Abstract
Diabetic macular edema (DMO) is a leading cause of blindness in the working age population. Although anti-vascular endothelial growth factor (VEGF) therapy provided a major advance in treatment of DMO for many patients, there is a significant proportion of patients who maintain persistent DMO and have minimal response to anti-VEGF treatment. Iluvien (fluocinolone acetonide 0.19 mg [FAc]) is an important additional treatment option for DMO. In this review we describe the clinical context and the evidence for the use of the FAc implant in treating DMO, from pilot to randomized controlled studies, to later phase real world data. These studies indicate that the FAc implant is effective, well tolerated and a cost-effective option in the treatment of insufficiently responsive DMO.
Similar content being viewed by others
Introduction
It is estimated that diabetes affects 422 million adults worldwide [1] and 3.7 million people in the UK (2016–2017 data) [2]. Diabetic macular edema (DMO) is the leading cause of blindness in people of working age in developed countries, and an estimated 12% and 27% of patients with type 1 and type 2 diabetes mellitus, respectively, have DMO [3]. DMO occurs due to impairment in the blood retinal barrier and increased leakage from blood vessels due to the loss of supportive cells, cell dysfunction, and inflammatory changes [4]. One key regulator in this process is vascular endothelial growth factor (VEGF), which has been successfully targeted with anti-VEGF monoclonal antibodies such as ranibizumab, aflibercept and bevacizumab. Corticosteroids provide an alternative therapeutic strategy by blocking leukotriene and prostaglandin synthesis via glucocorticoid receptors and subsequently acting on the arachidonic acid pathway. In addition, corticosteroids inhibit and interfere with other pro-inflammatory molecules, such as VEGF-alpha, interleukin-6 and intercellular adhesion molecule-1, and increase vasoconstriction by nitric oxide inhibition [4,5,6,7,8].
The tradition clinical treatment of DMO was largely dependent on macular laser (either focal or ‘grid’ distribution) treatment, supplemented with unlicensed short-acting steroid injections. Over the last decade care has been transformed by a shift to intravitreal anti-VEGF injections [9], with or without macular laser treatment, as the primary treatment for DMO. There are however a significant proportion of patients who have insufficiently responsive DMO despite anti-VEGF treatment, for whom other treatment options are required.
This article does not contain any studies with human participants or animals performed by any of the authors.
Brief Overview of the Iluvien™ Fluocinolone Acetonide Implant
The Iluvien™ fluocinolone acetonide (FAc) 0.19 mg intravitreal implant (Alimera Sciences Ltd., Alpharetta, Georgia, USA) is a slow-release preparation containing fluocinolone acetonide 0.19 mg (C24H30F2O6) [8]. The implant is injected intravitreally and maintains a slow release of 0.2 μg per day for up to 36 months. The FAMOUS study demonstrated sustained release, as assessed by the concentrations of FAc in the aqueous humor, with levels of slightly more than 2 ng/ml for approximately the first 3 months followed by a maintained concentration of 0.5–1.0 ng/ml through a period of 36 months [10].
Overview of the FAME Trials
Efficacy
The FAc 0.19 mg implant was approved for treatment of DMO after it was shown to be effective for the treatment of DMO in the Fluocinolone Acetonide in diabetic Macular Edema trials (FAME A and B studies) [11, 12], which were two parallel multicenter, randomized, sham-injection-controlled trials evaluating the safety and efficacy of the FAc implant in DMO.
Participants with persistent DMO and at least one previous macular laser treatment were randomly assigned to receive high-dose FAc (0.5 µg/day) (n = 393), low-dose FAc (0.2 µg/day) (n = 375), or sham injection (n = 185; sham group) in the ratio 2:2:1. All participants were eligible for rescue laser after 6 weeks from baseline injection. The primary efficacy endpoint was percentage of patients with best corrected visual acuity (BCVA) ≥ 15 letter (L) gain from baseline at 24 months using the early treatment diabetic retinopathy study (ETDRS) chart. Secondary outcomes were visual function and foveal thickness. An additional study drug or sham could be given after 1 year if study retreatment criteria were met. During the study clinicians were able to give ‘off protocol’ therapies, such as anti-VEGF, at their own discretion, and these patients were not excluded from the trial or analysis.
In both FAc implant groups, there was a significantly higher percentage of participants with ≥ 15L gain compared to the control at month 24: 28.7% in the low-dose treatment arm, 28.6% in the high-dose treatment arm and 16.2% in the sham treatment arm (p = 0.002); at 36 months the percentage was 28.7% (low dose), 27.8% (high dose) and 18.9% (sham) (p = 0.018). When only those left in the trial at month 36 were considered in the analysis, the percentage increased to 33.0, 31.9 and 21.4%, respectively (p = 0.030). Benefit was seen in both dosages at week 3 and at all subsequent time points. Mean letter gain at month 24 was 4.4L in the low-dose group, 5.4 L in the high-dose group and 1.7L in the sham group (p = 0.02 and p = 0.016 vs. sham). This benefit was maintained at 3 years, with a mean letter gain at month 36 of 5.3L in both FAc groups and 2.0 in the sham (p ≤ 0.018).
