Abstract
Purpose of Review
This article aims to review the anatomy and ultrasound techniques of common interfascial plane blocks used for cardiac surgeries along with the current available evidence for regional analgesia.
Recent Findings
Thoracic erector spinae plane block (ESPB) has a beneficial role in studies when compared with intravenous pain medications or control groups without blocks for cardiac surgeries. Some retrospective studies showed variable analgesic benefits with ESPB, and a recent meta-analysis did not show promising benefits over thoracic epidural analgesia. Serratus anterior plane block (SAPB) is beneficial with minithoractomy incisions for minimally invasive cardiac surgeries, while para sternal blocks (PSB) or parasternal intercostal plane (PIP) blocks are useful for sternotomy incisions. Pectolaris nerve blocks (PECS) have also been used for various cardiac surgeries with a promising role in cardiac pacemaker and ICD surgeries.
Summary
There is an increasing trend in the usage of fascial plane blocks for cardiac surgeries. Most can be used as components of multimodal analgesia and play a key role in enhanced recovery after cardiac surgery (ERACS) programs. The choice of these fascial plane blocks as opioid-sparing regional analgesia techniques depends on the incision and type of cardiac surgery. A combination of various fascial plane blocks can be used to increase the efficacy of these blocks, but caution should be exercised in limiting the total quantity of the local anesthetic administered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enhanced recovery after surgery (ERAS) programs are being implemented in almost all specialties of surgery, with cardiac surgery being no exception. One of the major components of ERAS protocols is pain control using multimodal “opioid-sparing” analgesic regimens. However, the implementation of multimodal protocols in cardiac surgery patients is challenging due to complex comorbidity profiles, anticoagulation status, and the risk of acute kidney injury, to name a few [1, 2]. Traditionally, anesthetic management relied on high-dose opioid-based regimens for pain control and the cardioprotective effect of opioids, leading to their widespread use. In the current era of the opioid epidemic, we are looking for alternative strategies to reduce the perioperative use of opioids, which include promoting the use of non-opioid agents, including acetaminophen, gabapentin, ketamine, magnesium, and intravenous lidocaine, and incorporating nonopioid measures, such as regional anesthesia [3]. Historically, thoracic epidural analgesia (TEA) and paravertebral blocks (PVBs) were the most used regional techniques for postoperative pain management, but the increased risk of epidural hematomas caused by systemic heparinization, hemodynamic instability due to sympathetic blockade, technical difficulty, and challenges with postoperative pain management with neuraxial blocks has constrained the usage of TEA and PVBs in the cardiac surgical patient population [4,5,6]. This has led to the research and development of various newer regional analgesic techniques, including ultrasound-guided fascial plane blocks. In this review, we discuss several thoracic anterior, lateral, and posterior interfascial plane blocks that can be used in the perioperative pain management of patients undergoing cardiac surgery.
Thoracic Erector Spinae Plane Block
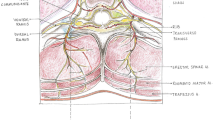
Erector spinae plane block (ESPB) was first introduced in 2016 for the treatment of thoracic neuropathic pain by Forero et al. Since the inception of ESPB, it has gained popularity with the success of its use in the pain management of patients with rib fractures, postsurgical pain in breast surgery, thoracic surgery, and is recently being used in cardiac surgery patients. The thoracic erector spinae plane block (ESPB) provides analgesia from the posterior midline to the posterior axillary line in the craniocaudal direction by blocking the dorsal ramus of the spinal nerve as it traverses over the transverse process. Some studies report that the spread of local anesthetic to the paravertebral space also blocks the ventral ramus and sympathetic ganglia, providing analgesia, which remains controversial. Its usage has gained popularity due to issues with the placements of neuraxial blocks in these cardiac surgical patients due to anticoagulation. ESPB is considered a superficial fascial plane block that can be safely administered using ultrasound guidance in these high-risk patients.
The ESPB is performed with the patient in prone, lateral, or sitting position. The probe is placed in a parasagittal orientation over the selected transverse process (Fig. 1). The needle is then advanced in a caudal or cranial direction towards the corner of the transverse process, below the erector spinae muscle group. After confirming with hydro dissection that the needle is in the correct fascial plane, local anesthetic is injected below the erector spinae muscle group above the transverse process (Fig. 2 and Video 1). In the adult thoracic spine, 20–30 mL of dilute local anesthetic spreads approximately 4 levels above and 4 levels below the site of injection. For midline incision, bilateral ESPBs need to be performed, and catheters can be placed for prolonged postoperative analgesia.
