little focus on the drug dispensing and administration phases. However, in studies examining the whole medication use process, MEs were commonly associated with both prescribing and administration phases. Thus, we recommend the frequency, type, and severity of medication administration errors and dispensing errors as a priority area for further research to effectively guide efforts to reduce such events across both ICU settings. A systematic review published in 2015, which assessed the effectiveness of existing interventions to reduce medication administration errors in hospitalised children including PICU patients, found that the true impact of available approaches remains limited [32]. The review concluded that medication administration is a multi-faceted process, which includes, for example, preparation technique and the correct patient, drug and dose, which may need better understanding to target the most susceptible stage to errors.
It is apparent that MEs/preventable ADEs in patients admitted to PICUs/NICUs were more frequently examined in the USA (10/35, 28.6% of included studies) than other countries. There is still little focus on these patient populations in the rest of the world. For example, only three of included studies were undertaken in Africa and none from Australia. More work is needed in individual countries to understand the frequency and nature of MEs/preventable ADEs in PICUs and NICUs to support the current World Health Organization’s call to reduce these events.
The focus of future studies should also include investigating the underlying causes of prescribing errors. This is because high rates of such errors were found in a number of studies conducted across both ICU settings, with little change between recent studies and those 10 years previously [19, 25, 53, 68]. Paediatric and neonatal ICUs are complex and dynamic environments with a strong humanistic element. A recent qualitative study explored causative factors in prescribing errors in PICUs and identified that systems in PICUs to support safe medication practice were ineffective [96]. This study supported the findings of Manias et al.’s review, which also identified limited effectiveness of interventions to mitigate ME in PICUs [84]. The complex interplay of systems also means that the consideration of prescribing errors, dispensing errors and medication administration errors as separate processes and phenomena may be misleading and lead to tokenistic interpretation of the causes. Therefore, a multi-system approach (based on precise estimates that used robust definitions) to understanding the underlying causes of MEs in critically ill children may be an area for investigation. These recommendations would support efforts in the development of effective interventional approaches and successful prioritisation of their implementation.
The key strength of this review is the inclusion of both MEs and related preventable ADEs together providing a more complete overview of the risks associated with medication use in children’s intensive care settings. This will help to better target attention towards interventions that might reduce preventable events and associated harm.
The limitations of this systematic review include the limited ability to meta-analyse data across studies owing to marked levels of observed heterogeneity across included studies. We acknowledge that there might be some preventive policies/interventions already implemented in specific study settings to reduce MEs in our included studies [97, 98], which may have influenced the rates of MEs, including interventional research studies where we only extracted baseline rates. Unfortunately, detailed data on such policies/interventions were not routinely reported. In addition, this systematic review did not assess quantitatively the impact of differences in ME definitions, error detection methods and hospital context (differences in units examined) on the error rates across included studies.
Because of the marked variations in study methods we observed between included studies, we could not directly compare findings between high- and low-quality studies. In addition, several conference abstracts were included in our systematic review that only provided basic information, hence limiting our ability to assess study quality. High-quality research in the future is needed to acquire a better estimate of the prevalence and nature of these events in children’s intensive care settings.
Only English language publications were included in this systematic review. The search terms we used were limited to English and therefore terms used in other languages may have been missed.
5 Conclusions
To our knowledge, this is the first systematic review exploring the prevalence and nature of both MEs and preventable ADEs across neonatal and paediatric intensive care settings worldwide. We identified 35 unique studies and found that preventable medication-related events are an enduring threat to the safety of children in intensive care. We have identified potentially important targets such as dosing errors and anti-infective medications that could help set an improvement agenda for clinicians, healthcare leaders and researchers. However, we also acknowledge the pressing need for standardisation of study design and definitions owing to high levels of observed heterogeneity and we recognise a need for future research to explore issues such as medication administration errors and preventable ADEs in more detail as there is little attention to these in the current body of evidence.
References
MacFie CC, Baudouin SV, Messer PB. An integrative review of drug errors in critical care. J Intensive Care Soc. 2016;17(1):63–72.
Wilmer A, Louie K, Dodek P, Wong H, Ayas N. Incidence of medication errors and adverse drug events in the ICU: a systematic review. Qual Saf Health Care. 2010;19(5):e7. https://doi.org/10.1136/qshc.2008.030783.