In a pre-planned subgroup analysis looking at patients with chronic DMO, the percentage with a ≥ 15L gain at month 36 compared to sham was doubled: 34.0% in the low-dose group (p < 0.001), 28.8% in the high-dose group (p = 0.002) and 13.4% in the sham group, suggesting more significant improvement in the more chronic cases [13].
Adverse Effects
The main ocular adverse effects revealed by the FAME trials were the development of visually significant cataract and rise in intraocular pressure (IOP) or glaucoma. The development of cataract and the percentage of participants requiring cataract surgery were significantly higher in both the low- and high-dose treatment arms than in the control (sham) group. In the low-dose group 81.7% patients developed cataracts by month 36, with 80.0% undergoing cataract surgery; in the high-dose group, 88.7% developed cataracts, with 87.2% undergoing surgery; in the sham group, 50.7% developed cataracts, with 27.3% undergoing surgery. While there was a higher proportion requiring cataract surgery in the FAc groups than in the sham group, visual improvement post cataract surgery was similar to that of the baseline pseudophakic patients, and BCVA at 36 months was similar in those phakic and pseudophakic patients at baseline [14].
A rise in IOP is a major concern to clinicians who are considering the use of intraocular corticosteroids and, therefore, the IOP profile of the FAc implant revealed in the FAME trials was of particular interest. Overall IOP-related adverse events were more common in the FAc groups than in the sham group. At 36 months the proportion of patients who had had elevated IOP requiring at least 7 days of topical IOP-lowering drops was 38.4% for the low-dose treatment group and 47.3% for the high-dose treatment group, compared to 14.1% in the sham group. An IOP of ≥ 30 mmHg developed in 16.3% of participants in the treatment groups by month 24 and in 18.4% by month 36. The development of elevated IOP that required incisional surgery at month 24 and 36 was 1.6 and 4.8% in the low-dose group, respectively, 6.4 and 8.1% in the high-dose group, respectively, and 0.5% in the sham group. It should be noted that these rates for incisional surgery at 24 months are based on the trial data on file; they are lower than the rates published by Campochiaro et al. as their analysis included some cases with a follow-up between 24 and 36 months due to the trial analysis not being truncated at month 24 [11, 12].
Patients who had been treated with corticosteroid previously were eligible to be included in the study provided that they did not have a prior history of corticosteroid-induced ocular hypertension (sometimes termed ‘steroid responders’). A further post hoc analysis of patients who had previously received corticosteroid treatment and were thus known to be ‘non-responders’ (n = 72) found that none of this group of patients required incisional surgery to lower IOP, whereas 6.1% of those who had not received prior corticosteroid, required IOP-lowering surgery (n = 18) [15]. Further assessment of changes in the optic nerve head using the fundus photographs used in the FAME trials concluded that treatment with FAc was not associated with significant glaucomatous changes with or without elevated IOP [16]. Endophthalmitis occurred in 0.2% (2 eyes) with the FAc implant compared to none in the sham group.
Overall the trials showed that both the low-dose and high-dose FAc implants produced a significant improvement in BCVA over 3 years. The benefit-to-risk ratio showed the low-dose implant to be superior to the high-dose implant, and the authors of the trials concluded that the former would provide a substantial visual benefit and provide a valuable addition to treatment options available in managing patients with DMO. Following these pivotal studies, the FAc 0.19 mg implant (0.2 µg per day) was licensed for use in the USA and Europe [17,18,19,20]. Treatment is the UK is guided by the National Institute for Health and Care Excellence (NICE) (UK) Technology Appraisal (TA301) which restricts its use to the treatment of insufficiently responsive DMO (defined as inadequate response to conventional therapy [laser and/or anti-VEGF], either no reduction in central retinal thickness or minimal reduction from treatment and a persistence in macular edema) only where the eye is pseudophakic and where the manufacturer provides the FAc implant at the discount agreed in the patient access scheme [17].
Real World Data and Long-Term Safety Outcomes
To date there have been numerous papers looking at the real world efficacy and safety profile of the FAc implant at years 1 and 2 and data is emerging for 3 years. Since the FAME trials the face of DMO management has changed, with anti-VEGF becoming the predominant first-line treatment in most areas; consequently most published studies have looked at clinical efficacy, side effect profile and use of the FAc implant when the patient is insufficiently responsive to the current gold standard treatment. An overview of these publications is given in Table 1.
