Abstract
Purpose of Review
To review the role of molecular imaging modalities in the evaluation of low back pain and identification of active pain generators.
Recent Findings
Low back pain is a common condition associated with high utilization of imaging. Identification of a pain source in patients with nonspecific low back pain is an important clinical challenge. However, there is inadequate correlation between anatomic findings on CT and MRI with symptoms of back pain, or clinical response to therapeutic procedures including injection or surgery. In contrast, molecular imaging modalities including single-photon emission-computed tomography (SPECT) or positron emission tomography (PET) with bone-targeting radiotracers like Tc-99m methylene diphosphonate (MDP) and 18F-NaF paired with CT or MRI demonstrate promise to improve test specificity in identification of pain generators in the spine. An accurate identification of pain source in patients with back pain is important in guiding therapeutic interventions including injection and surgery.
Summary
Molecular imaging modalities have demonstrated improved diagnostic accuracy in identifying active pain generators and predicting response to therapeutic intervention compared to anatomic imaging alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low back pain is an important priority for our healthcare system. Symptomatic low back pain affects nearly three in four people over a lifetime, and approximately one third of adults in the past 3 months in the United States [1, 2]. Pain can originate from numerous overlapping osseous and soft tissue structures of the spine and surrounding tissues, resulting in up to 85% of cases lacking a precise pathoanatomic diagnosis [3], termed nonspecific low back pain. More serious underlying pathologies causing low back pain are identified by “red flag” features (weight loss, fevers, night pain, etc.) and are much less common than nonspecific low back pain [4], with a 4% incidence of compression fracture, 0.7% incidence of neoplasm, and a 0.01% incidence of infection [3]. Given the prevalence of nonspecific low back pain and the low rate of acute pathology, routine imaging of low back pain without “red flag” features is not recommended [3, 5]. However, approximately one quarter of patients presenting to primary care clinics and one half of patients presenting to the emergency department with a chief complaint of low back pain receive imaging studies [6]. Therapeutic interventions for patients with low back pain have increased steadily over the past decades. Percutaneous injections for patients with persistent symptoms and spondylotic changes on imaging are becoming increasingly common; facet joint interventions in Medicare patients increased 386% between 2000 and 2011 and remain more common than in prior decades, despite a slight decrease in recent years [7]. Surgery for low back pain has more than doubled during the same period with an increase in the invasiveness of surgical interventions [8]. Imaging to prove accuracy of identifying a pain generator for nonspecific low back pain would be valuable in guiding an evidence-based approach to non-operative and operative interventions.
Lumbar spine radiographs offer moderate sensitivity and varying specificity for neoplastic (sensitivity 60%, specificity 95–99.5%) and infectious (sensitivity 82%, specificity 57%) etiologies of back pain [9], but findings of degenerative changes on radiographs have, at best, a weak correlation with the clinical symptoms of low back pain [10, 11]. Cross-sectional anatomic imaging modalities have improved sensitivity and specificity for spondylotic changes associated with back pain [9, 12,13,14,15]. However, the high prevalence of these changes in asymptomatic patients [16,17,18,19] highlights the “specificity challenge.” Advanced imaging is most useful when it identifies a specific pathology that leads to a precise and focused approach to care. Many studies have demonstrated only a limited association between findings on CT and/or MRI and the presence/severity of low back pain [18,19,20,21,22,23∙]. Furthermore, there is no clear correlation between spondylotic changes on CT/MRI (specifically, facet arthropathy and spinal canal stenosis) and a clinical response to subsequent percutaneous steroid injections [24,25,26]. Similarly, surgery for low back pain has been unreliable, and imaging has had limited utility in guiding surgery for nonspecific low back pain [27, 28].
Molecular imaging with bone-targeted agents such as Tc-99m methylene diphosphonate (MDP) and 18F-sodium fluoride (NaF) can identify areas of active bone remodeling through selectively increased radiotracer uptake in osteoblastic activity and synovial hyperemia [29, 30]. When used in conjunction with anatomic imaging, molecular imaging with single-photon emission-computed tomography (SPECT) or positron emission tomography (PET) shows promise to more accurately identify active pain generators [31,32,33]. Several retrospective and prospective studies evaluating the ability of SPECT and SPECT/CT to identify pain generators and predict response to treatment have demonstrated promising results. However, there still remains some controversy regarding the specificity of molecular imaging to identify a source of back pain [29, 33,34,35,36,37,38,39]. Additional molecular imaging modalities such as PET/CT and PET/MRI using NaF and fluorodeoxyglucose (FDG) radiotracers have been reported in the literature, and the preliminary studies have shown similarly favorable results [40,41,42,43]. In this article, we present a review of the current literature regarding the imaging of back pain to guide management, with a focus on molecular imaging (Fig. 1).
Workup and Management of Low Back Pain
Current Anatomic Imaging Modalities
Precise evaluation of sensitivity and specificity for disk and facet pathology on lumbar spine-imaging studies is somewhat limited given the lack of a universally accepted gold standard test for identifying the source of lower back pain [20]. Within this limitation, the diagnostic accuracies of commonly used imaging modalities are discussed below.
