Abstract
Background and Objectives
Olaratumab is a recombinant human monoclonal antibody that binds to platelet-derived growth factor receptor-α (PDGFRα). In a randomized phase II study, olaratumab plus doxorubicin met its predefined primary endpoint for progression-free survival and achieved a highly significant improvement in overall survival versus doxorubicin alone in patients with advanced or metastatic soft tissue sarcoma (STS). In this study, we characterize the pharmacokinetics (PKs) of olaratumab in a cancer patient population.
Methods
Olaratumab was tested at 15 or 20 mg/kg in four phase II studies (in patients with nonsmall cell lung cancer, glioblastoma multiforme, STS, and gastrointestinal stromal tumors) as a single agent or in combination with chemotherapy. PK sampling was performed to measure olaratumab serum levels. PK data were analyzed by nonlinear mixed-effect modeling techniques using NONMEM®.
Results
The PKs of olaratumab were best described by a two-compartment PK model with linear clearance (CL). Patient body weight was found to have a significant effect on both CL and central volume of distribution (V 1), whereas tumor size significantly affected CL. A small subset of patients developed treatment-emergent anti-drug antibodies (TE-ADAs); however, TE-ADAs did not have any effect on CL or PK time course of olaratumab. There was no difference in the PKs of olaratumab between patients who received olaratumab as a single agent or in combination with chemotherapy.
Conclusion
The PKs of olaratumab were best described by a model with linear disposition. Patient body weight and tumor size were found to be significant covariates. The PKs of olaratumab were not affected by immunogenicity or chemotherapeutic agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A mathematical/statistical model to describe the disposition of olaratumab was developed using data from four clinical studies. |
The model describes the time course of olaratumab disposition in the body of all patients included in the analysis and predicts disposition in additional patient populations. |
The described model is the most comprehensive understanding of the pharmacokinetic properties of olaratumab. |
1 Introduction
Platelet-derived growth factor receptor-α (PDGFRα) and its downstream signaling pathways have been implicated in cancer cell proliferation, metastasis, and the tumor microenvironment. PDGF/PDGFR signaling has an important role in mesenchymal stem cell differentiation, growth of mesenchymal cells, angiogenesis, and wound healing under normal physiological conditions [1,2,3]. PDGF/PDGFRα signaling has also been shown to be involved in the pathogenesis of multiple cancers, including osteosarcoma, chondrosarcoma, prostate cancer, breast cancer, and ovarian cancer, where the PDGF/PDGFRα complex has been shown to promote tumor growth and proliferation as well as tumor vasculature [4].
Olaratumab is a recombinant human immunoglobulin G subclass 1 (IgG1) monoclonal antibody (mAb) that binds specifically to PDGFRα. In vitro studies have demonstrated that olaratumab inhibits PDGFRα pathway signaling in tumor and stromal cells. In addition, in vivo studies have shown that olaratumab disrupts the PDGFRα pathway in tumor cells and inhibits tumor growth. An open-label, phase Ib and randomized phase II clinical study with olaratumab plus doxorubicin versus doxorubicin alone in patients with advanced or metastatic soft tissue sarcoma (STS) recently met its predefined primary endpoint for progression-free survival and achieved a highly significant improvement of 11.8 months in median overall survival over doxorubicin alone [5]. The study also demonstrated that a dose of 15 mg/kg (administered on days 1 and 8 of a 21-day cycle), which resulted in mean steady-state concentrations between 123 and 487 µg/mL, resulted in an acceptable safety profile in patients with advanced STS.
Given the clinically positive benefit-risk observed in the phase 1b/II study in advanced STS, a better understanding of the pharmacokinetics (PKs) of olaratumab was needed to ensure that serum levels associated with improved clinical outcomes can be achieved in as many patients as possible in the clinical setting. In this study, we present a population PK analysis of olaratumab in cancer patients enrolled in four phase II studies carried out in the US and EU. The primary objective was to analyze the PK data by means of nonlinear mixed-effect modeling (NONMEM) in order to estimate the typical PK properties and interpatient variability (IPV) in the cancer patient population. The PK model developed was also used to examine the effect of patient factors, including immunogenicity, as well as the effect of chemotherapy on the PKs of olaratumab in cancer patients.
