Abstract
To increase understanding of the epidemiology, risks, consequences and resource utilization of Clostridium difficile infection (CDI) in Japan, a systematic literature review was undertaken of relevant publications from January 2006 to November 2017. Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and methods, 55 articles met the criteria for full review. The majority (58%) of studies were from a single site, with the most recent data from 2015. The incidence, reported prevalence and recurrence rate of CDI in Japan were 0.8–4.71/10,000 patient-days, 0.3–5.5/1000 patients and 3.3–27.3%, respectively, and varied according to setting, population, CDI definition and detection method. Most C. difficile isolates associated with CDI in Japan were toxin A+B+, with a low level of C. difficile binary toxin-positive (CDT+) strains (0–6.8% reported across studies). The most common C. difficile PCR ribotypes associated with infection in Japan were smz/018, 002, 052 and 369. Data regarding the impact of CDI on length of hospital stay were limited. Reported all-cause mortality in patients with CDI ranged from 3.4 to 15.1% between 2007 and 2013. Two studies assessed risk factors for CDI recurrence, identifying malignant disease, intensive care unit hospitalization and use of proton pump inhibitors as factors increasing the risk of initial and/or recurrent CDI. No study analyzed initial CDI treatment in relation to recurrence. More comprehensive surveillance and coordinated studies are needed to map trends, understand risk factors, and recognize the extent and impact of CDI in Japanese patients.
Funding
Astellas Pharma, Inc.
Plain Language Summary
Plain language summary available for this article.
Similar content being viewed by others
Plain Language Summary
Clostridium difficile (C. difficile) is a bacterium that often lives without causing harm in people’s gut. However, when a person has antibiotic treatment for another infection, this can cause an imbalance in the normal levels of bacteria in the gut, and the C. difficile can grow and replace many of the normal bacteria, causing C. difficile infection (CDI). Symptoms include diarrhea, fever and pain. Although CDI is often mild, it can be very serious, particularly in older people, and, if untreated, can be fatal. This review looked at studies published from 2006 to 2017 to investigate patterns of CDI sickness (epidemiology) in Japan. A total of 55 studies were useful for our review and showed that, in general, CDI occurred less commonly in Japan than in Western countries. However, there was wide variation in the tests used to detect infection and the methods used to identify specific types of C. difficile bacteria responsible for the infections. Because of this variety, there was a difference in the reliability of the results from the different studies, which made it difficult to make comparisons between studies. However, there seemed to be consistent results showing that certain types of C. difficile were common in Japan. The studies were not able to tell us whether the types of C. difficile varied over time. More studies that use reliable high-quality tests, and greater detailed analysis in Japan to map patterns of CDI over time are needed. This would help us to understand the importance of CDI in Japan.
Introduction
Clostridium difficile is the most common infective cause of nosocomial diarrhea, implicated in 20–30% of cases of antibiotic-associated diarrhea [1, 2]. Appropriate patient care requires rapid and accurate diagnosis to support optimal management and prevent the spread of infection. Furthermore, knowledge of specific risk factors for C. difficile infection (CDI) in different clinical settings is essential.
No national CDI surveillance system has been implemented in Japan, and therefore it is challenging to grasp the trend in epidemiology over time using a standardized method. A review of CDI in Asia published in 2013 found only a few molecular-typing studies providing contemporary epidemiological information [3]. According to a questionnaire-based survey of 2537 hospitals in Japan in 2013, which had valid responses from 321 hospitals, CDI incidence varied between centers [4], and there was little information on the specific strains causing infection.
There have been several important changes in CDI diagnosis and treatment in Japan. First, a new diagnostic kit detecting toxin A and B plus “common” antigen (glutamate dehydrogenase; GDH) became available in April 2011. Second, oral and injectable metronidazole were indicated for CDI in August 2012 [5] and September 2014 [6], respectively, although unlicensed use of oral metronidazole for CDI had occurred in Japan prior to 2012. Third, in 2015, the Japanese Association for Infectious Diseases and Japanese Society of Chemotherapy released guidelines for the treatment of enteric infection, in which oral metronidazole was designated as the first-line treatment for CDI [7]. Vancomycin was recommended for severe cases and/or second and subsequent recurrences [7].
Considering these recent changes in the diagnosis and treatment of CDI, there is a greater need to understand and update the epidemiology of CDI, the predominant strains causing the infection, and the consequences, risks and resource utilization associated with CDI in clinical settings in Japan. This literature review was undertaken to summarize published epidemiological data on CDI in Japan from January 2006 to November 2017, to describe definitions of CDI applied, molecular typing and diagnostic methods used, and key risk factors and expected outcomes.
Methods
The recent literature was reviewed in a systematic fashion to identify studies and reports relating to the epidemiology of CDI in Japan. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to inform search terms, and the literature review process was conducted using the PRISMA Checklist and PRISMA Flow diagram.