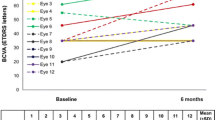
Bertelmann et al. reported a case of bilateral FAc implant in a phakic patient with refractory DMO of over 20 years duration (left eye at baseline, right eye at month 6), that resulted in an improvement in retinal thickness and BCVA, with a reduction in central retinal thickness (CRT) in the left eye from 642 to 372 µm at month 13, and from 473 to 334 µm in the right eye at month 6 [21]. Early outcomes were also described by Elaraoud et al. in 22 eyes across three sites in the UK [22]. These showed a mean reduction in CRT of 148.9 µm at 3 months and mean visual gain of 6.4L. Data at 6 and 12 months were also reported in another paper by Elaraoud et al. in patients with bilateral implants and DMO, with a mean increase in BCVA of 10L at 6 months and of 10.5L at 12 months, as well as sustained CRT reduction at months 6 and 12 [23, 24].
The results of a prospective phase IV study in France by Massin et al. [25] which enrolled patients with chronic DMO further supported the safety and efficacy data on the FAc implant for up to 12 months of follow-up. Patients included in the study were given the FAc implant at baseline, and clinical outcomes were monitored for over 1 year. Study participants were separated into two groups: chronic DMO insufficiently responsive to laser (group 1) with or without anti-VEGF (group 2). The patients were excluded from the study if they had one or more of the following: IOP of > 21 mmHg at screening; history of steroid response; need for > 2 IOP-lowering drops at screening; pre-existing glaucoma. Of the 16 patients, six patients (7 eyes) were in group 1 and ten (10 eyes) were in group 2. Twelve eyes were pseudophakic. Eyes of patients in group 1 had a significantly longer duration of DMO than those of patients in group 2 (7.6 vs 3.6 years, respectively). A reduction in CRT was seen at week 1, and the mean CRT reduction was 239 and 147 µm at month 1 for groups 1 and 2, respectively. This reduction was maintained at month 6 (281 [group 1] and 167 µm [group 2]), month 9 (295 [group 1] and 172 µm [group 2]) and month 12 (299 [group 1] and 251 µm [group 2]). There was a mean gain in BCVA at all time points, with a gain of 2.0L and 6.4L in groups 1 and 2, respectively, at month 1, and gain at month 12 of 5.6L and 0.9L, respectively. Four patients required top-up therapy. The IOP increased in three eyes, and the increase was controlled with IOP-lowering drops alone. Better CRT reduction and BCVA gain at month 12 were seen in the patients in group 1, which had the more chronic DMO, thereby supporting the FAME data.
The results of a study by Alfaqawi et al. supported the 12-month data of Massin et al. [25]. These authors reported outcomes from a UK cohort, looking at 28 eyes treated with the FAc implant at a single center [26]. There was an overall mean gain in BCVA at month 12 of 8L, with 25% (n = 7) of patients gaining at least 15L and 36% (n = 10) gaining at least 10L. The mean CRT decreased by 198 µm at 12 months. The authors noted that two eyes required additional anti-VEGF after 10 months and that three eyes developed IOP that was managed with drops alone. One eye developed vitreous hemorrhage and one eye developed endophthalmitis.
The RESPOND trial in Portugal, an open label, phase IV, multicenter, non-randomized trial involving 12 patients (4 phakic, 8 pseudophakic) showed rapid improvement in CRT and gain in BCVA after 1 week which were sustained at all time points up to 12 months. Mean visual gain was 3.7L across all patients, with greater improvement in the pseudophakic eyes (increase of 6.8L) compared to phakic eye (decrease of 2.5L) [27]. Two patients developed a rise in IOP, which was managed with drops alone. A limiting factor of this study is the lack of BCVA analysis post cataract surgery which does not allow full assessment of the efficacy of the FAc implant on vision. In a subsequent UK study, El-Ghrably et al. showed a mean reduction in CRT of 126 µm and BCVA gain of 5.1L at month 12 in patients [28]. Eyes that developed an increased IOP were managed with topical IOP-lowering drops alone.
The main limitation of all these early outcome studies is limited numbers; however clinical efficacy and adverse effects appear to be comparable to those of the FAME trials.
More recently, the Iluvien Clinical Evidence Study (ICE-UK) study evaluated UK visual outcomes and IOP in eyes treated with the FAc implant between April 2013 and April 2015 at 13 ophthalmology centers in the UK [29]. The study evaluated 12 months pre- and post-FAc implant results for 208 patients (233 eyes), of which 89% (n = 207 eyes) were pseudophakic at time of implant. Overall, by month 12, the improvement in visual acuity (VA) was at least 5L in 44% of patients, at least 10L in 30% of patients and at least 15L in 18% of patients. A significant improvement in VA was seen at all time points post implant in those with a baseline VA of > 55L; however, a non-significant improvement was seen in those with a baseline VA of < 55L.