2 Materials and Methods
2.1 Study Design and Study Population
The analysis of olaratumab PKs was based on data collected from four phase II studies in various cancer patient populations: nonsmall cell lung cancer (NSCLC), glioblastoma multiforme (GBM), STS, and gastrointestinal stromal tumors (GISTs). In STS patients (n = 95), olaratumab was tested at a dose of 15 mg/kg administered as a 60-min infusion on days 1 and 8 of a 21-day cycle, combined with 75 mg/m2 doxorubicin on day 1 of the cycle for up to 8 cycles (53 patients received olaratumab monotherapy after disease progression in the control arm). In NSCLC patients (n = 50), olaratumab was tested at a dose of 15 mg/kg administered as a 30-min infusion on days 1 and 8 of a 21-day cycle in combination with 200 mg/m2 paclitaxel and AUC 6 carboplatin. In GBM (n = 7) and GIST patients (n = 19), olaratumab was tested as a single agent at a dose of 20 mg/kg administered as a 90- to 60-min infusion every 14 days. In all studies, olaratumab was administered until disease progression or unacceptable toxicity was observed. In the NSCLC, GIST and STS studies, efficacy assessment, including tumor assessments, were performed according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) guidelines every 6 weeks. No tumor size data were available from the seven patients enrolled in the GBM study.
Rich and/or sparse sampling was conducted in patients. Rich sampling was generally limited to cycles 1 and 3, and peak and trough concentrations were collected for most of the remaining treatment cycles. Clinical data were collected by a series of questions contained in the clinical report forms, while information such as date of birth, habits (e.g. alcohol consumption, smoking), historical diagnoses, and chronic conditions were collected by patient self-report. Clinical parameters such as weight, blood pressure, and pulse were measured at specific visits at investigator sites. Laboratory tests to measure standard clinical chemistry panel, such as total bilirubin, albumin, etc., were conducted at investigator laboratories or a sponsor-designated laboratory. Serum samples of olaratumab were analyzed using a validated modified enzyme-linked immunosorbent assay (ELISA) method at ICON Development Solutions (Whitesboro, NY, USA). The lower limit of quantitation was 1 μg/mL and upper limit of quantification was 100 μg/mL. Samples above the limit of quantification were diluted with 0.2% human serum with Blocker™ BLOTTO in Tris-buffered saline to yield results within the range of quantification. The potential formation of treatment-emergent anti-drug antibodies (TE-ADAs) in serum was assessed using a validated ELISA following a four-tier approach: identification of putative positive samples (Tier 1); confirmation of detected antibodies (Tier 2); titer of detected antibodies (Tier 3); and characterization as neutralizing or non-neutralizing (Tier 4). Samples that were identified as being positive for ADAs were further evaluated in the neutralizing ADA assay.
2.2 Model Development and Analysis
The PK model development, evaluation, and validation generally follow recent guidelines for PKPD modeling [6]. The PKs of olaratumab were characterized by means of nonlinear mixed-effect modeling using NONMEM (version 7.3; ICON Development Solutions, Gaithersburg, MD, USA). A series of compartmental models were evaluated to best describe olaratumab concentration–time data. Given the exposure-dependent disposition of monoclonal antibodies targeting membrane-bound antigens, such as olaratumab, both linear and Michaelis–Menten (MM) clearance (CL) terms were tested. IPV was investigated for all parameters, and covariance between parameters was assessed using an omega block. Proportional and combined additive and proportional error models were also evaluated. Estimates of the PK parameters and error terms were obtained using the first-order conditional (FOCE) with epsilon-eta interaction estimation method in all analyses. Missing data, PK data below the quantification limit of the assay, or incomplete data items (i.e. missing time/date entries) were excluded from the analysis. Missing values of independent variables (patient characteristic data) were imputed within a given patient, using the last observation carried forward (LOCF) method.
Selection of the most appropriate PK base model structure was based on agreement between predicted and observed serum concentrations, lack of pattern (i.e. randomness) in the weighted residuals versus the predicted values, changes in the IPV, and significant decreases in the minimum objective function (MOF). A visual predictive check (VPC) was also performed on the base model to investigate the agreement between the observed and predicted concentrations.