Radiography is mainly useful for identifying severe underlying osseous pathology. Plain films of the lumbar spine have demonstrated 60% sensitivity and 95–99% specificity for malignancy, 82% sensitivity, and 57% specificity for infection, and 26–45% sensitivity and 100% specificity of ankylosing spondylitis [9]. Plain films are also useful and accurate for identification of spinal deformity including scoliosis, spondylolisthesis, and rotatory subluxation. For nonspecific low back pain, there are no clinically validated sensitivity and specificity values of lumbar spine radiographs reported in the literature. Furthermore, up to 75% of lumbar spine radiographs yield no information regarding a precise cause of low back pain [44]. Two systematic reviews have shown an association between low back pain and degenerative changes on radiographs characterized by disk space narrowing [10, 11], endplate sclerosis, and osteophytosis, with the odds ratios for these associations ranging from 1.2 to 3.3 [11]. However, several of the included studies were identified as having potential significant biases leading to an overestimation of association [11]. No association has been demonstrated between low back pain and spondylolisthesis, spondylolysis, transitional vertebrae, Scheuermann’s disease [11], and facet disease [10] on radiographs.
Computed tomography of the lumbar spine has improved sensitivity and specificity compared to plain radiography. CT has been reported to have 86.7% sensitivity and 78.6% specificity for facet disease [15]; 62–90% sensitivity and 70–87% specificity for herniated intervertebral disk [9]; and 90% sensitivity and 80–96% specificity for spinal canal stenosis [9]. Despite the relatively high sensitivity and specificity for these degenerative processes, a cross-sectional study by Kalichman et al. demonstrated no statistically significant association between these findings on CT and self-reported low back pain. The only feature strongly associated with low back pain was spinal stenosis, and this relationship did not reach statistical significance (OR 2.87 [95% CI 0.93–8.87]) [45].
Magnetic resonance imaging of the lumbar spine is typically considered to be the gold standard for anatomic imaging of the spine. With superior soft tissue resolution compared to CT, MRI is able to distinguish different structures within the intervertebral disk, visualize ligaments, assess bone marrow composition, and evaluate the contents of the spinal canal [9]. MRI has a reported 87–93% sensitivity and 64.3–87% specificity for facet disease [13, 15]. However, one study demonstrated a decreased sensitivity of 40–53% when attempting to precisely localize the painful facet [13], suggesting a poor or incomplete correlation between facet arthropathy and active pain generators. For evaluation of herniated disk, the MRI literature varies widely, reporting a range of 60–100% sensitivity and 43–97% specificity [9]. However, a systematic review of 8 studies demonstrated 75% sensitivity and 77% specificity of MRI for herniated disk on a pooled analysis. The diagnostic accuracy for associated nerve root compression was evaluated in two of the included studies which demonstrated high sensitivity of 81 and 92% and variable specificity of 52 and 100% [14]. MRI has 77–96% sensitivity and 68–100% specificity for lumbar spinal stenosis [12, 14].
While CT and MRI have good sensitivity and specificity for symptomatic nerve compression or facet arthropathy, the utility of CT and MRI in identifying a pain generator in nonspecific low back pain is limited [4]. Many spondylotic changes, so called “degenerative findings,” seen on lumbar spine CT and MRI are also highly prevalent in asymptomatic patients, indicating that they may represent normal age-related changes [17, 45]. For example, Boden et al. demonstrated that within a cohort of asymptomatic patients older than 60, 36% had a herniated disk, 21% had spinal stenosis, and more than 90% had disk degeneration on MRI [16]. Similar prevalence was demonstrated on CT in a nonselected group of patients older than 60; 87.5% had facet arthropathy, 16.3% had spinal stenosis, 35.4% had degenerative spondylolisthesis, and 83.3% had disk space narrowing [45]. Given the high frequency of degenerative lumbar spine findings in asymptomatic individuals, using a specific anatomic finding to definitively diagnose the cause of low back pain is of limited value [18, 19].
A meta-analysis evaluating the ability of MRI findings to identify the facet joints, intervertebral disk, and/or sacroiliac joint as the cause of lower back pain determined that no MRI features could significantly predict facet or sacroiliac sources of pain. Furthermore, the absence of disk degeneration could only moderately rule out a discogenic pain source [20]. Additional studies comparing MRI findings with Oswestry Disability Index demonstrated only a weak correlation of symptoms with disk degeneration and specifically right L5–S1 facet disease, and concluded that imaging alone is insufficient to explaining sources of low back pain [21, 22].