Identification
Searches of MEDLINE-PubMed and EMBASE® were made using the following primary search terms: C. difficile infection; pseudomembranous colitis; epidemiology; Japan. Secondary search terms were as follows: C. difficile diarrhea; C. difficile colitis; enterocolitis; toxic megacolon; hospital-acquired diarrhea; nosocomial diarrhea; antibiotic-associated diarrhea; incidence; Japan. The publications were limited to the English language from 1 January 2006 to 27 November 2017.
Selection
Identified abstracts were reviewed by a single reviewer to remove duplicates and to identify publications meeting the pre-defined inclusion and exclusion criteria. Inclusion criteria were: Japanese patients or human samples with CDI; observational or non-randomized interventional studies; cross-sectional surveys; cohort studies; case–control studies; pharmacy records or claims databases; electronic registers or electronic medical/health records; insurance or administrative claims databases studies; registry studies; prospective or retrospective studies; longitudinal or follow-up studies; and reviews. Publications were included if they reported on: CDI epidemiology (incidence/prevalence); CDI risk factors; CDI definitions; diagnostic and laboratory test methods; CDI strains; length of hospital stay (LOS); intensive care unit admission; CDI recurrence; and mortality. There was reliance on the individual publications to define CDI and no minimum (discriminatory) definition was used during the selection process. Exclusion criteria were: animal studies; in vitro studies; case reports; editorials, commentaries and letters; congress abstracts; and non-English language publications. All publications that met criteria for the review were obtained as full articles, reassessed and reviewed.
Quality Determination and Data Extraction
A single reviewer assessed the quality of each paper/study according to Oxford Centre for Evidence-Based Medicine – Levels of Evidence [8] (Enhanced Supplementary Material). As most of the captured studies did not fall strictly within a given category, references were also assessed by the same individual to ensure consistent application of the criteria across all publications.
Data from the selected studies were extracted by a single reviewer and used to populate summary tables.
Compliance with Ethics Guidelines
The analysis in this article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by either of the authors.
Results
Identification of Relevant Publications
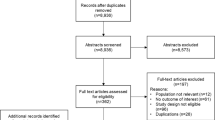
A total of 385 potential articles were identified, of which 55 were defined as relevant, after applying the pre-defined inclusion and exclusion criteria (Fig. 1). One article was an erratum [9] of a previously identified study [10], therefore the study was counted only once. The assigned grades of literature per relevant article are summarized in the Enhanced Supplementary Material.
Assessment of search results to identify key papers for review and data extraction. Asterisk did not meet inclusion criteria in relation to study population or design (see “Selection”). Hash did not include reports of: Clostridium difficile infection (CDI) epidemiology (incidence/prevalence); CDI risk factors; CDI definitions; diagnostic and laboratory test methods; CDI strains; length of hospital stay; intensive care unit admission; CDI recurrence; or mortality. Dagger one identified article was an erratum of a previously identified study, therefore the study was counted only once
Many papers were insufficiently specific: for example, several papers reported on the validation of novel C. difficile diagnostic assays or laboratory testing methods in Japan, but did not include clinical data. Others were excluded owing to a small sample size (n ≤ 8), a focus on pre-clinical evaluation of CDI testing methods, or for reporting CDI contamination in non-patient groups [11,12,13,14,15]. Several papers were reviews or editorials with limited relevance to Japanese CDI epidemiology [3, 16, 17].
Incidence and Prevalence
Twenty-four papers reported data relating to the incidence and prevalence of CDI, or to C. difficile-related disease or diarrhea in Japanese cohorts (Table 1). Most reports were based on retrospective chart reviews (n = 16), eight were prospective studies; either observational studies or randomized controlled trials (RCTs), and one was a systematic review and meta-analysis. Most of the papers described and defined CDI in terms of clinical diagnosis (‘diarrhea’) and laboratory findings (‘toxin positivity’). The papers differed in their approach to testing for CDI: some actively investigated C. difficile colonization across cohorts whilst others tested for C. difficile only in patients with clinical symptoms suggestive of CDI. Testing methods also varied. Because of these differences and the heterogeneity in the patient populations examined in the publications, including hematopoietic stem cell transplant (HSCT) patients, rheumatology patients and those with Helicobacter pylori-positive peptic ulcer, it was not possible to examine trends over time in the incidence of CDI.
Very few papers reported incidence in terms of cases per 10,000 patient-days; most reported observed CDI ratios (as percentages), with CDI variously defined, or prevalence within specific patient subgroups or populations (Table 1). Figure 2 depicts reported CDI incidence from retrospective chart reviews in different patient cohorts, where CDI was defined based on diarrhea and laboratory detection of fecal C. difficile toxin.