Upon establishment of an appropriate structural and statistical model, the effects of patient factors were assessed for their influence on the disposition of olaratumab. The patient factors tested comprised both continuous and categorical covariates (Table 1). Given there was an acceptable level of ETA shrinkage, selection of the covariates began with visual inspection of covariate effects on the IPV of relevant parameters. Covariates that exhibited correlation in the distribution of IPV of PK parameters were then selected for further evaluation. Stepwise covariate modeling (SCM) was implemented using Perl-Speaks NONMEM (PsN) [7]. Continuous covariates were tested using linear, power, or exponential models, as shown in Eq. (1) through Eq. (3). Categorical covariates were tested using a categorical model, as shown in Eq. (4).
where P is the individual’s estimate of the parameter (e.g. CL, V), \( \varTheta_{ 1} \) represents the typical value of the parameter, \( \varTheta_{ 2} \) represents the effect of the covariate, COV is the value of the covariate, and MED is the population median of the covariate. IND is an indicator variable with a value of either 0 or 1 assigned for values of a dichotomous categorical covariate (e.g. female or male) and 1 to n for various values of a categorical covariate ranging from 1 to n, where n is the number of categories (e.g. n geographies).
The criteria for the selection of covariates in the forward selection was a statistically significant (p < 0.01) drop in MOF (≥6.635), whereas the criteria for backward elimination were more stringent (p < 0.001), with a greater drop in MOF (≥10.828). Model convergence, reasonable estimates of parameter values, and parameter precision were all additional factors for covariate selection. Once statistically significant covariates were identified, individual analysis was performed for each continuous covariate to ensure the inclusion of the covariate results in a ≥2% decrease in the IPV of the corresponding model parameter. To demonstrate clinical relevance, covariates were only retained if their effect on the corresponding parameter was >15% for a dichotomous covariate, or >15% at the highest or lowest observed covariate value for a continuous covariate. In addition, model performance evaluated through goodness-of-fit plots and VPCs was taken into consideration during the selection process. Only those covariates that met the above criteria were included in the final PK model. Certain covariates deemed clinically significant were included regardless of inclusion criteria. For instance, given that olaratumab was administered based on body weight, the influence of body weight at time of study entry (WTE) on CL and V 1 was incorporated as a power function (Eq. 2). Allometric scaling was also tested, with a fixed power coefficient of 0.75 for CL parameters, and 1 for volume of distribution parameters [8].
Once the final PK model was established, a VPC was performed to ensure that the model maintained fidelity with the observed PK data and to ensure the inclusion of the covariates visibly improved model performance. The stability and precision of final PK model parameter estimates were assessed through a bootstrap analysis.
3 Results
3.1 Data Summary
Of the four studies included in this analysis, a total of 1748 data points from 196 patients were collected. Overall, 188 observations (10.8%) were below the quantifiable limit of the PK assay and were therefore excluded from the analysis. Only 18 observations (1.0%) were taken after the start of treatment. Another 59 observations (3.3%) were also excluded due to missing data items. In total, 1501 olaratumab serum concentration observations from 171 patients (NSCLC: 50 patients, 272 observations; GBM: 7 patients, 37 observations; STS: 95 patients, 1132 observations; GIST: 19 patients, 60 observations) were retained in the analysis. Continuous and categorical characteristics, as well as details on the number of patients per treatment regimen, are provided in Table 2. The number of samples per patient ranged from 1 to 54, with a median of five samples. Olaratumab serum concentration–time profiles relative to the first olaratumab dose and time after dose are illustrated in Fig. 1, along with the relative contributions of data from the four studies. The blood sampling scheme adopted in the studies facilitated the capturing of increasing olaratumab serum concentrations as they approached their steady state levels, as well as the elimination after the last dose. Across the entire analysis database, the median number of treatment cycles with olaratumab ranged from 4 to 5. A total of 32 patients experienced at least one dose reduction.
3.2 Base Model Development
The time course data of olaratumab serum concentrations was best described with a two-compartment PK model with linear clearance parameterized in terms of clearance (CL), central volume of distribution (V 1), peripheral volume of distribution (V 2), and intercompartmental clearance (Q). Parameter estimates for the base PK model are presented in Table 3. During the initial model development, a term describing target-mediated drug disposition (TMDD) was incorporated into the model in the form of an MM approximation. However, this mixed CL model showed instability and poor precision in parameter estimation, therefore the MM clearance term was removed from the model. Log-normally distributed IPV was estimated with high precision for V 1 and CL. There was no significant correlation between the IPV of V 1 and CL. Interoccasion variability (IOV) was also tested but was removed from the model due to lack of precision in the parameter estimate. Residual variability was best characterized by an additive/proportional error model. Evaluations of diagnostic goodness-of-fit plots (data not shown) indicated good agreement between model-predicted and observed PK data, as well as random distribution of residual error.