Nagaraja et al. [7] conducted a prospective randomized controlled trial (RCT) including 50 patients undergoing elective cardiac surgeries and compared continuous thoracic epidural analgesia (TEA) with bilateral ESPB with catheters. They showed that the visual analog scale (VAS) pain scores were comparable in the two groups up until 12 h postextubation (P > 0.05), and VAS pain scores at rest and during cough were better in the ESPB catheter group at 24-h, 36-h, and 48-h marks (P < 0.05). There was no difference in the total intraoperative fentanyl usage, postoperative incentive spirometry numbers, rescue analgesic requirement, ventilator duration, or length of ICU stay in either group. In another well-designed, single-blinded prospective RCT in 106 elective cardiac surgical patients undergoing coronary artery bypass graft, mitral valve replacement, or atrial septal defect, Krishna et al. [8•] compared ultrasound-guided bilateral ESPB with intravenous (IV) pain medications. The primary outcome measure of pain scores evaluated using the 11-point numeric rating scale postextubation up to 12 h was far better in the ESPB group than in the IV pain medication group (P = 0.0001). In addition, ESPB patients had used fewer rescue analgesics (P = 0.0001), less total opioid (P = 0.0001) and were extubated at a significantly earlier time (63.09 ± 1.30 min to 102.62 ± 1.30 min). Macaire et al. [9], in a patient-matched controlled before and after trial with continuous ESPB, also reported a significant decrease in opioid usage in the first 48 h following surgery (40 mg in the control group to 0 mg in the ESPB catheter group with a P < 0.001) and decrease in time to first mobilization post-extubation (median time of 2 h vs 3.5 h with P < 0.05).
Gaweda et al. [10] conducted a prospective double-blinded RCT to study the effect of the addition of pectoralis nerve (PECS) blocks to ESPB in patients undergoing minithoracotomy for mitral or tricuspid valve repair. Patients in the PECS + ESPB group used less oxycodone than the individuals in the ESPB group [median 12 (interquartile range (IQR): 6–16) mg vs 20 (IQR: 18–19) mg P = 0.0004]. Secondary outcomes of pain intensity assessed by VAS scores were appreciably lower, and patient satisfaction (P = 0.0007) was greater in the PECS + ESPB group.
Despite multiple recent studies showing the benefit of ESPB over no regional anesthetic in off-pump [11] and on-pump [12] cardiac surgeries, King et al. [13••], in their meta-analysis of five studies, reported that ESPB did not significantly impact postoperative pain scores, intraoperative opioid usage, time-to-extubation, or ICU length of stay (LOS). There are several retrospective studies that have reported limited benefits of ESPB for cardiac surgeries. Zhou et al. [14••], in their meta-analysis of 65 RCTs, concluded that TEA seems to be the most effective postoperative regional anesthesia for patients undergoing cardiac surgery. Further high-quality RCTs are needed to study the impact of ESPB in cardiac surgery patients.
Serratus Anterior Plane Blocks: Superficial and Deep Approaches
Serratus anterior plane block (SAPB) provides analgesia to the anterolateral chest wall by blocking the lateral cutaneous branches of the T2 to T9 intercostal nerves, the intercostobrachial nerve, and the thoracodorsal nerve. The long thoracic nerve is also blocked with the superficial approach. SAPB is being used for postoperative pain management following breast surgery, rib fractures, and thoracotomy.
The block is performed with the patient supine or lateral with the arm abducted. A linear probe is placed in the mid-axillary line at the T4 level (Fig. 3). In this position, ribs 5 and 6 are visualized, and the serratus muscle is seen immediately above the ribs. The wedge-shaped latissimus dorsi muscle lies above the serratus muscle, and the thoracodorsal artery can be seen in the fascial plane between the latissimus dorsi and serratus anterior muscles. With the superficial approach, local anesthetic is injected in an anterior to posterior direction in the fascial plane between the serratus anterior and latissimus dorsi muscles (Fig. 4 and Video 2). With the deep approach, local anesthetic is injected in an anterior to posterior direction between the serratus anterior muscle and the rib (Fig. 5 and Video 3). The plane between the serratus anterior muscle and the rib may be easier to visualize in patients with challenging anatomy. The deep approach spares the long thoracic nerve. In total, approximately 20 to 40 mL of dilute local anesthetic is injected while being mindful of the maximum dosage of local anesthetic that each patient can safely receive. Hydrodissection with saline is advised when performing this block so that local anesthetic is spared from intramuscular injection when confirming that the block needle is in the correct fascial plane.