Multiple longitudinal studies following asymptomatic patients demonstrated limited associations between the development of new MRI findings and incident axial low back pain [18, 19, 23∙], with one study finding a stronger association with depression than with new imaging findings [18]. New annular fissures were the only MRI finding that demonstrated a statistically significant association with incident axial low back pain in one study (OR 6.0 [95% CI 1.1–33.1]). However, symptoms only developed in half of the cases [19] and this relationship was not seen in other studies [18, 23∙]. Additionally, in Javrik, et al., the only statistically significant association with MRI findings was the presence of a disk protrusion as a negative predictor for future back pain (HR 0.5 [95% CI 0.3–0.9)]. Disk extrusion, nerve root contact, and central canal stenosis were associated with incident low back pain (HRs ranging from 1.2 to 2.2) but were not statistically significant [18]. While no strong correlation between MRI findings and low back pain was demonstrated in these longitudinal studies, they were all limited by small sample size and low incidence of new MRI findings. Conversely, a meta-analysis by Brinjikji et al. demonstrated that in adults 50 years of age and younger, certain MRI findings were more prevalent in symptomatic compared to asymptomatic individuals—disk bulge (OR 7.54; 95% CI 1.28–44.56; P = 0.03), spondylolysis (OR 5.06; 95% CI 1.65–15.53; P < 0.01), disk extrusion (OR 4.38; 95% CI 1.98–9.68; P < 0.01), Modic 1 changes (OR 4.01; 95% CI 1.10–14.55; P = 0.04), disk protrusion (OR 2.65; 95% CI 1.52–4.62; P < 0.01), and disk degeneration (OR 2.24; 95% CI 1.21–4.15; P = 0.01). Of note, several of these findings (disk bulge, disk protrusion, and disk degeneration) demonstrated I2 values > 62% suggesting substantial heterogeneity in reported results [46]. This suggests that some anatomic findings may have an association with back pain in a younger adult population, perhaps prior to development of more ubiquitous degenerative changes in later decades of life. The review of the literature highlights the limitation of anatomic imaging in addressing the “specificity challenge” of identifying a specific pain generator on a background of asymptomatic or noncontributory spondylotic changes, especially in the older adult population.
Several studies have shown no significant correlation between the pathoanatomic findings of facet arthropathy on MRI and CT with the clinical response to targeted facet joint infiltration [24,25,26] or rhizotomy [47]. Despite the poor ability of pathoanatomic findings on CT and MRI to predict if a particular facet is a pain generator, recent systematic reviews have shown fair evidence for therapeutic infiltrations [48]. Likewise, facet joint denervation with radiofrequency ablation (RFA) has demonstrated a pain relief success rate of 55–68% at 1 year [49]. Of note, use of posttreatment pain relief as a diagnostic gold standard is limited due to a high false-positive rate of a single injection, up to 38% for intra-articular injections [50] and 45% for single medial branch blocks [51]. The false-positive rate was determined by comparing the response rate of an initial lidocaine injection to a subsequent confirmatory bupivacaine injection in the same location. In both studies, a significant number of patients did not demonstrate reproducible symptomatic improvement on confirmatory injection—26 of the 83 patients for intra-articular injection [50] and 83 of the 150 patients for medial branch block [51]. This high rate may be secondary to leakage of injectate into periarticular structures [24] and anesthesia of the muscles, ligaments, and periosteum also innervated by the medial branch nerve [25∙], respectively.
Diphosphonate SPECT/CT
Disphosphonate SPECT/CT can be performed for molecular imaging of back pain. Methyldiphosphonate (MDP) and hydroxydiphosphonate (HDP) are phosphate analogues which complex with the hydroxyapatite mineral phase of bone, a process known as chemisorption. These compounds can be labeled with Tc-99m for single-photon emission-computed tomography (SPECT) imaging to identify areas of active bone remodeling, characterized by increased osteoblastic activity and synovial hyperemia [29, 30]. This environment is seen in facet joints undergoing osteoarthritic changes [29]. The degree of uptake can differentiate an actively growing osteophyte from stable, mature osseous growth which could account for differences in response to therapy, with sites of active remodeling responding better than mature changes [39]. SPECT can be fused with CT which, in theory, combines the high sensitivity of SPECT with the anatomic specificity of CT, allowing for superior spatial resolution and more accurate localization of the diseased structure [31,32,33]. A retrospective study by Matar et al. found that SPECT/CT could identify potential pain generators in 92% of cervical spine scans and 86% of lumbar spine scans with precise localization of target facet joints in 65% of patients [32]. When used in conjunction with cross-sectional imaging, SPECT provides additional specificity for facet joint arthropathy by differentiating the frequent incidental, asymptomatic findings from the significant ones [31]. Additionally, SPECT/CT has been shown to have high diagnostic accuracy in the evaluation of sacroiliac joint dysfunction with reported sensitivity 95% of and specificity of 99% [52] as well as for the identification of hardware loosening in postoperative patients with recurrent back pain (sensitivity 100%, specificity 89.7%) [53].
In the literature, the diagnostic accuracy of SPECT and SPECT/CT is limited and differs if intra-articular facet injection or medial branch block is used as the comparative gold standard. Compared with responses to facet injections, the sensitivity and specificity of SPECT for facet disease are 79–100% and 70–71%, respectively [34, 35, 54]. Compared to medial branch block, the sensitivity and specificity of SPECT are 96–100% and 45–50%, respectively [37]. In contrast, the one study determining the accuracy of SPECT/CT compared with medial branch block demonstrated at sensitivity of 57% and a specificity of 77% [36].