In the 12 months prior to implant, 191 (82%) eyes had had at least 1 anti-VEGF injection, 101 eyes (43%) had received steroid treatment and 146 eyes (63%) had at least one macular laser treatment. By month 12, adjunctive treatments had been given to 69 eyes (30%). Five eyes required cataract surgery within the first 3 months. IOP-lowering drops were required in 15% of patients with no prior history; none required surgical management.
Further analysis of fellow eyes in the ICE-UK study showed that a letter gain of at least 15L was achieved by more often by treated eyes than by fellow eyes (18 vs. 4%) and that the mean reduction in CRT was 113 µm in treated eyes compared to − 13 µm in fellow eyes [30]. Comparison of retinal thickness in these patients revealed that eyes with a CRT of at least 400 µm or greater were significantly more likely to develop at least a 10, 25 and 50% reduction in CRT compared to those with CRT of < 400 µm [31].
Although this study had large numbers, there were numerous limitations, including the retrospective nature of the study, incomplete data sets and inconsistent documentation in medical records. These were acknowledged by the authors who made attempts to minimize their effect.
Longer Term Real World Data
Extended clinical benefit from the FAc implant from 12 to 24 months was first reported by Quhill et al. in a pseudophakic patient treated with a FAc implant for DMO unresponsive to laser and anti-VEGF treatments [32]. These authors noted an initial rapid reduction in CRT of 507 µm at 1 week and gain in BCVA of 15L which was maintained up to 24 months. No top-up treatment was required and there was no recurrence of fluid.
In their large cohort study, Bailey et al. looked at visual and safety outcomes for up to 24 months in 345 eyes treated with the FAc implant at 14 sites across the UK using data extraction from a single electronic medical records system [9]. Of the patients, 91% had received prior therapy for DMO, with 28.4% having had prior macular laser therapy and 84.6% having had prior anti-VEGF treatment. The mean gain in BCVA was 4.5L at 18 months (n = 120) and 5.3L at 24 months (n = 53). A minimum gain of 15.0L had been achieved by 15% of eyes at 12 months, by 15% of eyes at 18 months and by 20.8% of eyes at month 24. Mean CRT at baseline and last observation was 451.2 and 355.5 µm, respectively. The percentage of eyes requiring treatment post FAc implant was reduced, with the number of eyes requiring anti-VEGF treatment, laser treatment and steroid treatment reduced by 51.3, 2.1 and 2.5%, respectively. Regarding the IOP, 13.9% of eyes required IOP-lowering drops, 7.2% had an IOP of > 30 mmHg, and 0.3% required surgery to manage IOP and had a prior history of IOP event. Prior to the FAc implant, 14.2% of eyes were receiving IOP-lowering medication at baseline. In eyes previously treated with steroid and no IOP problems, there were no new IOP events. Despite large numbers, data extraction from electronic medical record systems relies on complete and accurate data entry and due to the retrospective nature of analysis, missing data could not be obtained, which is a limitation of the study.
Fusi-Rubiano et al. looked at real world 2-year outcomes in a UK pseudophakic cohort and found an improvement in BCVA and reduction in CRT with the FAc implant that was sustained at 1 and 2 years [33]. Additional 3-year data in this publication was broadly similar to the longer term results seen in the FAME trials, with 50% (n = 3) improving by 15L. Supplementary treatment was needed in most cases; however, the overall burden of treatment had been reduced. Only two eyes required IOP-lowering drops in this cohort, and none required incisional surgery. Although limited by small numbers, especially for the 36-month outcomes, this study does show some of the first results for a 3-year follow-up.
Vitrectomized Eyes
Since the advent of intravitreal agents there has been discussion over their use in vitrectomized eyes, particularly in terms of pharmacokinetics and drug clearance. Results from some studies using animal models have suggested that anti-VEGF intravitreal agents have a shorter half-life and are cleared more quickly in vitrectomized eyes [34]. In contrast, analysis of 3-year data in a DRCR.net trial of ranibizumab and prompt or deferred laser treatment found that there was no significant difference in long-term outcome at annual time points in vitrectomized eyes when compared to non-vitrectomized eyes [35]. Although the results of this study are not directly comparable to those of two studies looking at sustained-release dexamethasone [36, 37], it may be worth noting that these studies found no statistically significant difference in the vitreoretinal pharmacokinetics between vitrectomized and non-vitrectomized eyes [36] and showed good clinical efficacy in vitrectomized eyes [37].