Berthoud et al. [15] extended the application of SAPB for pain management in minimally invasive heart surgery (MIHS) patients. In a retrospective study, they compared the use of SAPB with a continuous wound infiltration (CWI) catheter inserted into the subcutaneous space at the site of the surgical incision. The primary outcome of total morphine consumption during the first 48 h after surgery was markedly lower in the SAPB group than in the CWI group [median 21 vs 11 mg (P < 0.01)]. Moreover, the VAS scores in the first 6 h post extubation (P < 0.01) and the length of stay in the ICU (median 41 vs 24 h, P = 0.03) and the hospital (median 8 vs 6 days, P = 0.03) were also strikingly lower in the SAPB group, proving its superiority over CWI. Contrary to Berthould et al.’s results, Moll et al. [16] reported no benefit of SAPB when compared with no block and increased opioid consumption when compared with paravertebral block (PVB) in patients undergoing robotic CABG. In another prospective cohort study, Toscano et al. [17] compared the utilization of continuous deep SAPB for mitral valve surgery via a right mini-thoracotomy approach in 63 patients to conventional IV morphine use. They found that patients in the SAPB group had lower pain scores on the numeric rating scale (NRS) at 48 h (mean 1.77 [95% CI 0.99–2.54] vs 3.23 [95% CI 2.13–4.33] P = 0.03), and the total morphine consumption (mean 2.22 [95% CI 0.99–3.44 mg] vs 12.98 [95% CI 10.90–15.05 mg] P < 0.001) was strikingly lower in comparison to the control group. Although the pain scores at 24 h were lower in the SAPB group (mean 2.15 [95% confidence interval (CI) 1.22–3.09] vs 3.23 [95% CI 2.28–4.29] P = 0.07), they did not reach statistical significance. No differences were noted between the study groups in terms of the sedation evaluated by the Richmond Agitation Sedation Scale, mild adverse effects (namely, nausea and catheter malpositioning) and duration of mechanical ventilation, intensive care unit (ICU) stay, and hospital length of stay. The same group from Italy [18] further compared the use of continuous SAPB vs ESPB for minimally invasive mitral valve surgery and showed them to be equipotent with mild superiority of ESPB in reducing postoperative NSAID and antiemetic use.
Parasternal Blocks: PIFB (Superficial) and TTPB (Deep)
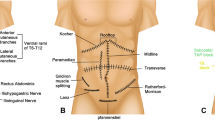
The parasternal block (PSB) provides analgesia to the medial chest by blocking the T2 to T6 anterior cutaneous branches located directly adjacent to the sternum. These branches travel deep into the internal intercostal muscle above the transversus thoracic muscle, and then pierce through the internal intercostal muscle and the pectoralis major muscle just lateral to the sternum. Most of these interfascial plane blocks are now standardized with new nomenclature to avoid confusion with these different blocks and to enable more uniformity with description for all future studies [19•].
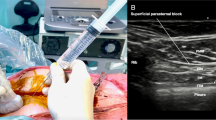
The superficial approach to the PSB, previously named the pecto-intercostal fascial block (PIFB), now named the superficial parasternal intercostal plane (PIP) block, consists of injecting local anesthetic in the fascial plane between the internal intercostal and the pectoralis major muscles. A linear probe is placed in sagittal orientation over the sternum and then moved laterally until the costal cartilages are identified (Fig. 6). The needle is then advanced in-plane in a caudal or cranial direction through the pectoralis major muscle. After confirming that the needle is in the correct fascial plane with hydrodissection between the pectoralis major and internal intercostal muscles, local anesthetic is injected (Fig. 7 and Video 4). The pectoralis major muscle lifts off the costal cartilages with injection of local anesthetic. For superficial parasternal single-shot blocks on adult patients, two equidistant injections of 10 mL dilute local anesthetic are placed on each side of the sternum. For superficial parasternal catheters, a bolus injection of 20 mL on each side of the sternum is sufficient.
The deep approach to the PSB, previously named the transversus thoracis plane block (TTPB), now named the deep parasternal intercostal plane (PIP) block, consists of injecting local anesthetic in the fascial plane between the transversus thoracic and the internal intercostal muscles (Fig. 8 and Video 5). The transversus thoracic muscle is very small, and all that may be visualized below the internal intercostal muscle is the pleura and mammary vessels. To ensure that the mammary vessels are clearly visualized in the short axis, the probe is placed in a transverse orientation next to the sternum (Fig. 9). The internal mammary vessels and the anterior intercostal nerve traverse in the same fascial plane targeted for TTPB; therefore, great care should be taken to identify the vessels, especially the laterally lying vein, using ultrasound imaging while performing the block [20]. The needle is then advanced in a lateral to medial direction below the internal intercostal muscle using careful hydrodissection to avoid the pleura and mammary vessels. The pleura is displaced with an injection below the internal intercostal muscle. The fascial plane of the deep parasternal approach is more compliant than that of the superficial approach, and a single injection of 15–20 mL of dilute local anesthetic on either side of the sternum is sufficient for adult patients.