Wilson DG, McArtney R, Newcombe RG, McArtney R, Gracie J, Kirk C, et al. Medication errors in paediatric practice: insights from a continuous quality improvement approach. Eur J Pediatr. 1998;157(9):769–74.
Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–20.
Conroy S, McIntyre J, Choonara I. Unlicensed and off label drug use in neonates. Arch Dis Child Fetal Neonatal Ed. 1999;80(2):F142–4.
Nir-Neuman H, Abu-Kishk I, Toledano M, Heyman E, Ziv-Baran T, Berkovitch M. Unlicensed and off-label medication use in pediatric and neonatal intensive care units: no change over a decade. Adv Ther. 2018;35(7):1122–32. https://doi.org/10.1007/s12325-018-0732-y.
Santesteban E, Arenas S, Campino A. Medication errors in neonatal care: a systematic review of types of errors and effectiveness of preventive strategies. J Neonatal Nurs. 2015;21(5):200–8.
Turner S, Gill A, Nunn T, Hewitt B, Choonara I. Use of “off-label” and unlicensed drugs in paediatric intensive care unit. Lancet. 1996;347(9000):549–50.
Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol. 2001;52(1):77–83.
Conroy S. Association between licence status and medication errors. Arch Dis Child. 2011;96(3):305. https://doi.org/10.1136/adc.2010.191940.
World Health Organization. Promoting safety of medicines for children. In: Patient safety. World Health Organization; 2007. http://apps.who.int/medicinedocs/en/m/abstract/Js14235e/. Accessed 15 Jan 2019.
Franke HA, Woods DM, Holl JL. High-alert medications in the pediatric intensive care unit. Pediatr Crit Care Med. 2009;10(1):85–90.
World Health Organization. WHO global patient safety challenge: medication without harm. In: Patient safety. World Health Organization; 2017. https://www.who.int/patientsafety/medication-safety/en/. Accessed 19 June 2019.
Sutcliffe K, Stokes G, O’Mara A, Caird J, Hinds K, Bangpan M, et al. Paediatric medication error: a systematic review of the extent and nature of the problem in the UK and international interventions to address it. London: EPPI-Centre, Social Science Research Unit, Institute of Education, University of London; 2014.
Cliff-Eribo KO, Sammons H, Choonara I. Systematic review of paediatric studies of adverse drug reactions from pharmacovigilance databases. Expert Opin Drug Saf. 2016;15(10):1321–8.
Smyth RL, Peak M, Turner MA, Nunn AJ, Williamson PR, Young B, et al. ADRIC: Adverse Drug Reactions In Children: a programme of research using mixed methods. Chapter 4. Systematic review of paediatric adverse drug reactions. In: National Institute for Health Research; 2014. https://www.ncbi.nlm.nih.gov/books/NBK262753/. Accessed 3 Aug 2018.
Silva DC, Araujo OR, Arduini RG, Alonso CF, Shibata AR, Troster EJ. Adverse drug events in a paediatric intensive care unit: a prospective cohort. BMJ Open. 2013;3(2):e001868.
Otero P, Leyton A, Mariani G, Cernadas JMC. Medication errors in pediatric inpatients: prevalence and results of a prevention program. Pediatrics. 2008;122(3):e737–43.
Ewig CL, Cheung HM, Kam KH, Wong HL, Knoderer CA. Occurrence of potential adverse drug events from prescribing errors in a pediatric intensive and high dependency Unit in Hong Kong: an observational study. Pediatr Drugs. 2017;19(4):347–55.
Glanzmann C, Frey B, Meier CR, Vonbach P. Analysis of medication prescribing errors in critically ill children. Eur J Pediatr. 2015;174(10):1347–55.
Haghbin S, Shahsavari S, Vazin A. Medication errors in pediatric intensive care unit: incidence, types and outcome. Trends Pharm Sci. 2016;2(2):109–16.
Likhi D, Phatak A, Morgaonkar V, Ganjiwale J, Nimbalkar S. Audit of medication errors in a neonatal intensive care unit of a tertiary care hospital. In: India Western, editor. 6th Congress of the European Academy of Paediatric Societies. Amsterdam: Springer; 2016. p. 1874–5.
Machado AP, Tomich CS, Osme SF, Ferreira DM, Mendonça MA, Pinto RM, et al. Prescribing errors in a Brazilian neonatal intensive care unit. Cad Saude Publica. 2015;31(12):2610–20.