In one of the first publications looking solely at the treatment of vitrectomized eyes with the FAc implant, Kumar et al. described two cases of vitrectomized eyes previously treated with anti-VEGF and triamcinolone that showed complete resolution of DMO up to 1 year post implant [38]. Meireles et al. looked at the efficacy and safety of the FAc implant in 26 eyes with prior vitrectomy from six European countries [39]. Mean time to insertion was 24.2 months post vitrectomy, and mean follow-up time was 255 days. Mean gain in BCVA was 11.7L, and there was a mean reduction in CRT of 233.5 µm. There was a mean rise of IOP from baseline of 1.4 mmHg, and eight eyes required IOP-lowering drops or had been on them prior to FAc implant. The authors concluded that the FAc implant was safe and effective in vitrectomized eyes.
In a further retrospective analysis of 43 eyes, comparing vitrectomized (n = 24) and non-vitrectomized (n = 19) eyes treated with FAc implant for DMO, Pessoa et al. found a gain of at least 15L in 37.5% of vitrectomized eyes (group 1) and in 36.8% of non-vitrectomized eyes (group 2) [40]. In addition, there was a mean reduction in CRT from baseline of 217.7 and 155.6 µm in groups 1 and 2, respectively; however there was no statistical difference between the two groups. There was no significant difference for IOP and the need for IOP-lowering medications between the groups. Although these results appear to show no significant difference between the two groups, the mean follow-up was 8.1 months, and thus further long-term follow-ups and studies are needed.
All of these studies are limited by small numbers and their retrospective nature. There is limited evidence for real world outcomes in vitrectomized eyes, and further studies are needed in these patients.
Despite good outcomes, care should be taken in vitrectomized eyes. The migration of the implant into the anterior chamber in patients who had had prior vitrectomy was seen in two eyes in the series by El-Ghrably et al., where posterior capsular defect had been identified previously [28]. There were no long-term adverse effects for these eyes. Moisseiev et al. also reported a case of ‘floater’ in a patient with previous vitrectomy where the implant had centralized in the visual axis with vitreous attachment [41]. It should be noted that YAG vitreolysis was required to break the adhesion, but this treatment resolved the patient’s symptoms. In another case, Andreatta et al. reported a dislodgment of FAc implant into the infusion cannula during vitrectomy [42].
How Does the FAc 0.19 mg Implant (Iluvien™) Fit into Current Treatment Regimens, Patient Management and Guidelines
Regulatory Approvals
In 17 European countries the FAc 0.19 mg implant has been approved for the treatment of patients with DMO insufficiently responsive to available therapies [17,18,19,20]. In some countries, such as the UK, Italy and Spain, reimbursement is restricted for use only in eyes with an intraocular (pseudophakic) lens [17, 19, 20]. In the USA, the implant is approved for patients with DMO who have had prior treatment with corticosteroid and found not to have a clinically significant rise in IOP [18]. It is contraindicated in patients with confirmed or suspected ocular or periocular infections, patients with glaucoma with a CDR ≥ 0.8 and patients with a known hypersensitivity to any component of the implant [18].
DMO is multifactorial disease and as such there should be a variety of treatment options available to tackle it. Anti-VEGF is now the gold standard in the treatment of DMO; however, not all patients respond or are able to maintain monthly anti-VEGF treatments. In the study of Gonzalez et al. [43], almost 40% of patients treated with anti-VEGF had a minimal response (less than 5L gain) in BCVA after 3 months, and this was associated with worse long-term BCVA. The FAc implant as a corticosteroid is an effective treatment option for the management of DMO and, as suggested by the European Society of Retinal Specialists (EURETINA), should be considered for patients lacking a response to current first-line treatments [44]. These guidelines also advise that retreatment with FAc implant can be considered after 1 year. Although the FAc 0.19 mg implant IS particularly appropriate for pseudophakic patients, phakic patients are being treated but should be advised of the high risk of cataract formation.
Will It Be Cost Effective and Beneficial for My Patients?
Cost-effect analyses have shown the FAc implant to be a cost-effective treatment in insufficiently responsive DMO in both phakic and pseudophakic patients [45, 46] because of its long-acting efficacy, safety profile and tolerability. The requirement for adjunctive treatment post implant within 3 years is lower than that for average anti-VEGF treatment regimes [9, 33]. A recent comparative study of anti-VEGF treatments in DMO reported that a median of nine injections of aflibercept and ten injections of ranibizumab/bevacizumab were required to control DMO over a 12-month period [47].