PIFB use for post sternotomy pain in a patient who underwent coronary artery bypass grafting with internal thoracic artery dissection was first reported by Liu et al. [21]. In the following few years, a few RCTs were conducted to learn the efficacy and safety of PIFB in a cardiac surgical patient population. Kumar and colleagues [22•], in a well-structured, single-blinded RCT, have demonstrated that bilateral PIFB reduces postoperative pain scores and rescue opioid use in patients undergoing CABG and valvular surgery. Khera et al. [23] orchestrated a quadruple-blinded, placebo-controlled RCT looking at bilateral PIFB use at 2 time points, on postoperative day (POD) 0 immediately after cardiac surgery and on POD 1. Although the cumulative opioid consumption was lower in the intervention group than in the placebo group, it did not reach statistical significance [49.1 ± 22.4 mg vs 49.1 ± 26.9 mg; P = 0.14]. Self-reported pain scores were remarkably lower in the bupivacaine group (4.8 ± 2.7 vs 5.1 ± 2.6; P < 0.001), but other secondary outcomes of the length of ICU stay, hospital stay, and incidence of in-hospital postoperative delirium were comparable between both groups. In the same manner, a recent RCT by Zhang et al. [24] comparing continuous PIFB to sham block and a prospective study by Pascarella et al. [25] comparing superficial PSB to no block have proclaimed decreased pain scores, intraop, postop opioid use, and a reduction in intraop opioid consumption, respectively. To improve the efficacy of fascial plane blocks, anesthesiologists are moving toward combination blocks. Dost et al. [26] compared ESPB combined with superficial PSB and ESPB alone to show the superiority of the combination block at improving pain scores and reducing rescue analgesic use. Analogously, Wang et al. [27] combined PIFB with rectus sheath block to show reduced postop opioid consumption until 48 h when compared with PIFB alone.
Aydin et al. [28] were the first to mastermind a prospective double-blinded, placebo-controlled RCT to study the efficacy of TTPB in a cardiac surgical patient population after a pilot study by Fujii et al. [29]. In this feasibility study, Fujii et al. reported no adverse events up to 48 h of follow-up, and the data advocating the TTPB group experienced lower pain scores at the 12-h mark without any significant difference in the 24-h hydromorphone usage. Aydin et al. [28] showed that the 24-h fentanyl consumption was significantly lower in the bupivacaine group than in the placebo group [median 255 mcg (IQR 235–305) vs 465 mcg (415–585), respectively; P < 0.001]. Moreover, there were substantial differences in the time of first rescue analgesic requirement (P < 0.001), VAS scores (P < 0.05), and postoperative nausea (P = 0.04) favoring the bupivacaine group [28]. In a similarly designed RCT, Zhang et al. [30] studied the efficacy of TTPB in 100 pediatric patients undergoing cardiac surgery. They also documented a decrease in pain scores measured by the modified objective pain score (MOPS) in the first 24 h after surgery and a significant decrease in intraoperative and postoperative fentanyl requirements (P < 0.01), time to extubation, duration of ICU stay, and hospital stay (P < 0.01) in the ropivacaine group. Interestingly, Kaya et al. [31••] compared PIFB and TTPB and proved that both are equally effective in managing acute poststernotomy pain.
Pectoralis Nerve (PECS) Blocks
PECS I: Interpectoral Plane Block
The interpectoral plane (IPP) block, formerly named the PECS I block, is an injection of local anesthetic between the pectoralis major and minor muscles. This results in analgesia of the medial and lateral pectoral nerves.
To perform the interpectoral block, the arm is abducted 90°, and a linear probe is placed on the anterior chest wall in a sagittal orientation just medial to the coracoid process. The brachial plexus is first visualized at the infraclavicular level. The probe is tilted medially, bringing the second rib into view. Next, the probe is moved inferior and posterolateral to the level of the third rib (Fig. 10). The pectoralis major and minor muscles are visualized as well as a branch of the thoracoacromial artery between the pectoralis muscles. The needle is advanced in an anteromedial to posterolateral direction using hydrodissection to confirm the fascial plane between the pectoralis major and minor muscles. In adults, 10 mL of local anesthetic is injected to complete the interpectoral block (Fig. 11 and Video 6).
PECS II: Pectoserratus Plane Block
The pectoserratus plane (PSP) block, formerly named PECS II block, is an injection of local anesthetic between the pectoralis minor and serratus anterior muscles. This results in analgesia of the lateral cutaneous branches of T2 to T6.
Continuing the probe position for the interpectoral block, the probe is translated further inferior and posterolateral to the level of the fourth rib. Here, we visualized the serratus anterior muscle over the fourth and fifth ribs and the pectoralis muscles overlying the serratus anterior muscle. The needle is advanced in an anteromedial to posterolateral direction using hydrodissection to confirm the fascial plane between the serratus anterior and pectoralis minor muscles. In adults, 20 mL of local anesthetic is injected into this fascial plane, completing the pectoserratus block (Fig. 12 and Video 7).