Truter A, Schellack N, Meyer JC. Identifying medication errors in the neonatal intensive care unit and paediatric wards using a medication error checklist at a tertiary academic hospital in Gauteng, South Africa. S Afr J Child Health. 2017;11(1):5–10.
Palmero D, Di Paolo ER, Beauport L, Pannatier A, Tolsa JF. A bundle with a preformatted medical order sheet and an introductory course to reduce prescription errors in neonates. Eur J Pediatr. 2016;175(1):113–9. https://doi.org/10.1007/s00431-015-2607-4.
Leung JS, Johnson DW, Sperou AJ, Crotts J, Hartling L, Stang A. A systematic review of adverse drug events associated with administration of common asthma medications in children. PLoS One. 2017;12(8):e0182738.
Koumpagioti D, Varounis C, Kletsiou E, Nteli C, Matziou V. Evaluation of the medication process in pediatric patients: a meta-analysis. J Pediatr (Rio J). 2014;90(4):344–55.
Khan LM. Comparative epidemiology of hospital-acquired adverse drug reactions in adults and children and their impact on cost and hospital stay: a systematic review. Eur J Clin Pharmacol. 2013;69(12):1985–96.
Gates PJ, Meyerson SA, Baysari MT, Lehmann CU, Westbrook JI. Preventable adverse drug events among inpatients: a systematic review. Pediatrics. 2018;142(3):e20180805.
Van Rosse F, Maat B, Rademaker CM, van Vught AJ, Egberts AC, Bollen CW. The effect of computerized physician order entry on medication prescription errors and clinical outcome in pediatric and intensive care: a systematic review. Pediatrics. 2009;123(4):1184–90.
Gonzales K. Medication administration errors and the pediatric population: a systematic search of the literature. J Pediatr Nurs. 2010;25(6):555–65.
Ameer A, Dhillon S, Peters MJ, Ghaleb M. Systematic literature review of hospital medication administration errors in children. Integr Pharm Res Pract. 2015;4:153–65. https://doi.org/10.2147/iprp.S54998.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Mateen FJ, Oh J, Tergas AI, Bhayani NH, Kamdar BB. Titles versus titles and abstracts for initial screening of articles for systematic reviews. Clin Epidemiol. 2013;5:89–95. https://doi.org/10.2147/clep.s43118.
Ghaleb MA, Barber N, Franklin BD, Wong ICK. The incidence and nature of prescribing and medication administration errors in paediatric inpatients. Arch Dis Child. 2010;95(2):113–8.
Jain S, Basu S, Parmar V. Medication errors in neonates admitted in intensive care unit and emergency department. Indian J Med Sci. 2009;63(4):145–51.
Larsen GY, Donaldson AE, Parker HB, Grant MJC. Preventable harm occurring to critically ill children. Pediatr Crit Care Med. 2007;8(4):331–6.
Kilbridge PM, Noirot LA, Reichley RM, Berchelmann KM, Schneider C, Heard KM, et al. Computerized surveillance for adverse drug events in a pediatric hospital. J Am Med Inform Assoc. 2009;16(5):607–12.
Flynn EA, Barker KN, Pepper GA, Bates DW, Mikeal RL. Comparison of methods for detecting medication errors in 36 hospitals and skilled-nursing facilities. Am J Health Syst Pharm. 2002;59(5):436–46.
Allan E, Barker K. Fundamentals of medication error research. Am J Health Syst Pharm. 1990;47(3):555–71.
Alsulami Z, Conroy S, Choonara I. Medication errors in the Middle East countries: a systematic review of the literature. Eur J Clin Pharmacol. 2013;69(4):995–1008.
McLeod MC, Barber N, Franklin BD. Methodological variations and their effects on reported medication administration error rates. BMJ Qual Saf. 2013;22(4):278–89.
Alshehri GH, Keers RN, Ashcroft DM. Frequency and nature of medication errors and adverse drug events in mental health hospitals: a systematic review. Drug Saf. 2017;40(10):871–86.
Ghaleb MA, Barber N, Franklin BD, Yeung VW, Khaki ZF, Wong IC. Systematic review of medication errors in pediatric patients. Ann Pharmacother. 2006;40(10):1766–76.