There have been multiple studies showing favorable results regarding the use of SPECT and SPECT/CT to identify pain generators that are likely to respond to injection. An early prospective study in 1996 by Dolan et al. compared the outcome of 58 patients who underwent SPECT and then received facet injections directed by SPECT findings if the scan was positive and clinical exam if the scan was negative. The positive SPECT group demonstrated significant improvement in VAS (visual analog scale) and McGill pain scores compared to the SPECT negative group; of those with positive scans, 95% reported symptomatic improvement at 1 month compared to 47% in the negative scan group [35]. In a randomized control trial by Pneumaticos et al. in 2006, patients with back pain received facet joint injections either guided by SPECT findings or clinical exam findings (n = 47). For the SPECT group, 87% of patients treated at the site of increased tracer uptake had significant symptom relief compared with 13% of those with a negative SPECT and 31% of the control group. Additionally, the number of therapeutic injections was decreased from 60 to 27 in patients with positive scans [39]. A prospective study by McDonald et al. in 2007 performed SPECT/CT on 37 patients with clinically diagnosed facetogenic back pain who then underwent facet joint injection and/or rhizotomy targeted to specific facet joints identified on SPECT-CT. 36 of the 37 patients experienced symptomatic improvement with a mean improvement of 4.4 ± 1.6 VAS (visual analog scale) points [33]. A similar prospective study by Koh et al. in 2011 obtained SPECT on 33 patients with suspected facetogenic chronic low back pain and performed a medial branch block based on imaging (for SPECT-positive patients) or clinical exam (for SPECT negative patients). 85.7% of the SPECT-positive patients were responders at 2 weeks versus 20% of the SPECT negative patients; at 2 weeks, the VAS score of the SPECT-positive group was 3.13 ± 1.54 and 5 ± 2.28 for the SPECT negative group, decreased from 6.8 ± 1.8 and 6.33 ± 1.87, respectively [37]. An additional randomized control trial by Jain in 2015 (n = 80) compared pain relief after nerve block between patients receiving a pre-procedural SPECT/CT and a control group using only clinical features and radiologic findings to arrive at a clinical diagnosis. The number of patients reporting significant pain relief was higher in the SPECT/CT group with 80% obtaining > 50% improvement and 70% obtaining 70–100% improvement. In contrast, the control group demonstrated 52.5% obtaining > 50% improvement and 25% obtaining 70% improvement [55]. A retrospective study by Lee et al. in 2014 reviewed 175 patients who had undergone SPECT/CT for chronic low back pain. Of the patients that underwent target specific treatment, an 82.6% response rate was seen for those with definite focal uptake on SPECT versus a 54.8% response rate for the patients with no or mild uptake [29]. A more recent case series in 2019 by Kato et al. reviewed 5 patients with low back pain initially diagnosed with adult spinal deformity based on clinical exam and anatomic imaging. All 5 patients had planned extensive spinal fusion, however pre-operative SPECT/CT demonstrated more localized degenerative pain generators and even isolated endplate and transverse process fractures. Based on these findings, 3 patients underwent minor segmental fusion and 2 patients were treated conservatively; all patients achieved significant reduction in pain based on an 11-point pain scale, from 8.4 ± 0.5 to 2.2 ± 1.3 [56∙∙].
Conversely, a study by Lehman et al. in 2014 demonstrated a discrepancy between SPECT/CT findings and treatment. In this retrospective study of 74 patients, only 47% of the treated facets were positive on SPECT/CT, and only 70% of facets with increased uptake were selected for treatment, suggesting that findings do not always translate into changes of clinical practice [38]. Additionally, Freirmuth et al. performed a randomized control trial in which 29 patients underwent medial branch blocks based on clinical exam followed by the unblinding of the proceduralist to the SPECT/CT with additional blocks performed at untreated levels if the initial infiltrations were negative. In contrast to the previously discussed studies, there was only a modestly increased response rate, from 17 to 24%, with SPECT/CT. The sensitivity and specificity were found to be 0.57 and 0.77 [36].
Of note, a study by Ackerman et al. demonstrated that there was a significantly greater improvement in pain and disability scores with intra-articular injections compared with medial branch blocks in patients with SPECT-positive facets [34]. This difference may account for some of the variability seen in the above studies.
There have been multiple cases at our institution where findings on MDP SPECT/CT have been used to guide clinical management, including surgical interventions. Several such cases are presented which highlight the use of molecular imaging in identifying facet arthropathy (Fig. 2), degenerative disk disease (Fig. 3), sacroiliitis (Fig. 4), adjacent segment disease (Fig. 5), and pseudoarthrosis (Fig. 6). The impact of molecular imaging on clinical outcomes remains to be demonstrated.