In a UK study, Quhill and Beiderbeck undertook a cost analysis of the FAc implant looking at the overall cost of treatment with the FAc implant and one additional ranibizumab treatment per year, compared to ranibizumab as needed, using an average of 14 lucentis treatments over 3 years [46] (based on the RESTORE extension study [48]). These authors calculated a cost saving of around £6068 in pseudophakic eyes using the FAc implant and £5341 in phakic eyes (taking into account the added cost of cataract surgery). Additionally, reducing the need for treatment is an important benefit for patients, as the number of injections has been shown to affect quality of life, with a higher frequency causing an increase in work leave and anxiety [49]. In addition, vital treatment time and workload are reduced in already overstretched ophthalmology departments.
Conclusion
The FAc 0.19 mg implant (Iluvien™) is a licensed treatment for use in DMO and has been shown to be effective, to have good tolerability and to be a cost-effective option for insufficiently responsive DMO, with a long-acting duration up to 36 months in both vitrectomized and non-vitrectomized eyes. It should be considered as a second-line option for treatment of DMO in patients whose eyes do not sufficiently respond to first-line treatment (unless otherwise contraindicated). It is also a valuable alternative in cases where anti-VEGF treatment is not a viable option. Clinicians may consider evaluating ‘IOP steroid responsiveness’ prior to treatment with FAc implant to reduce the risk of IOP-related events in patients. Ongoing studies will help to further define the patients with DMO most likely to benefit from the FAc implant, but this implant already has a valuable place in our treatments for this sight-threatening disease.
Change history
07 February 2020
A Correction to this paper has been published: https://doi.org/10.1007/s40123-020-00231-3
References
World Health Organization (WHO). Global Report on Diabetes. 2016. http://www.who.int/diabetes/global-report/en/ Accessed 28 May 2018.
Diabetes UK. Diabetes Prevalence 2017. https://www.diabetes.org.uk/professionals/position-statements-reports/statistics/diabetes-prevalence-2017 Accessed 3 May 2018.
Romero-Aroca P. Managing diabetic macular edema: the leading cause of diabetes blindness. World J Diabetes. 2011;2(6):98–104.
Amoaku WM, Saker S, Stewart EA. A review of therapies for diabetic macular oedema and rationale for combination therapy. Eye (Lond). 2015;29(9):1115.
Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341(2–3):309–15.
Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103.
Tamura H, Miyamoto K, Kiryu J, Miyahara S, Katsuta H, Hirose F, Musashi K, Yoshimura N. Intravitreal injection of corti-costeroid attenuates leukostasis and vascular leakage in experimental diabetic retina. Invest Ophthalmol Vis Sci. 2005;46(4):1440–4.
PubChem [database on the Internet]. Compound summary for CID 6215. Bethesda: National Center for Biotechnology Information, US Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/fluocinolone_acetonide#section=Top. Accessed May 23, 2018.
Bailey C, Chakravarthy U, Lotery A, Menon G, Talks J, Medisoft AG. Real-world experience with 0.2 μg/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye (Lond). 2017;31(12):1707–15. https://doi.org/10.1038/eye.2017.125.
Campochiaro PA, Nguyen QD, Hafiz G, Bloom S, Brown DM, Busquets M, Ciulla T, Feiner L, Sabates N, Billman K, Kapik B. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013;120(3):583–7.
Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, Tolentino M, Gupta A, Duarte L, Madreperla S, Gonder J. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626–35.
Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, Garretson B, Gupta A, Hariprasad SM, Bailey C, Reichel E. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–32.
Cunha-Vaz J, Ashton P, Iezzi R, Campochiaro P, Dugel PU, Holz FG, Weber M, Danis RP, Kuppermann BD, Bailey C, Billman K. Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology. 2014;121(10):1892–903.
Yang Y, Bailey C, Holz FG, Eter N, Weber M, Baker C, Kiss S, Menchini U, Moreno JR, Dugel P, Lotery A. Long-term outcomes of phakic patients with diabetic macular oedema treated with intravitreal fluocinolone acetonide (FAc) implants. Eye (Lond). 2015;29(9):1173–80.
Parrish RK, Campochiaro PA, Pearson PA, Green K, Traverso CE, FAME Study Group. Characterization of intraocular pressure increases and management strategies following treatment with fluocinolone acetonide intravitreal implants in the FAME trials. Ophthalmic Surg Lasers Imaging. Retina. 2016;47(5):426–35.
Parrish RK, Traverso CE, Green K, Dabis RP, FAME Study Group. Quantitative assessment of optic nerve changes in patients with diabetic macular edema treated with fluocinolone acetonide vitreous implants. Ophthalmic Surg Lasers Imaging Retina. 2016;47(5):418–25.
National Institute for Health and Care Excellence (NICE) guidelines [TA301]; 2013 [online]. https://www.nice.org.uk/guidance/TA301/chapter/1-Guidance. Accessed 29 May 2018.