Kunac DL, Kennedy J, Austin N, Reith D. Incidence, preventability, and impact of adverse drug events (ADEs) and potential ADEs in hospitalized children in New Zealand. Pediatr Drugs. 2009;11(2):153–60.
Kunac DL, Reith DM. Preventable medication-related events in hospitalised children in New Zealand. N Z Med J. 2008;121(1272):17–32.
Fortescue EB, Kaushal R, Landrigan CP, McKenna KJ, Clapp MD, Federico F, et al. Prioritizing strategies for preventing medication errors and adverse drug events in pediatric inpatients. Pediatrics. 2003;111(4):722–9.
Benkirane RR, Redouane R, Haimeur CC, El Kettani SSEC, Azzouzi AA, Alaoui AAD, et al. Incidence of adverse drug events and medication errors in intensive care units: a prospective multicenter study. J Patient Saf. 2009;5(1):16–22.
Khoo TB, Tan JW, Ng HP, Choo CM, Teh SH. Paediatric in-patient prescribing errors in Malaysia: a cross-sectional multicentre study. Int J Clin Pharm. 2017;39(3):551–9.
Sakuma M, Ida H, Nakamura T, Ohta Y, Yamamoto K, Seki S, et al. Adverse drug events and medication errors in Japanese paediatric inpatients: a retrospective cohort study. BMJ Qual Saf. 2014;23(10):830–7.
Potts AL, Barr FE, Gregory DF, Wright L, Patel NR. Computerized physician order entry and medication errors in a pediatric critical care unit. Pediatrics. 2004;113(1):59–63.
Buckley MS, Erstad BL, Kopp BJ, Theodorou AA, Priestley G. Direct observation approach for detecting medication errors and adverse drug events in a pediatric intensive care unit. Pediatr Crit Care Med. 2007;8(2):145–52.
Cimino MA, Kirschbaum MS, Brodsky L, Shaha SH, Initiative CHA. Assessing medication prescribing errors in pediatric intensive care units. Pediatr Crit Care Med. 2004;5(2):124–32.
Agarwal S, Classen D, Larsen G, Tofil NM, Hayes LW, Sullivan JE, et al. Prevalence of adverse events in pediatric intensive care units in the United States. Pediatr Crit Care Med. 2010;11(5):568–78.
Kaushal R, Bates DW, Abramson EL, Soukup JR, Goldmann DA. Unit-based clinical pharmacists’ prevention of serious medication errors in pediatric inpatients. Am J Health Syst Pharm. 2008;65(13):1254–60.
Booth R, Sturgess E, Taberner-Stokes A, Peters M. Zero tolerance prescribing: a strategy to reduce prescribing errors on the paediatric intensive care unit. Intensive Care Med. 2012;38(11):1858–67.
Sutherland A, Barber R, Talken-Sinclair J. Reducing prescribing errors in a British PICU. Pediatr Crit Care Med. 2011;12:A148.
Isaac RE, Martin J, Reynolds F. Prevalence and characteristics of prescribing errors in a paediatric intensive care unit (PICU). Pediatr Crit Care Med. 2014;15:206.
Morris S, Rivett C. The development of a quality improvement system to monitor, assess and feedback prescribing errors in paediatric intensive care. Arch Dis Child. 2016;101(9):e2.
Warrick C, Naik H, Avis S, Fletcher P, Franklin BD, Inwald D. A clinical information system reduces medication errors in paediatric intensive care. Intensive Care Med. 2011;37(4):691–4.
Maat B, Bollen C, van Vught A, Egberts A, Rademaker C. Errors in medication prescriptions in paediatric intensive care patients. Arch Dis Child. 2012;97(Suppl 2):A-62A.
Alagha HZ, Badary OA, Ibrahim HM, Sabri NA. Reducing prescribing errors in the paediatric intensive care unit: an experience from Egypt. Acta Paediatr. 2011;100(10):e169–74.
Kadmon G, Bron-Harlev E, Nahum E, Schiller O, Haski G, Shonfeld T. Computerized order entry with limited decision support to prevent prescription errors in a PICU. Pediatrics. 2009;124(3):935–40.
Morriss FH, Abramowitz PW, Nelson SP, Milavetz G, Michael SL, Gordon SN, et al. Effectiveness of a barcode medication administration system in reducing preventable adverse drug events in a neonatal intensive care unit: a prospective cohort study. J Pediatr. 2009;154(3):363–8.e1.