Facet arthropathy. A 54-year-old woman with chronic back pain had an MRI lumbar spine (a) at an outside facility which revealed multilevel degenerative disk disease (white arrowhead) and facet arthropathy (white arrow) without a definite level of most severe disease. Based on the nonspecific MRI findings and clinical evaluation, she underwent an epidural spinal injection which provided no improvement in symptoms. After referral to our spine center, a SPECT/CT (b) was performed which demonstrated focal increased uptake in the left L4–L5 and L5–S1 facet joints (yellow arrow), suggesting a facetogenic source of pain. Subsequent bilateral facet injections at these levels provided symptomatic relief (Color figure online)
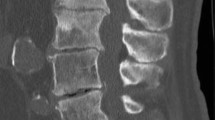
Degenerative disk disease. A 84-year-old woman with chronic neck pain after a remote injury while white-water rafting underwent MRI cervical spine (a) which showed severe multilevel degenerative disk disease worst at C4–C5 and C6–C7 as well as facet arthropathy worst on the right C3–C4 and C7–T1 (white arrowhead). Given the degree of facet arthropathy and clinical presentation, the patient was referred for facet joint injection. Over the course of three visits, the patient had numerous CT-guided facet injections (b, c) of the bilateral C2–C3, right C3–C4 and C4–C5, bilateral C5–C6, C6–C7, and C7–T1 facet joints (white arrows at right C3–C4 and right C7–T1) with suboptimal symptomatic relief. After the third procedure, an SPECT/CT was obtained (d) which revealed intense focal uptake centered in the C4–C5 disk space (yellow arrowhead), consistent with a discogenic rather than facetogenic pain generator. These findings prompted a CT-guided epidural steroid injection (e) at C6–C7 (yellow arrow) which resulted in marked improvement of pain (Color figure online)
Sacroiliitis. A 74-year-old man status post complex spine reconstruction resulting in fusion from C5–S1 fusion and a right sacroiliac bolt presented with new low back pain radiating into the left hip. Radiography demonstrates extensive fusion of the cervical spine through pelvis without evidence of hardware complication or pseudoarthrosis (a). On subsequent SPECT/CT (b), there is isolated increased uptake in the left sacroiliac joint (white arrows) without associated morphologic changes, likely secondary to altered biomechanics following fusion. In the context of the SPECT/CT findings, the patient was taken back to the OR for revision of the spinal hardware with new fusion across the left sacroiliac joint (c). Patient reported improvement in pain following revision
Adjacent segment disease. A 67-year-old woman presenting with low back pain and right leg pain with a history of L4–L5 total disk replacement for recurrent disk herniations > 15 years prior. An initial CT (a) identified the L4–L5 disk replacement (white arrows), bilateral facet arthropathy at L4–L5 (yellow arrows), and degenerative disk disease at L2–L3 and L3–L4 (black arrows). Given the clinical findings, her back pain was attributed to the facet arthropathy. However, a SPECT/CT obtained prior to intervention (b) demonstrated markedly increased uptake within the L3–L4 disk space (white arrowhead), most consistent with adjacent segment disease. The facet joints and L4–L5 disk space did not have significant uptake to suggest facetogenic pain generators or hardware complication. The patient subsequently underwent posterior spinal fusion spanning L2–L5 with significant symptomatic improvement (Color figure online)
Pseudoarthrosis. A 62-year-old man with 7 years of low back pain for which he had a microdiscectomy and ALIF at L5–S1 which provided only partial symptom relief. An outside CT (a) revealed facet arthropathy of the bilateral L5–S1 facet joints (white arrows) and no evidence of hardware failure. His pain was felt to be facetogenic in nature and bilateral L5–S1 facet injections were performed with transient resolution of pain. A subsequent SPECT/CT was obtained, (b) which demonstrated intense uptake at the level of the L5–S1 fusion (yellow arrows), compatible with pseudoarthrosis. Based on these findings, the patient underwent L5–S1 posterior spinal fusion and decompression with excellent postoperative pain relief (Color figure online)
18F-FDG and 18F-NaF PET/CT and PET/MRI
Like SPECT/CT, PET can be combined with CT or MRI to provide superimposed metabolic and anatomic information. Due to the higher intrinsic spatial resolution of PET compared to planar and SPECT imaging, PET/CT or PET/MRI may identify pathologies more accurately with shorter imaging times [57]. Several smaller studies have investigated the use of 18F-NaF and 18F-fluorodeoxyglucose (18F-FDG) radiotracers in the evaluation of back pain.
NaF localizes to the hydroxyapatite matrix of bone in areas of osseous remodeling via hydroxyl ion exchange, similar to MDP, though with significantly less binding to serum proteins, allowing for earlier image acquisition [41, 57]. Several early studies have shown promising results of NaF PET/CT and PET/MRI in identifying potential spinal pain, with a sensitivity as high as 88% [43]. A retrospective study by Mabray et al. demonstrated a weak positive correlation between degree of NaF uptake and Pathria CT morphologic grade of facet arthropathy. There was a wide range of SUVs within each Pathria grade, and some Pathria grade 0 (normal) facets demonstrated uptake while some grade 4 (advanced disease) facets demonstrated minimal to no uptake; these findings suggest a discrepancy between molecular and anatomic imaging with possible metabolic changes preceding morphologic changes [41]. Similarly, a prospective study evaluating low back pain patients with NaF PET/MRI showed a strong correlation between a kinetic measure of dynamic NaF uptake (Ki_Patlak) and Oswestry disability index scores, but only a weak correlation with MRI arthropathy grade [42∙]. An additional case series performed by Pouldar et al. focused on NaF PET/CT performed in patients with persistent back pain following surgery. In 18 of the 25 cases, the results of the PET/CT changed the diagnosis and/or management, including 6 cases of pseudoarthrosis and 5 cases of hardware impingement or loosening that were not seen on CT or MRI [58]. While these studies show encouraging results, they are significantly limited by small sample sizes and the lack of confirmatory anesthetic joint injections or nerve blocks.