3.3 Covariate Analysis and Final Model
The effect of the covariates listed in Table 1 on V 1 and CL was initially assessed visually (data not shown). Since none of the examined covariates changed over time, the analysis was conducted using their values at the time of initial assessment. Since olaratumab is administered per kilogram of body weight, the effect of WTE was incorporated into the model prospectively. Evaluation of the body weight effect on CL and V 1 showed that using estimated power coefficients for body weight resulted in the best fit and was thus retained. After implementing the prespecified inclusion/exclusion criteria for the remaining covariates, only tumor size effect on CL using a linear model was retained in the final model. Incorporation of WTE effect on CL and V 1 resulted in decreases in IPV of 2.6 and 6.6% points for CL and V 1, respectively. Subsequent incorporation of tumor size effect on CL resulted in an additional decrease of 2.3% points in IPV of CL. Overall, the incorporation of WTE effect on CL and V 1, and tumor size effect on CL, led to a total decrease in IPV of 4.9 and 6.6% points for CL and V 1, respectively.
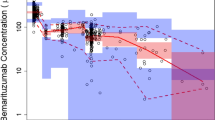
The final population PK model parameters were all estimated with high precision (Table 3). Goodness-of-fit plots show good agreement between model-predicted and observed PK values, as well as randomness in residual variability (electronic supplementary Fig. S1). The VPC of the final PK model (Fig. 2) showed good agreement between predicted and observed values in all prediction intervals in the early half of sampling times where data are rich. However, at later sampling times after drug treatment where data are sparse, the variability in the model prediction increases as expected. Bootstrap analysis of the final model showed the model was stable and that model parameters were all estimated with high precision (Table 3).
Visual predictive check of the final olaratumab population pharmacokinetic model. a Full time course of data available after dose. b Early time points where rich data are available. Black circles indicate observed data, dashed lines depict the observed 5th, 50th, and 95th percentiles, and the blue shaded areas define 90% confidence intervals of the 5th, 50th and 95th percentiles of the stimulated model predictions. Actual time from dose was rounded to the nearest 200 h to facilitate percentage calculation. Conc concentration
3.4 Immunogenicity
Across the four studies, a total of nine subjects tested positive for TE-ADAs, corresponding to an incidence of 5% of the total patient population. An overlay of the time course of olaratumab serum concentration and ADA titers in TE-ADA-positive patients showed no correlation between olaratumab concentration and ADA titers (Fig. 3a). Furthermore, there was no difference between the individual CL estimates in patients who tested positive versus those who tested negative for TE-ADAs (Fig. 3b). The effect of ADAs on the CL of olaratumab was thus not included in the model.
Effect of anti-drug antibody titers on olaratumab pharmacokinetics. a Sample time course of olaratumab serum concentration (grey) overlaid with time course of anti-drug antibody titer (red) in patients tested positive for treatment-emergent anti-drug antibody. b Post hoc clearance estimates of patients negative for TE-ADAs versus those positive for TE-ADAs. ADA anti-drug antibody, CL clearance, NSCLC nonsmall cell lung cancer, PK pharmacokinetic, STS soft tissue sarcoma, TE-ADAs treatment-emergent anti-drug antibodies
3.5 Drug–Drug Interaction
Potential drug–drug interaction (DDI) of olaratumab with paclitaxel/carboplatin and doxorubicin was explored using the same PK analysis dataset, which contained olaratumab serum data collected from patients who received olaratumab as a single agent (n = 53), as well as in combination with paclitaxel/carboplatin (n = 45) or doxorubicin (n = 73). Model estimates of individual patient CL and V 1 across the three groups are graphically presented in Fig. 4. No difference in olaratumab CL or V 1 was observed between individuals who received olaratumab alone or in combination with either a doxorubicin or paclitaxel/carboplatin regimen.