Jozefczyk KG, Kennedy WK, Lin MJ, Achatz J, Glass MD, Eidam WS, et al. Computerized prescriber order entry and opportunities for medication errors: comparison to tradition paper-based order entry. J Pharm Pract. 2013;26(4):434–7.
Ridges S, Huggins F. Comparison of medication order entry error rates in a neonatal intensive care unit versus an adult intensive care unit in a predominantly adult hospital. In: ASHP Midyear Clinical Meeting. 2009. https://www.ashp.org/Meetings-and-Events/Meetings-and-Conferences/Midyear-Clinical-Meeting-and-Exhibition/Midyear-Clinical-Meeting-Abstracts-Archive. Accessed 19 June 2019.
Fordham T, Green H, Badeaa Q, Ibrahim H, Subhedar NV. Reduction in prescription errors in a neonatal intensive care unit: a completed audit cycle. London: BMJ Publishing Group; 2015. p. A160.
Campino A, Lopez-Herrera MC, Lopez-de-Heredia I, Valls-i-Soler A. Medication errors in a neonatal intensive care unit. Influence of observation on the error rate. Acta Paediatr. 2008;97(11):1591–4.
Chedoe I, Molendijk H, Hospes W, Van den Heuvel ER, Taxis K. The effect of a multifaceted educational intervention on medication preparation and administration errors in neonatal intensive care. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F449–55.
Raja Lope R, Boo N, Rohana J, Cheah F. A quality assurance study on the administration of medication by nurses in a neonatal intensive care unit. Singapore Med J. 2009;50(1):68–72.
American Society of Health-System Pharmacists. ASHP standard definition of a medication error. Am J Hosp Pharm. 1982;39(2):321.
National Co-ordinating Council for Medication Error Reporting and Prevention. NCCMERP index for categorising medication errors. http://www.nccmerp.org/categorizing-medication-errors-index-color. Accessed 12 Feb 2018.
Billstein-Leber M, Carrillo CJD, Cassano AT, Moline K, Robertson JJ. ASHP guidelines on preventing medication errors in hospitals. Am J Health Syst Pharm. 2018;75(19):1493–517. https://doi.org/10.2146/ajhp170811.
Wolfe D, Yazdi F, Kanji S, Burry L, Beck A, Butler C, et al. Incidence, causes, and consequences of preventable adverse drug reactions occurring in inpatients: a systematic review of systematic reviews. PLoS One. 2018;13(10):e0205426.
Elliott RCE, Campbell F, Jankovic D, Martyn St James M, Kaltenthaler E, Wong R, et al. Prevalence and economic burden of medication errors in the NHS in England. In: Policy Research Unit in Economic Evaluation of Health and Care Interventions. Universities of Sheffield and York; 2018. http://www.eepru.org.uk/prevalence-and-economic-burden-of-medication-errors-in-the-nhs-in-england-2/. Accessed 19 June 2019.
Gates PJ, Meyerson SA, Baysari MT, Westbrook JI. The prevalence of dose errors among paediatric patients in hospital wards with and without health information technology: a systematic review and meta-analysis. Drug Saf. 2019;42(1):13–25. https://doi.org/10.1007/s40264-018-0715-6.
Conroy S, Sweis D, Planner C, Yeung V, Collier J, Haines L, et al. Interventions to reduce dosing errors in children. Drug Saf. 2007;30(12):1111–25.
Wong ICK, Ghaleb MA, Franklin BD, Barber N. Incidence and nature of dosing errors in paediatric medications. Drug Saf. 2004;27(9):661–70. https://doi.org/10.2165/00002018-200427090-00004.
Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr. 2005;164(9):552–8.
Czarniak P, Bint L, Favie L, Parsons R, Hughes J, Sunderland B. Clinical setting influences off-label and unlicensed prescribing in a paediatric teaching hospital. PLoS One. 2015;10(3):e0120630. https://doi.org/10.1371/journal.pone.0120630.
Conroy S, McIntyre J, Choonara I, Hull PS. Unlicensed and off label drug use in neonates. Arch Dis Child Fetal Neonatal Ed. 1999;80(2):F142–5.
Bellis JR, Kirkham JJ, Thiesen S, Conroy EJ, Bracken LE, Mannix HL, et al. Adverse drug reactions and off-label and unlicensed medicines in children: a nested case–control study of inpatients in a pediatric hospital. BMC Med. 2013;11:238. https://doi.org/10.1186/1741-7015-11-238.