Dr. Blanco [32] was the first to describe the use of PECS block for analgesia after breast surgery. There have been several metanalyses performed since then proving the superiority of PECS block over conventional systemic analgesia and being equivalent in efficacy to other regional anesthetic techniques, such as PVB, but mostly in patients undergoing breast surgery [33, 34]. Literature on the use of PECS block in major cardiac surgery is very scarce. Kumar et al. [35] conducted a single-blinded RCT enrolling 40 patients undergoing either CABG or valve surgery using midline sternotomy to compare the analgesic efficacy of bilateral PECS blocks with conventional intravenous analgesia. They showed that VAS pain scores at rest and with cough were significantly lower in the PECS block group up to 18 h post extubation (P ≤ 0.0001), and the duration of mechanical ventilation was also shorter in the block group [108.5 ± 24.33 vs 206.3 ± 47.04; P < 0.0001]. In a recently published retrospective study, Vinzant et al. [36] compared PECS II block with PVB to show that PECS II is a highly safe and effective analgesic option for robotic mitral valve surgery, and that its efficacy is comparable to that of PVB. As mentioned previously, in contemporary regional anesthesia practice, the combination of different fascial plane blocks is becoming common. Two of the following studies of the combination of PECS II with SAPB for minimally invasive cardiac surgery revealed contrasting results. Torre et al. [37] studied 78 patients undergoing minithoracotomy and examined the primary outcome of the critical care pain observation tool (CPOT) score and opioid consumption. They reported better pain control and reduced use of rescue analgesia in the PECS II + SAPB group. In dire contrast, Afirevic et al. [38] claimed that the combination of PEC II + SAPB did not improve postoperative analgesia, cumulative opioid consumption, or respiratory mechanics during the initial 3 days after robotic mitral valve surgery, despite using liposomal bupivacaine along with normal bupivacaine.
PECS block has been utilized in the setting of alternative access transcatheter aortic valve replacement (TAVR). Block et al. [39] utilized PECS II to perform alternative access TAVR with subclavian cut down while administering sedation. The PECS technique allowed the avoidance of general anesthesia to facilitate close monitoring of neurologic status following valve deployment. The use of PECS block is growing and being extrapolated to perioperative analgesic management of cases such as pacemaker and ICD implantation in children [40, 41]. Arasu et al. [42] broadened the use of PECS I block in combination with TTPB for CIED implantation in adults. They affirm the superiority of combination block over PECS I alone.
Conclusions
Systemic anticoagulation has limited the use of traditional neuraxial blockade in cardiac surgery. The use of novel interfascial plane blocks such as the erector spinae plane block (ESPB), serratus anterior plane block (SAPB), parasternal intercostal plane (PIP) block, and pectoralis nerve block (PECS) has increased in the last decade. Fascial plane blocks play a major role in ERACS programs and can facilitate opioid-sparing regional analgesia for cardiac surgeries. There are numerous fascial plane blocks with the choice of a particular block based on the type and complexity of cardiac surgery. These ultrasound-guided fascial plane blocks are safe and easy to perform while overcoming the limitations of neuraxial blocks. Catheters can be placed for these thoracic fascial plane blocks to prolong postoperative analgesia and improve patient satisfaction. A total safe dose of local anesthetic should be taken into account while performing bilateral or multiple interfascial plane blocks to prevent local anesthetic systemic toxicity (LAST).
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Engelman DT, Ben Ali W, Williams JB, Perrault LP, Reddy VS, Arora RC, et al. Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. 2019. https://doi.org/10.1001/jamasurg.2019.1153.
Gregory AJ, Grant MC, Manning MW, Cheung AT, Ender J, Sander M, et al. Enhanced recovery after cardiac surgery (ERAS cardiac) Recommendations: An Important first step-but there is much work to be done. J Cardiothorac Vasc Anesth. 2020;34(1):39–47. https://doi.org/10.1053/j.jvca.2019.09.002.
Koepke EJ, Manning EL, Miller TE, Ganesh A, Williams DGA, Manning MW. The rising tide of opioid use and abuse: the role of the anesthesiologist. Perioper Med (Lond). 2018;7:16. https://doi.org/10.1186/s13741-018-0097-4.
Scarfe AJ, Schuhmann-Hingel S, Duncan JK, Ma N, Atukorale YN, Cameron AL. Continuous paravertebral block for post-cardiothoracic surgery analgesia: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2016;50(6):1010–8. https://doi.org/10.1093/ejcts/ezw168.