Alimera Sciences, Inc. ILUVIEN® (fluocinolone acetonide intravitreal implant) 0.19 mg for diabetic macular edema (DME)—product overview. https://alimerasciences.com/products/iluvien-for-diabetic-macular-edema-dme/. Accessed online 30 May 2018.
Agenzia Italiana del Farmaco. Riclassificazione del medicinale per uso umano « Iluvien » , ai sensi dell’articolo 8, comma 10, della legge 24 dicembre 1993, n. 537 (Determina n. 33/2017) (17A00755); 2017 [online]. www.gazzettaufficiale.it/eli/id/2017/02/01/17A00755/sg;jsessionid=rIdm7JHMmJi5EZJXuRAUHw__.ntc-as4-guri2a. Accessed online 17 July 2018.
Alimera Sciences, Inc. Alimera announces the reimbursement of iluvien in Spain [online]. https://www.prnewswire.com/news-releases/alimera-sciences-announces-the-reimbursement-of-iluvien-in-spain-300656891.html. Accessed online 17 July 2018.
Bertelmann T, Schulze S. Long-term follow-up of patient with diabetic macular edema receiving fluocinolone acetonide intravitreal implant. Ophthalmol Therapy. 2015;4(1):51–8.
Elaraoud I, Andreatta W, Kidess A, Bhatnagar A, Tsaloumas M, Quhill F, Yang Y. Use of flucinolone acetonide for patients with diabetic macular oedema: patient selection criteria and early outcomes in real world setting. BMC Ophthalmol. 2016;16(1):3.
Elaraoud I, Attawan A, Quhill F. Case series investigating the efficacy and safety of bilateral fluocinolone acetonide (ILUVIEN®) in patients with diabetic macular edema. Ophthalmol Therapy. 2016;5(1):95–104.
Elaraoud I, Quhill H, Quhill F. Case series investigating the efficacy and safety of bilateral fluocinolone acetonide (Iluvien®) in patients with diabetic macular edema: 10 eyes with 12 months follow-up. Ophthalmol Therapy. 2016;5(1):105–9.
Massin P, Erginay A, Dupas B, Couturier A, Tadayoni R. Efficacy and safety of sustained-delivery fluocinolone acetonide intravitreal implant in patients with chronic diabetic macular edema insufficiently responsive to available therapies: a real-life study. Clin Ophthalmol (Auckland, NZ). 2016;10:1257.
Alfaqawi F, Lip PL, Elsherbiny S, Chavan R, Mitra A, Mushtaq B. Report of 12-months efficacy and safety of intravitreal fluocinolone acetonide implant for the treatment of chronic diabetic macular oedema: a real-world result in the United Kingdom. Eye (Lond). 2017;31(4):650.
Figueira J, Henriques J, Amaro M, Rosas V, Alves D, Cunha-Vaz J. A nonrandomized, open-label, multicenter, phase 4 pilot study on the effect and safety of ILUVIEN® in chronic diabetic macular edema patients considered insufficiently responsive to available therapies (RESPOND). Ophthalmic Res. 2017;57(3):166–72.
El-Ghrably I, Steel DH, Habib M, Vaideanu-Collins D, Manvikar S, Hillier RJ. Diabetic macular edema outcomes in eyes treated with fluocinolone acetonide 0.2 µg/d intravitreal implant: real-world UK experience. Eur J Ophthalmol. 2017;27(3):357.
Holden SE, Currie CJ, Owens DR. Evaluation of the clinical effectiveness in routine practice of fluocinolone acetonide 190 µg intravitreal implant in people with diabetic macular edema. Curr Med Res Opin. 2017;33[Suppl 2]:5–17.
Currie CJ, Holden SE, Berni E, Owens DR. Evaluation of the clinical effectiveness of fluocinolone acetonide 190 µg intravitreal implant in diabetic macular edema: a comparison between study and fellow eyes. Curr Med Res Opin. 2017;33[Suppl 2]:19–31.
Currie CJ, Holden SE, Owens DR. Patterns of retinal thickness prior to and following treatment with fluocinolone acetonide 190 µg intravitreal implant for diabetic macular edema. Curr Med Res Opin. 2017;33[Suppl 2]:33–43.
Quhill H, Quhill F. Real-life ILUVIEN (Fluocinolone Acetonide) case study: rapid drying of the macula and improved vision within 2 years after therapy Initiation. Case Rep Ophthalmol. 2016;7(3):579–85.
Fusi-Rubiano W, Mukherjee C, Lane M, Tsaloumas MD, Glover N, Kidess A, Denniston AK, Palmer HE, Manna A, Morjaria R. Treating diabetic macular oedema (DMO): real world UK clinical outcomes for the 0.19 mg fluocinolone acetonide intravitreal implant (Iluvien™) at 2 years. BMC Ophthalmol. 2018;18(1):62.