Pharmacokinetic model estimates across treatments. Comparison of individual CL (left) and V 1 (right) estimates in patients who received olaratumab as a single agent or in combination with either PTX and CP or Dox. CL clearance, CP carboplatin, Dox doxorubicin, PTX paclitaxel, V 1 central volume of distribution
3.6 Body Weight-Based versus Fixed Dosing
Since body weight was a significant covariate for olaratumab CL and volume of distribution, the model developed in this study was used to evaluate the effect of body weight-based and fixed dosing strategies on the variability of olaratumab concentrations between patients. Specifically, a dose of 15 mg/kg and a flat dose of 1200 mg, infused on days 1 and 8 of a 21-day cycle, were simulated using post hoc individual PK parameter estimates of all patients from the four studies. The distribution of the simulated trough concentration after cycle 1 (C min1) and the average concentration (C avg) showed no visible difference between a weight-based or flat dosing strategy (Fig. 5). As expected, plots of C min1 and C avg versus WTE show that weight-based and flat dosing could lead to different olaratumab serum levels in patients with very low or very high body weights.
Weight-based versus flat dosing effect on olaratumab concentrations. Overlay of simulated olaratumab C min1 (left) and C avg (right) following weight-based dose of 15 mg/kg or flat dose of 1200 mg in the current study patient population. C avg average serum concentration, C min1 trough serum concentration during cycle 1, CV coefficient of variation
4 Discussion
4.1 Study Overview
The objectives of this work were to develop a population PK model to characterize the PKs of olaratumab in cancer patients. The PK model would be used to characterize the IPV of olaratumab PKs, and identify patient factors, including immunogenicity, that may influence olaratumab disposition. As olaratumab was used either as a single agent or in combination with doxorubicin or paclitaxel/carboplatin, the model was also used to assess the effect of chemotherapy on the disposition of olaratumab.
4.2 Structural Model
The final PK model for olaratumab was a two-compartment model with IPV in CL and V 1. The CL of olaratumab was found to be linear at the doses tested, with an elimination half-life estimated to be approximately 11 days, which is typical of therapeutic monoclonal antibodies [9]. As the disposition of an mAb is influenced by the relative expression and turnover of the target, commonly referred to as TMDD [10], TMDD was evaluated during model development. Although numerous models were previously developed to describe TMDD for mAbs [11,12,13], an MM approximation [14, 15] was used, as is commonly done with clinical PK data due to parameter identifiability concerns [16]. The MM parameters could be identified, but were estimated with poor precision and the model exhibited instability during bootstrap validation. These findings suggest that the MM parameters associated with TMDD were only supported by data from a small subset of the patient population, and were no longer identifiable when these were replaced during the bootstrap analysis. The linear PK model was thus retained as the final structural model, which in turn indicates that the doses of 15 and 20 mg/kg administered on days 1 and 8 of a 21-day cycle or every 14 days, respectively, yield olaratumab serum levels likely to achieve full target saturation.
4.3 Covariate Model
The effect of different covariates (including sex, age, body weight, race, albumin, hepatic function, and renal function) on the disposition of olaratumab was also investigated in the analysis. As olaratumab is administered in milligrams per kilogram, WTE was prospectively added as a covariate and was found to have a significant effect on both CL and V 1. Most importantly, the effect of WTE was less than directly proportional on either CL or V 1, with exponent values of approximately 0.5. Compared with flat dosing, the body weight-based dosing paradigm currently adopted for olaratumab is therefore not expected to inflate PK variability on either CL or V 1 [17]. This was confirmed by simulations using our population PK model, which showed that the distributions of olaratumab concentrations (C min1 and C avg) are similar under the two different dosing strategies. However, it should be noted that our analysis is based on data collected from studies carried out in the US, therefore the distribution of WTE may not be fully descriptive of that in the worldwide population. The effect of weight-based versus flat dosing on olaratumab serum levels and its activity in patients with extreme body weight values could thus not be fully understood, which supported carrying body weight-based dosing into the global STS phase III study (ClinicalTrials.Gov identifier: NCT02451943). Tumor size was also found to have a significant effect on CL, whereby a larger tumor burden was associated with higher CL. Several potential contributing factors, such as variation in tumor biology, target expression [18], and the uneven distribution of the number of patients in the four trials, may explain this observation. Since the PKs of olaratumab are best described by a model with linear disposition within the range of serum levels observed in our dataset, it is unlikely that CL in patients with larger tumors increased because of a higher level of target expression and TMDD. The distribution of CL and V 1 across the different tumor types was also analyzed in order to rule out a potential contribution of uneven tumor type representation during the SCM; no differences were found (data not shown). Overall, although inclusion of WTE and tumor size as covariates fulfilled all statistical criteria, comparison of base and final model goodness-of-fit plots indicates that their overall contribution to variability remains limited. IPV on the PK parameters of the final population PK model was low to moderate (33.3% for CL, 15.6% for V 1), as commonly observed with monoclonal antibodies [16]. The lack of correlation between liver or renal status and CL can be expected given the known mechanisms involved for clearance of antibodies [16, 19, 20].