Bellis J, Kirkham J, Pirmohamed M. Adverse drug reactions and off-label and unlicensed medicines in children: a nested case–control study of paediatric inpatients. Arch Dis Child. 2013;98(6):e1.
Manias E, Kinney S, Cranswick N, Williams A, Borrott N. Interventions to reduce medication errors in pediatric intensive care. Ann Pharmacother. 2014;48(10):1313–31.
Rinke ML, Bundy DG, Velasquez CA, Rao S, Zerhouni Y, Lobner K, et al. Interventions to reduce pediatric medication errors: a systematic review. Pediatrics. 2014;134(2):338–60.
Maaskant JM, Vermeulen H, Apampa B, Fernando B, Ghaleb MA, Neubert A, et al. Interventions for reducing medication errors in children in hospital. Cochrane Database Syst Rev. 2015;3:CD006208. https://doi.org/10.1002/14651858.cd006208.pub3.
Nguyen M-NR, Mosel C, Grzeskowiak LE. Interventions to reduce medication errors in neonatal care: a systematic review. Ther Adv Drug Saf. 2018;9(2):123–55.
Manias E. Detection of medication-related problems in hospital practice: a review. Br J Clin Pharmacol. 2013;76(1):7–20.
Lao KS, Chui CS, Man KK, Lau WC, Chan EW, Wong IC. Medication safety research by observational study design. Int J Clin Pharm. 2016;38(3):676–84.
Dean B, Schachter M, Vincent C, Barber N. Prescribing errors in hospital inpatients: their incidence and clinical significance. Qual Saf Health Care. 2002;11(4):340–4.
Keers RN, Williams SD, Cooke J, Ashcroft DM. Prevalence and nature of medication administration errors in health care settings: a systematic review of direct observational evidence. Ann Pharmacother. 2013;47(2):237–56.
Lewis PJ, Dornan T, Taylor D, Tully MP, Wass V, Ashcroft DM. Prevalence, incidence and nature of prescribing errors in hospital inpatients. Drug Saf. 2009;32(5):379–89.
Nolan TW. System changes to improve patient safety. BMJ. 2000;320(7237):771–3.
Davis K, Stremikis K, Squires D, Schoen C. Mirror, mirror on the wall. In: How the performance of the US health care system compares internationally. New York (NY): Common Wealth Fund; 2014. https://www.commonwealthfund.org/publications/fund-reports/2010/jun/mirror-mirror-wall-how-performance-us-health-care-system. Accessed 19 June 2019.
Illingworth J. Continuous improvement of patient safety. In: The case for change in the NHS; 2015. https://www.health.org.uk/publications/continuous-improvement-of-patient-safety. Accessed 19 June 2019.
Sutherland A, Ashcroft DM, Phipps DL. Exploring the human factors of prescribing errors in paediatric intensive care units. Arch Dis Child. 2019;104(6):588–95. https://doi.org/10.1136/archdischild-2018-315981.
Greenberg RG, Smith PB, Bose C, Clark RH, Cotten CM, DeRienzo C. National survey of neonatal intensive care unit medication safety practices. Am J Perinatol. 2018;35(14):1419–22.
Matti N, Nguyen MR, Mosel C, Grzeskowiak LE. Utilization of neonatal medication error prevention strategies: a clinical practice survey of Australian and New Zealand neonatal units. Ther Adv Drug Saf. 2018;9(11):609–17. https://doi.org/10.1177/2042098618796952.
Acknowledgements
We are thankful to Ghadah H. Alshehri for her help with creating the systematic review protocol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Anwar A. Alghamdi gratefully acknowledges the King Abdulaziz University for funding his PhD programme at the University of Manchester. No sources of funding were received for the conduct of this systematic review or preparation of this article.
Conflict of interest
Anwar A. Alghamdi, Richard N. Keers, Adam Sutherland and Darren M. Ashcroft have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
This research does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Alghamdi, A.A., Keers, R.N., Sutherland, A. et al. Prevalence and Nature of Medication Errors and Preventable Adverse Drug Events in Paediatric and Neonatal Intensive Care Settings: A Systematic Review. Drug Saf 42, 1423–1436 (2019). https://doi.org/10.1007/s40264-019-00856-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-019-00856-9