In contrast, FDG localizes to areas of increased glucose utilization, and its use in bones has traditionally been to identify metastatic lesions and infection. However, the FDG accumulation can also be seen within foci of inflammation such as active degenerative changes [59]. A retrospective study on 150 patients undergoing FDG PET/CT for evaluation of any known or suspected malignancy demonstrated a weak but statistically significant correlation between the degree of degenerative disk and facet changes and FDG avidity, with substantial variability in the intensity of FDG uptake in areas of severe degenerative change [59]. Like the similar studies done with NaF PET, this may represent the degree of active bone remodeling at the time of imaging superimposed on the more chronic morphological changes that develop over years. Sawicki et al. performed a prospective trial in which 10 patients underwent FDG PET/MRI of the cervical spine followed by facet injection targeted to focal FDG uptake, if present, versus clinical localization and level of maximum morphological arthrosis. The 6 PET-positive patients had significantly reduced posttreatment pain levels (7.5 ± 1.0 pretreatment to 2.3 ± 1.0 at 3 h), while the 4 PET-negative patients demonstrated no significant pain reduction (6.75 ± 2.1 pretreatment to 6.0 ± 1.8 at 3 h) [40]. This study’s outcomes mirror the findings seen in several of the prospective trials on MDP SPECT/CT-targeted injections, although they are limited by the small number of patients.
Conclusion
Molecular imaging, including SPECT/CT, PET/CT, and PET/MRI, have demonstrated improved diagnostic accuracy in identifying active pain generators compared to anatomic imaging alone. MDP SPECT/CT has been the most studied molecular imaging modality with a majority of the literature supporting its improved sensitivity and specificity. PET/CT and PET/MRI using NaF, and to a lesser extent FDG, demonstrate promising preliminary data; however, additional large prospective randomized trials are needed to confirm these results. If supported, these PET modalities have even greater utilization potential given the higher intrinsic spatial resolution and shorter imaging times compared to SPECT.
References
Papers of particular interest, published recently have been highlighted as: ∙ Of importance ∙∙ Of major importance
Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2021;85:343–50.
Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates. Spine. 2006;31:2724–7.
Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363–70.
Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017;389:736–47.
Koes BW, Van Tulder M, Lin CWC, Macedo LG, McAuley J, Maher C. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19:2075–94.
Tan A, Zhou J, Kuo YF, Goodwin JS. Variation among primary care physicians in the use of imaging for older patients with acute low back pain. J Gen Intern Med. 2016;31:156–63.
Manchikanti L, Falco F, Singh V, et al. Utilization of interventional techniques in managing chronic pain in the Medicare population: analysis of growth patterns from 2000 to 2011. Pain Physician. 2012;15:E969.
Martin B, Mirza S, Spina N, Spiker W, Lawrence B, Brodke D. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. 2019;44:369–76.
Jarvik JGDR. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586–97.
Raastad J, Reiman M, Coeytaux R, Ledbetter L, Goode AP. The association between lumbar spine radiographic features and low back pain: a systematic review and meta-analysis. Semin Arthritis Rheum. 2015;44:571–85.
Van Tulder MW, Assendelft WJJ, Koes BW, Bouter LM. Spinal radiographic findings and nonspecific low back pain: a systematic review of observational studies. Spine. 1997;22:427–34.
Roudsari B, Jarvik JG. Lumbar spine MRI for low back pain: indications and yield. Am J Roentgenol. 2010;195:550–9.
Freund W. Magnetic resonance imaging can detect symptomatic patients with facet joint pain. A retrospective analysis. J Clin Med Exp Images. 2017;1:027–36.
Wassenaar M, Van Rijn RM, Van Tulder MW, et al. Magnetic resonance imaging for diagnosing lumbar spinal pathology in adult patients with low back pain or sciatica: a diagnostic systematic review. Eur Spine J. 2012;21:220–7.
Zhou X, Liu Y, Zhou S, et al. The correlation between radiographic and pathologic grading of lumbar facet joint degeneration. BMC Med Imaging. 2016;16:27–27.
Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg. 1990;72:403–8.
Chou R, Deyo RA, Jarvik JG. Appropriate use of lumbar imaging for evaluation of low back pain. Radiol Clin North Am. 2012;50:569–85.
Jarvik JG, Hollingworth W, Heagerty PJ, Haynor DR, Boyko EJ, Deyo RA. Three-year incidence of low back pain in an initially asymptomatic cohort: clinical and imaging risk factors. Spine. 2005;30:1541–8.