4.4 Immunogenicity
The small incidence of immunogenicity in the database (5% across all four studies) did not allow the inclusion of immunogenicity in an integrated model [21]. However, the comparison of individual post hoc estimates for olaratumab CL showed no notable difference between patients who tested positive or negative for TE-ADAs, not unexpected considering that ADAs were of low titer. This is consistent with a visual analysis of the time profile of TE-ADA titers overlaid with that of olaratumab serum concentration, where increases in TE-ADA titers did not correspond with a decrease in olaratumab serum concentrations. Although not all TE-ADAs will affect the PKs of mAbs [22], in some cases the development of TE-ADAs can have profound effects on the disposition of mAbs [23]. Therefore, it was important to rule out the involvement of TE-ADAs in the PKs of olaratumab. These findings should also be confirmed using data from other clinical studies of olaratumab in a similar patient population [ClinicalTrials.Gov identifiers: NCT02326025 and NCT02451943].
4.5 Drug–Drug Interactions
It was possible to examine the potential effect of chemotherapy on the PKs of olaratumab based on olaratumab serum concentration data collected from patients who received olaratumab as a single agent as well as in combination with paclitaxel/carboplatin or doxorubicin, in the PK database. As expected for a biologic, no clinically relevant difference in olaratumab CL or V 1 was observed as a result of the combination with either chemotherapeutic regimen. These findings are in line with previous reports [24, 25] and, together with the results of a DDI study showing no effect of olaratumab on the PKs of doxorubicin [26], support the use of olaratumab in combination with chemotherapeutic agents without dose adjustments.
5 Conclusion
The population PK model developed in this study indicates that olaratumab exhibits linear drug disposition suggestive of full target saturation at the dose levels tested in the four studies included in the analysis. Olaratumab elimination half-life was approximately 11 days, which corresponds to a time to steady state of approximately 50 days. The disposition of olaratumab was found to be influenced by patient body weight and tumor size, but simulations using the final model indicate that the current weight-based dosing is adequate in limiting IPV of drug concentrations. Finally, neither the development of TE-ADAs nor the combination with chemotherapies affected the PK properties of olaratumab.
References
Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–312.
Li A, Xia X, Yeh J, Kua H, Liu H, Mishina Y, et al. PDGF-AA promotes osteogenic differentiation and migration of mesenchymal stem cell by down-regulating PDGFRalpha and derepressing BMP-Smad1/5/8 signaling. PLoS One. 2014;9(12):e113785.
Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112(2):295–307.
Shah GD, Loizos N, Youssoufian H, Schwartz JD, Rowinsky EK. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer. 2010;116(4 Suppl):1018–26.
Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–97.
Nguyen TH, Mouksassi MS, Holford N, Al-Huniti N, Freedman I, Hooker AC, et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol. 2017;6(2):87–109.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79(3):241–57.
Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36.
Leveque D, Wisniewski S, Jehl F. Pharmacokinetics of therapeutic monoclonal antibodies used in oncology. Anticancer Res. 2005;25(3c):2327–43.
Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs. 2011;3(1):61–6.
Gibiansky L, Gibiansky E, Kakkar T, Ma P. Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn. 2008;35(5):573–91.
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28(6):507–32.
Mager DE, Krzyzanski W. Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm Res. 2005;22(10):1589–96.
Peletier LA, Gabrielsson J. Dynamics of target-mediated drug disposition. Eur J Pharm Sci. 2009;38(5):445–64.
Yan X, Mager DE, Krzyzanski W. Selection between Michaelis–Menten and target-mediated drug disposition pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2010;37(1):25–47.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633–59.