Kakinoki M, Sawada O, Sawada T, Saishin Y, Kawamura H, Ohji M. Effect of vitrectomy on aqueous VEGF concentration and pharmacokinetics of bevacizumab in macaque monkeys. Invest Ophthalmol Vis Sci. 2012;53(9):5877–80.
Bressler SB, Melia M, Glassman AR, Almukhtar T, Jampol LM, Shami M, Berger BB, Bressler NM, Diabetic Retinopathy Clinical Research Network. Ranibizumab plus prompt or deferred laser for diabetic macular edema in eyes with vitrectomy prior to anti-vascular endothelial growth factor therapy. Retina. 2015;35(12):2516–28.
Chang-Lin JE, Burke JA, Peng Q, Lin T, Orilla WC, Ghosn CR, Zhang KM, Kuppermann BD, Robinson MR, Whitcup SM, Welty DF. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci. 2011;52(7):4605–9.
Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li XY, Liu CC, Lou J, Whitcup SM. Ozurdex CHAMPLAIN Study Group. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31(5):915–23.
Kumar A, Alfahad Q, Mitra A, Elsherbiny S, Lip PL. Intravitreal fluocinolone acetonide (Iluvien) for treatment of refractory diabetic macular oedema in vitrectomised eyes. Eye (Lond). 2016;30(5):763.
Meireles A, Goldsmith C, El-Ghrably I, Erginay A, Habib M, Pessoa B, Coelho J, Patel T, Tadayoni R, Massin P, Atorf J. Efficacy of 0.2 μg/day fluocinolone acetonide implant (ILUVIEN) in eyes with diabetic macular edema and prior vitrectomy. Eye (Lond). 2017;31(5):684–90.
Pessoa B, Coelho J, Correia N, Ferreira N, Beirão M, Meireles A. Fluocinolone acetonide intravitreal implant 190 μg (ILUVIEN®) in vitrectomized versus nonvitrectomized eyes for the treatment of chronic diabetic macular edema. Ophthal Res. 2018;59(2):68–75.
Moisseiev E, Morse LS. Fluocinolone acetonide intravitreal implant in the visual axis. JAMA Ophthalmol. 2016;134(9):1067–8.
Andreatta W, Elaraoud I, Mitra A. Dislogement of fluocinolone acetonide intravitreal implant into the infusion cannula during vitrectomy for retinal detachment. Retinal Cases Brief Rep. 2017. https://doi.org/10.1097/ICB.0000000000000678
Gonzalez VH, Campbell J, Holekamp NM, Kiss S, Loewenstein A, Augustin AJ, Ma J, Ho AC, Patel V, Whitcup SM, Dugel PU. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J Ophthalmol. 2016;1(172):72–9.
Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, Jonas J, Larsen M, Tadayoni R, Loewenstein A. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222.
Ch’ng SW, Brent AJ, Empeslidis T, Konidaris V, Banerjee S. Real-world cost savings demonstrated by switching patients with refractory diabetic macular edema to intravitreal fluocinolone acetonide (Iluvien): a retrospective cost analysis study. Ophthalmol Therapy. 2017;10:1–8.
Quhill F, Beiderbeck A. Cost advantage of fluocinolone acetonide implant (ILUVIEN®) versus ranibizumab in the treatment of chronic diabetic macular oedema. Glob Reg Health Technol Assess. 2017; 3(2). https://doi.org/10.5301/grhta.5000268
Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203.
Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, Gerstner O, Bouazza AS, Shen H, Osborne A, Mitchell P. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014;121(5):1045–53.
Sivaprasad S, Oyetunde S. Impact of injection therapy on retinal patients with diabetic macular edema or retinal vein occlusion. Clin Ophthalmol. 2016;10:939–46
Acknowledgements
During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from the comments received were made by the author based on their scientific and editorial merit.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
William Fusi-Rubiano has received sponsorship from Alimera to attend Euretina 2017. Rupal Morjaria has received sponsorship from Alimera to attend Euretina 2017. Mark Lane and Rebecca R. Blow have no disclosures to declare. Alastair K. Denniston receives a proportion of his funding from the Department of Health’s NIHR Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and UCL Institute of Ophthalmology, and from the Wellcome Trust, through a Health Improvement Challenge grant (200141/Z/15/Z). The views expressed in the publication are those of the author and not necessarily those of the Department of Health.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7022222.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Fusi-Rubiano, W., Blow, R.R., Lane, M. et al. Iluvien™ (Fluocinolone Acetonide 0.19 mg Intravitreal Implant) in the Treatment of Diabetic Macular Edema: A Review. Ophthalmol Ther 7, 293–305 (2018). https://doi.org/10.1007/s40123-018-0145-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-018-0145-7