Svircevic V, van Dijk D, Nierich AP, Passier MP, Kalkman CJ, van der Heijden GJ, Bax L. Meta-analysis of thoracic epidural anesthesia versus general anesthesia for cardiac surgery. Anesthesiology. 2011;114(2):271–82. https://doi.org/10.1097/ALN.0b013e318201d300.
Zhang S, Wu X, Guo H, Ma L. Thoracic epidural anesthesia improves outcomes in patients undergoing cardiac surgery: meta-analysis of randomized controlled trials. Eur J Med Res. 2015;20:25. https://doi.org/10.1186/s40001-015-0091-y.
Nagaraja PS, Ragavendran S, Singh NG, Asai O, Bhavya G, Manjunath N, Rajesh K. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Ann Card Anaesth. 2018;21(3):323–7. https://doi.org/10.4103/aca.ACA_16_18.
• Krishna SN, Chauhan S, Bhoi D, Kaushal B, Hasija S, Sangdup T, Bisoi AK. Bilateral erector spinae plane block for acute post-surgical pain in adult cardiac surgical patients: a randomized controlled trial. J Cardiothorac Vasc Anesth. 2019;33(2):368–75. https://doi.org/10.1053/j.jvca.2018.05.050. An RCT comparing ESPB with IV pain medicatiosn showing the beneficial effects of ESPB for cardiac surgery.
Macaire P, Ho N, Nguyen T, Nguyen B, Vu V, Quach C, et al. Ultrasound-guided continuous thoracic erector spinae plane block within an enhanced recovery program is associated with decreased opioid consumption and improved patient postoperative rehabilitation after open cardiac surgery-a patient-matched, controlled before-and-after study. J Cardiothorac Vasc Anesth. 2019;33(6):1659–67. https://doi.org/10.1053/j.jvca.2018.11.021.
Gaweda B, Borys M, Belina B, Bak J, Czuczwar M, Woloszczuk-Gebicka B, et al. Postoperative pain treatment with erector spinae plane block and pectoralis nerve blocks in patients undergoing mitral/tricuspid valve repair - a randomized controlled trial. BMC Anesthesiol. 2020;20(1):51. https://doi.org/10.1186/s12871-020-00961-8.
Kodali VRK, Shree S, Prasad M, Sambandam KKG, Karthekeyan RB, Vakamudi M. A Comparative study of bilateral erector spinae block versus intravenous dexmedetomidine for perioperative pain management in patients undergoing off-pump coronary artery bypass grafting - a single-blind randomized controlled trial. J Cardiothorac Vasc Anesth. 2022;36(11):4085–92. https://doi.org/10.1053/j.jvca.2022.07.015.
Athar M, Parveen S, Yadav M, Siddiqui OA, Nasreen F, Ali S, Haseen MA. A randomized double-blind controlled trial to assess the efficacy of ultrasound-guided erector spinae plane block in cardiac surgery. J Cardiothorac Vasc Anesth. 2021;35(12):3574–80. https://doi.org/10.1053/j.jvca.2021.03.009.
•• King M, Stambulic T, Servito M, Mizubuti GB, Payne D, El-Diasty M. Erector spinae plane block as perioperative analgesia for midline sternotomy in cardiac surgery: a systematic review and meta-analysis. J Card Surg. 2022;37(12):5220–9. https://doi.org/10.1111/jocs.17005. A systematic review and meta-analyisis of ESPB for midline sternotomy in cardiac surgery showing limited benefits.
•• Zhou K, Li D, Song G. Comparison of regional anesthetic techniques for postoperative analgesia after adult cardiac surgery: bayesian network meta-analysis. Front Cardiovasc Med. 2023;10:1078756. https://doi.org/10.3389/fcvm.2023.1078756. A network meta-analysis comparing regional anesthesia techniques for postoperative anlgesia after cardiac surgery shows the benefits of thoracic epidural analgesia.
Berthoud V, Ellouze O, Nguyen M, Konstantinou M, Aho S, Malapert G, et al. Serratus anterior plane block for minimal invasive heart surgery. BMC Anesthesiol. 2018;18(1):144. https://doi.org/10.1186/s12871-018-0614-5.
Moll V, Maffeo C, Mitchell M, Ward CT, Groff RF, Lee SC, et al. Association of serratus anterior plane block for minimally invasive direct coronary artery bypass surgery with higher opioid consumption: a retrospective observational study. J Cardiothorac Vasc Anesth. 2018;32(6):2570–7. https://doi.org/10.1053/j.jvca.2018.04.043.
Toscano A, Capuano P, Costamagna A, Burzio C, Ellena M, Scala V, et al. The serratus anterior plane study: continuous deep serratus anterior plane block for mitral valve surgery performed in right minithoracotomy. J Cardiothorac Vasc Anesth. 2020;34(11):2975–82. https://doi.org/10.1053/j.jvca.2020.05.021.