Zhang S, Shi R, Li C, Parivar K, Wang DD. Fixed dosing versus body size-based dosing of therapeutic peptides and proteins in adults. J Clin Pharmacol. 2012;52(1):18–28.
Shinjo K, Kondo Y. Targeting cancer epigenetics: linking basic biology to clinical medicine. Adv Drug Deliv Rev. 2015;95:56–64.
Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M. Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet. 2013;52(2):83–124.
Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12(1):33–43.
Ng CM, Loyet KM, Iyer S, Fielder PJ, Deng R. Modeling approach to investigate the effect of neonatal Fc receptor binding affinity and anti-therapeutic antibody on the pharmacokinetic of humanized monoclonal anti-tumor necrosis factor-alpha IgG antibody in cynomolgus monkey. Eur J Pharm Sci. 2014;51:51–8.
Kelley M, Ahene AB, Gorovits B, Kamerud J, King LE, McIntosh T, et al. Theoretical considerations and practical approaches to address the effect of anti-drug antibody (ADA) on quantification of biotherapeutics in circulation. AAPS J. 2013;15(3):646–58.
Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs. 2010;2(3):256–65.
Seitz K, Zhou H. Pharmacokinetic drug-drug interaction potentials for therapeutic monoclonal antibodies: reality check. J Clin Pharmacol. 2007;47(9):1104–18.
Zhao L, Ren TH, Wang DD. Clinical pharmacology considerations in biologics development. Acta Pharmacol Sin. 2012;33(11):1339–47.
Villalobos V, Agulnik M, Pollack SM, Rushing DA, Singh A, Van Tine BA, et al. Abstract CT145: a phase I open-label study to evaluate the effect of olaratumab on the pharmacokinetics (PK) of doxorubicin (Dox) in patients with advanced soft tissue sarcoma (STS). Cancer Res. 2016;76(14 Suppl):CT145.
Acknowledgements
The authors acknowledge the contributions of Randy Nichols in the preparation of the analysis datasets.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Eli Lilly and Company, USA, and/or one of its subsidiaries.
Conflicts of interest
At the time of performing this study, Gary Mo, John R. Baldwin, Debra Luffer-Atlas, Robert L. Ilaria Jr, Ilaria Conti, Michael Heathman, and Damien M. Cronier were all employees and shareholders of Eli Lilly and Company.
Research Involving Human Participants
The studies used in this analysis were done in compliance with the Declaration of Helsinki, International Conference on Harmonisation Guidelines for Good Clinical Practice, and applicable local regulations. The protocol was approved by the Ethics Committees of all participating centers and all patients provided written informed consent before study entry.
Additional information
Ilaria Conti: Former employee of Eli Lilly and Company.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40262_2017_562_MOESM1_ESM.pdf
Fig. S1 Goodness-of-Fit Plots of the Final Olaratumab Population Pharmacokinetic Model. Observed versus predicted olaratumab serum concentration and conditional weighted residuals for the final olaratumab PK model. Color points are used to identify data from the different tumor types. (PDF 67 kb)
40262_2017_562_MOESM2_ESM.pdf
Fig. S2 Inter-patient Variability versus Weight at Study Entry and Tumor Size. Inter-patient variability (η) for CL (upper two plots) and V1(lower two plots) are plotted against tumor size (left two plots) and weight at study entry (right two plots). Blue line is the loess regression analysis to show trend in data. (PDF 19 kb)
40262_2017_562_MOESM3_ESM.pdf
Fig. S3 Visual Predictive Check of the Final Olaratumab Population Pharmacokinetic Model by Tumor Type. Data from each tumor type are overlaid with model simulation from the full popPK model. Black circles indicate observed data, the dashed lines depict the observed 5th, 50th, and 95th percentiles, and the blue shaded areas define 90% confidence intervals of the 5th, 50th and 95th percentiles of the stimulated model predictions. Actual time from dose was rounded to the nearest 200 hours to facilitate percentage calculation. (PDF 174 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mo, G., Baldwin, J.R., Luffer-Atlas, D. et al. Population Pharmacokinetic Modeling of Olaratumab, an Anti-PDGFRα Human Monoclonal Antibody, in Patients with Advanced and/or Metastatic Cancer. Clin Pharmacokinet 57, 355–365 (2018). https://doi.org/10.1007/s40262-017-0562-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0562-0