Suri P, Boyko EJ, Goldberg J, Forsberg CW, Jarvik JG. Longitudinal associations between incident lumbar spine MRI findings and chronic low back pain or radicular symptoms: Retrospective analysis of data from the longitudinal assessment of imaging and disability of the back (LAIDBACK). BMC Musculoskelet Disord. 2014;15:152–152.
Hancock MJ, Maher CG, Latimer J, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J. 2007;16:1539–50.
Maataoui A. Association between facet joint osteoarthritis and the Oswestry Disability Index. World J Radiol. 2014;6:881–881.
Middendorp M, Vogl TJ, Kollias K, Kafchitsas K, Khan MF, Maataoui A. Association between intervertebral disc degeneration and the Oswestry Disability Index. J Back Musculoskelet Rehabil. 2017;30:819–23.
∙Tonosu J, Oka H, Higashikawa A, Okazaki H, Tanaka S, Matsudaira K. The associations between magnetic resonance imaging findings and low back pain: a 10-year longitudinal analysis. PLoS ONE. 2017;12:e0188057–e0188057. This reference updates the longitudinal analysis highlights the limited ability of MRI findings to predict low back pain
Gorbach C, Schmid MR, Elfering A, Hodler J, Boos N. Therapeutic efficacy of facet joint blocks. Am J Roentgenol. 2006;186:1228–33.
∙Hofmann UK, Keller RL, Walter C, Mittag F. Predictability of the effects of facet joint infiltration in the degenerate lumbar spine when assessing MRI scans. J Orthop Surg Res. 2017. https://doi.org/10.1186/s13018-017-0685-x. This reference demonstrates the limited correlation between MRI findings and response to facet joint infiltration
Schwarzer A, Wang S, O’Driscoll D, Harrington JT, Bogduk N, Laurent R. The ability of computed tomography to identify a painful zygapophysial joint in patients with chronic low back pain. Spine. 1995;20:907–12.
Yavin D, Casha S, Wiebe S, et al. Lumbar fusion for degenerative disease: a systematic review and meta-analysis. Neurosurgery. 2017;80:701–15.
Fairbank J, Frost H, Wilson-MacDonald J, Yu L, Barker K, Collins R. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. BMJ (Clinical research ed). 2005;330:1233.
Lee I, Budiawan H, Moon JY, et al. The value of SPECT/CT in localizing pain site and prediction of treatment response in patients with chronic low back pain. J Korean Med Sci. 2014;29:1711–6.
Perolat R, Kastler A, Nicot B, et al. Facet joint syndrome: from diagnosis to interventional management. Insights Imaging. 2018;9:773–89.
Harisankar CNB, Mittal BR, Bhattacharya A, Singh P, Sen R. Utility of single photon emission computed tomography/computed tomography imaging in evaluation of chronic low back pain. Indian J Nucl Med. 2012;27:156–63.
Matar HE, Navalkissoor S, Berovic M, et al. Is hybrid imaging (SPECT/CT) a useful adjunct in the management of suspected facet joints arthropathy? Int Orthop. 2013;37:865–70.
McDonald M, Cooper R, Wang MY. Use of computed tomography-single-photon emission computed tomography fusion for diagnosing painful facet arthropathy. Tech Neurosurg Focus. 2007;22:E2–E2.
Ackerman WE, Ahmad M. Pain relief with intraarticular or medial branch nerve blocks in patients with positive lumbar facet joint SPECT imaging: a 12-week outcome study. South Med J. 2008;101:931–4.
Dolan AL, Ryan PJ, Arden NK, et al. The value of SPECT scans in identifying back pain likely to benefit from facet joint injection. Rheumatology. 1996;35:1269–73.
Freiermuth D, Kretzschmar M, Bilecen D, et al. Correlation of <sup>99m</sup> Tc-DPD SPECT/CT scan findings and diagnostic blockades of lumbar medial branches in patients with unspecific low back pain in a randomized-controlled trial. Pain Med. 2015;16:1916–22.
Koh WU, Kim SH, Hwang BY, et al. Value of bone scintigraphy and single photon emission computed tomography (SPECT) in lumbar facet disease and prediction of short-term outcome of ultrasound guided medial branch block with bone SPECT. Korean J Pain. 2011;24:81–6.
Lehman VT, Murphy RC, Kaufmann TJ, et al. Frequency of discordance between facet joint activity on technetium Tc99m methylene diphosphonate SPECT/CT and selection for percutaneous treatment at a large multispecialty institution. Am J Neuroradiol. 2014;35:609–14.
Pneumaticos SG, Chatziioannou SN, Hipp JA, Moore WH, Esses SI. Low pain: Prediction of short-term outcome of facet joint injection with bone scintigraphy. Radiology. 2006;238:693–8.
Sawicki L, Schaarschmidt B, Heusch P, et al. Value of 18 F-FDG PET/MRI for the outcome of CT-guided facet block therapy in cervical facet syndrome: initial results. J Med Imaging Radiat Oncol. 2017;61:327–33.