Toscano A, Capuano P, Costamagna A, Canavosio FG, Ferrero D, Alessandrini EM, et al. Is continuous erector spinae plane block (ESPB) better than continuous serratus anterior plane block (SAPB) for mitral valve surgery via mini-thoracotomy? Results from a prospective observational study. Ann Card Anaesth. 2022;25(3):286–92. https://doi.org/10.4103/aca.aca_69_21.
• El-Boghdadly K, Wolmarans M, Stengel AD, Albrecht E, Chin KJ, Elsharkawy H, et al. Standardizing nomenclature in regional anesthesia: an ASRA-ESRA Delphi consensus study of abdominal wall, paraspinal, and chest wall blocks. Reg Anesth Pain Med. 2021;46(7):571–80. https://doi.org/10.1136/rapm-2020-102451. ASRA-ESRA Delphi consensus study of standardized nomenclature in regional anesthesia.
Gray AT. Transversus thoracis muscular plane block. In: Gray AT, editor. Atlas of ultrasound-guided regional anesthesia. 3rd ed. Elsevier; 2019. p. 244–8.
Liu V, Mariano ER, Prabhakar C. Pecto-intercostal fascial block for acute poststernotomy pain: a case report. A A Pract. 2018;10(12):319–22. https://doi.org/10.1213/XAA.0000000000000697.
• Kumar AK, Chauhan S, Bhoi D, Kaushal B. Pectointercostal fascial block (PIFB) as a novel technique for postoperative pain management in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2020. https://doi.org/10.1053/j.jvca.2020.07.074. An RCT showing the benefits of PIFB blocks for postopeartive pain management in patients undergoing cardiac surgery.
Khera T, Murugappan KR, Leibowitz A, Bareli N, Shankar P, Gilleland S, et al. Ultrasound-guided pecto-intercostal fascial block for postoperative pain management in cardiac surgery: a prospective, randomized, placebo-controlled trial. J Cardiothorac Vasc Anesth. 2020. https://doi.org/10.1053/j.jvca.2020.07.058.
Zhang Y, Min J, Chen S. Continuous pecto-intercostal fascial block provides effective analgesia in patients undergoing open cardiac surgery: a randomized controlled trial. Pain Med. 2022;23(3):440–7. https://doi.org/10.1093/pm/pnab291.
Pascarella G, Costa F, Nonnis G, Strumia A, Sarubbi D, Schiavoni L, et al. Ultrasound guided parasternal block for perioperative analgesia in cardiac surgery: a prospective study. J Clin Med. 2023;12(5):2060. https://doi.org/10.3390/jcm12052060.
Dost B, Kaya C, Turunc E, Dokmeci H, Yucel SM, Karakaya D. Erector spinae plane block versus its combination with superficial parasternal intercostal plane block for postoperative pain after cardiac surgery: a prospective, randomized, double-blind study. BMC Anesthesiol. 2022;22(1):295. https://doi.org/10.1186/s12871-022-01832-0.
Wang L, Jiang L, Jiang B, Xin L, He M, Yang W, et al. Effects of pecto-intercostal fascial block combined with rectus sheath block for postoperative pain management after cardiac surgery: a randomized controlled trial. BMC Anesthesiol. 2023;23(1):90. https://doi.org/10.1186/s12871-023-02044-w.
Aydin ME, Ahiskalioglu A, Ates I, Tor IH, Borulu F, Erguney OD, et al. Efficacy of ultrasound-guided transversus thoracic muscle plane block on postoperative opioid consumption after cardiac surgery: a prospective, randomized, double-blind study. J Cardiothorac Vasc Anesth. 2020;34(11):2996–3003. https://doi.org/10.1053/j.jvca.2020.06.044.
Fujii S, Roche M, Jones PM, Vissa D, Bainbridge D, Zhou JR. Transversus thoracis muscle plane block in cardiac surgery: a pilot feasibility study. Reg Anesth Pain Med. 2019;44(5):556–60. https://doi.org/10.1136/rapm-2018-100178.
Zhang Y, Chen S, Gong H, Zhan B. Efficacy of bilateral transversus thoracis muscle plane block in pediatric patients undergoing open cardiac surgery. J Cardiothorac Vasc Anesth. 2020;34(9):2430–4. https://doi.org/10.1053/j.jvca.2020.02.005.