Mabray M, Brus-Ramer M, Behr S, et al. (18)F-sodium fluoride PET-CT hybrid imaging of the lumbar facet joints: tracer uptake and degree of correlation to CT-graded arthropathy. World J Nucl Med. 2016;15:85–90.
∙Jenkins N, Talbott J, Shah V, et al. [18 F]-sodium fluoride PET MR-based localization and quantification of bone turnover as a biomarker for facet joint-induced disability. AJNR Am J Neuroradiol. 2017;38:2028–31. This reference identifies a strong correlation between facet NaF uptake and Oswestry disability score
Gamie S, El-Maghraby T. The role of PET/CT in evaluation of facet and disc abnormalities in patients with low back pain using (18)F-Fluoride. Nucl Med Rev Central Eastern Europe. 2008;11:17–21.
Scavone J, Latshaw R, Rohrer G. Use of lumbar spine films. Statistical evaluation at a university teaching hospital. JAMA. 1981;246:1105–8.
Kalichman L, Kim D, Li L, Guermazi A, Hunter D. Computed tomography-evaluated features of spinal degeneration: prevalence, intercorrelation, and association with self-reported low back pain. Spine J. 2010;10:200–8.
Brinjikji W, Diehn F, Jarvik J, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36:2394–9.
Cohen SP, Hurley RW, Christo PJ, Winkley J, Mohiuddin MM, Stojanovic MP. Clinical predictors of success and failure for lumbar facet radiofrequency denervation. Clin J Pain. 2007;23:45–52.
Falco FJE, Manchikanti L, Datta S, et al. An update of the systematic assessment of the diagnostic accuracy of lumbar facet joint nerve blocks. Pain Physician. 2012;15(6):E869.
Filippiadis DK, Kelekis A. A review of percutaneous techniques for low back pain and neuralgia: current trends in epidural infiltrations, intervertebral disk and facet joint therapies. Br Inst Radiol. 2016;89:20150357.
Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophysial joints. Pain. 1994;58:195–200.
Manchukonda R, Manchikanti KN, Cash KA, Pampati V, Manchikanti L. Facet joint pain in chronic spinal pain: an evaluation of prevalence and false-positive rate of diagnostic blocks. J Spinal Disord Tech. 2007;20:539–45.
Cusi M, Saunders J, Van der Wall H, Fogelman I. Metabolic disturbances identified by SPECT-CT in patients with a clinical diagnosis of sacroiliac joint incompetence. Eur Spine J. 2013;22:1674–82.
Hudyana H, Maes A, Vandenberghe T, et al. Accuracy of bone SPECT/CT for identifying hardware loosening in patients who underwent lumbar fusion with pedicle screws. Eur J Nucl Med Mol Imaging. 2016;43:349–54.
Holder LE, Machin JL, Asdourian PL, Links JM, Sexton CC. Planar and high-resolution SPECT bone imaging in the diagnosis of facet syndrome. J Nucl Med. 1995;36:37–44.
Jain A, Jain S, Agarwal A, Gambhir S, Shamshery C, Agarwal A. Evaluation of efficacy of bone scan with SPECT/CT in the management of low back pain: a study supported by differential diagnostic local anesthetic blocks. Clin J Pain. 2015;31:1054–9.
∙∙Kato S, Demura S, Matsubara H, et al. Utility of bone SPECT/CT to identify the primary cause of pain in elderly patients with degenerative lumbar spine disease. J Orthop Surg Res. 2019;14:1–6. This reference updates the randomized control trial demonstrates significantly higher rates of pain relief in the study group undergoing treatment targeted by SPECT/CT compared to the control group
Grant F, Fahey F, Packard A, Davis R, Alavi A, Treves S. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78.
Pouldar D, Bakshian S, Matthews R, Rao V, Manzano M, Dardashti S. Utility of 18F sodium fluoride PET/CT imaging in the evaluation of postoperative pain following surgical spine fusion. Musculoskelet Surg. 2017;101:159–66.
Rosen R, Fayad L, Wahl R. Increased 18F-FDG uptake in degenerative disease of the spine: characterization with 18F-FDG PET/CT. J Nucl Med. 2006;47:1274–80.
Acknowledgements
We thank Dr. Michael Schecht for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Other than as listed below, the authors have no relevant conflicts of interest or competing interests. Sigurd Berven receives royalties from Elsevier Publications and Stryker Spine; consulting fees from Medtronic spine, Stryker spine, Innovasis, Camber spine, and Globus Medical; support for meetings/travel from AO Spine; and serves in the following roles: SRS Board of Directors; NASS Program Chair. William Dillon holds a planned/issued/pending patent, “Position Guidance Device with Bubble Level” for spine interventions.
Research Involving Human and Animal Participants
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sweetwood, K., Junn, J.C., Qiao, Y. et al. Beyond Anatomy: The Role of Molecular Imaging in the Evaluation of Low Back Pain. Curr Radiol Rep 11, 142–152 (2023). https://doi.org/10.1007/s40134-023-00418-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40134-023-00418-z