•• Kaya C, Dost B, Dokmeci O, Yucel SM, Karakaya D. Comparison of ultrasound-guided pecto-intercostal fascial block and transversus thoracic muscle plane block for acute poststernotomy pain management after cardiac surgery: a prospective, randomized, double-blind pilot study. J Cardiothorac Vasc Anesth. 2022;36(8 Pt A):2313–21. https://doi.org/10.1053/j.jvca.2021.09.041. An RCT comparing PIFB and TTMPB showing similar effectiveness with both superfical and deep parasternal intercostal plane (PIP) blocks
Blanco R. The ‘pecs block’: a novel technique for providing analgesia after breast surgery. Anaesthesia. 2011;66(9):847–8. https://doi.org/10.1111/j.1365-2044.2011.06838.x.
Jin Z, Li R, Gan TJ, He Y, Lin J. Pectoral nerve (PECs) block for postoperative analgesia-a systematic review and meta-analysis with trial sequential analysis. Int J Physiol Pathophysiol Pharmacol. 2020;12(1):40–50.
Versyck B, van Geffen G-J, Chin K-J. Analgesic efficacy of the Pecs II block: a systematic review and meta-analysis. Anaesthesia. 2019;74(5):663–73. https://doi.org/10.1111/anae.14607.
Kumar KN, Kalyane RN, Singh NG, Nagaraja PS, Krishna M, Babu B, et al. Efficacy of bilateral pectoralis nerve block for ultrafast tracking and postoperative pain management in cardiac surgery. Ann Card Anaesth. 2018;21(3):333–8. https://doi.org/10.4103/aca.ACA_15_18.
Vinzant NJ, Christensen JM, Yalamuri SM, Smith MM, Nuttall GA, Arghami A, et al. Pectoral fascial plane versus paravertebral blocks for minimally invasive mitral valve surgery analgesia. J Cardiothorac Vasc Anesth. 2023;37(7):1188–94. https://doi.org/10.1053/j.jvca.2023.02.012.
Torre DE, Pirri C, Contristano M, Behr AU, De Caro R, Stecco C. Ultrasound-guided PECS II + serratus plane fascial blocks are associated with reduced opioid consumption and lengths of stay for minimally invasive cardiac surgery: an observational retrospective study. Life (Basel). 2022;12(6):805. https://doi.org/10.3390/life12060805.
Alfirevic A, Marciniak D, Duncan AE, Kelava M, Yalcin EK, Hamadnalla H, et al. Serratus anterior and pectoralis plane blocks for robotically assisted mitral valve repair: a randomised clinical trial. Br J Anaesth. 2023;130(6):786–94. https://doi.org/10.1016/j.bja.2023.02.038.
Block M, Pitchon DN, Schwenk ES, Ruggiero N, Entwistle J, Goldhammer JE. Left subclavian transcatheter aortic valve replacement under combined interscalene and pectoralis nerve blocks: a case series. A A Pract. 2018;11(12):332–5. https://doi.org/10.1213/XAA.0000000000000819.
Yang JK, Char DS, Motonaga KS, Navaratnam M, Dubin AM, Trela A, et al. Pectoral nerve blocks decrease postoperative pain and opioid use after pacemaker or implantable cardioverter-defibrillator placement in children. Heart Rhythm. 2020;17(8):1346–53. https://doi.org/10.1016/j.hrthm.2020.03.009.
Elhaddad AM, Hefnawy SM, El-Aziz MA, Ebraheem MM, Mohamed AK. Pectoral nerve blocks for transvenous subpectoral pacemaker insertion in children: randomized controlled trial. Korean J Anesthesiol. 2023. https://doi.org/10.4097/kja.22681.
Arasu T, Ragavendran S, Nagaraja PS, Singh NG, Vikram MN, Basappanavar VS. Comparison of pectoral nerve (PECS1) block with combined PECS1 and transversus thoracis muscle (TTM) block in patients undergoing cardiac implantable electronic device insertion - a pilot study. Ann Card Anaesth. 2020;23(2):165–9. https://doi.org/10.4103/aca.ACA_254_18.
Author information
Authors and Affiliations
Contributions
N.N. did the literature search and wrote the main manuscript text; J.G. and R.L. reviewed the manuscript; M.G. and H.K. prepared the figures and videos; H.K. wrote the abstract and conclusion, and helped with the literature search; All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
A) This article does not contain any studies with human or animal subjects performed by any of the authors.
b) All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MOV 1417 KB)
Supplementary file2 (MOV 2.46 MB)
Supplementary file3 (MOV 2.43 MB)
Supplementary file4 (MOV 1426 KB)
Supplementary file5 (MOV 2946 KB)
Supplementary file6 (MOV 3126 KB)
Supplementary file7 (MOV 3492 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nooli, N.P., Goldhammer, J.E., Linganna, R.E. et al. Fascial Plane Blocks as Regional Analgesia Techniques for Cardiac Surgeries: a Technical Description and Evidence Update. Curr Anesthesiol Rep 14, 63–74 (2024). https://doi.org/10.1007/s40140-023-00576-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-023-00576-y