Clostridium difficile infection (defined as diarrhea/CD toxin) reported in retrospective cohorts of Japanese patients. CRC colorectal cancer, GI gastrointestinal, HSCT hematopoietic stem cell transplantation, IBD inflammatory bowel disease, pts patients, RA rheumatoid arthritis. Patient numbers represent those diagnosed with Clostridium difficile infection
A chart review of over 22,800 inpatients at a single tertiary care center (of whom 851 were tested for C. difficile) reported on healthcare-facility onset CDI, defined as diarrhea and a positive toxin test (using C DIFF QUIK CHEK COMPLETE®) [18]. The CDI prevalence was 5.5 cases/1000 patients. The incidence of healthcare-facility onset CDI was 3.11 cases/10,000 patient-days, compared with 0.2 cases/10,000 patient-days for community-onset CDI [18]. The authors considered the CDI incidence (hospital- and community-onset) to be rather low, which they suggested may have been attributable to the relatively low frequency of testing for C. difficile and the relatively low sensitivity of the EIA toxin detection method. Another large-scale outpatient study (n = 2193) reported the incidence of community-acquired CDI as 1.4/100,000 patient-years (or 0.14/10,000 patient-years) [19], and a study of both actively tested in- and outpatients provided an incidence of 0.8 cases/10,000 patient-days [20]. A retrospective cohort study based on chart reviews at four tertiary care hospitals reported 160 patients aged at least 14 years with hospital-onset CDI, as defined according to clinical practice guidelines, giving an incidence of 1.04/10,000 patient-days (or 1.61/1000 admissions) [21]. The low incidence of hospital-onset CDI compared with that reported in studies from Western countries was suggested by the authors to be a consequence of the different strains prevalent in different regions, with outbreaks of hospital-acquired CDI in Western countries being attributed to highly virulent strains that are not prevalent in Japan. The study did not explore the strains responsible for the CDI episodes at the four hospitals. The authors also suggested that the low frequency of CDI may be a consequence of the low frequency of testing for C. difficile [21].
Few studies identified in our search described measures to control the incidence of C. difficile in hospitals. Although not strictly an infection control measure, the prophylactic use of pre- and probiotics has been suggested to maintain the colonic microbiota and potentially reduce the development of CDI [22]. A single-center RCT of 379 patients undergoing colorectal surgery evaluated the impact of perioperative synbiotics (combination of pro- and prebiotics) on post-surgical outcomes and fecal microbiota composition, finding that patients administered synbiotics before surgery had a lower incidence of C. difficile in their fecal microbiota compared with control patients. The incidence of CDI was low, with only one patient in the control group developing CDI, while none in the treated group did so. The authors suggested a potential role for synbiotics in suppressing overgrowth of C. difficile after surgery [22]. The literature search also identified a study reporting the impact of infection control interventions on CDI occurrence. A medical record-based C. difficile-associated disease at a single center was reported to have an incidence of 0.47 cases/1000 inpatient days, which fell to 0.11 cases/1000 patient-days after intensive infection control intervention [23]. Infection control measures included carbapenem restriction and continuous instruction to the ward staff on infection control measures [23].
Some of the epidemiological reports highlighted the incidence and prevalence of CDI in particular patient groups (Table 1; Fig. 2). For example, in patients undergoing HSCT, the cumulative incidence of CDI was 6.2% for all patients, compared with 9.2% in the allogeneic HSCT subpopulation, 1.0% in the autologous HSCT subpopulation and 9% in a cohort of HSCT recipients who received unrelated cord blood [24, 25]. In a cohort of liver transplant patients, CDI-associated diarrhea occurred at a rate of 4.5% [26]. Among rheumatology inpatients, CDI was observed in 0.16% [27]; in a large cohort of patients with sepsis, hospital-acquired CDI was observed in 1.3% of patients without and 1.4% with ulcer prophylaxis [28]. A retrospective database review of over 140,000 gastrointestinal (GI) surgery patients reported CDI (ICD-10 definition) in 0.28% of the study population, or a prevalence of 2.8 cases/1000 patients [29]. In a smaller cohort study of patients with active ulcerative colitis, 40.1% tested positive for possible CDI [30]. A study of pediatric patients with cancer who were hospitalized at a single center reported CDI (clinical symptoms and positivity for toxin EIA using TOXA/B QUIK CHEK) in 27% of the study population [31]. The authors suggested that the high incidence of CDI compared with other studies of similar patient populations in non-Japanese settings may be because of the much longer length of stay for patients with cancer in Japan compared with other countries [31].
Among the studies that reported on possible C. difficile colonization of patient groups was an epidemiological study of hospitalized pediatric patients. At least 1 in 10 pediatric patients harbored C. difficile asymptomatically, while fecal cytotoxin was found in 9% of otherwise healthy children and 23.1% of children with underlying disease [32]. However, these findings should be interpreted with caution as the inclusion of patients younger than 3 years old may increase the likelihood that the diarrhea had a cause other than C. difficile [33].
The reviewed studies included reports of both hospital and community patients. No papers were identified that specifically related to patients in long-term healthcare facilities and only one paper reported on changes in infection over time [23]. Therefore, depending on the patient group and methods used to define and assess CDI, the incidence varied between 0.8 and 4.71/10,000 patient-days, while the prevalence was between 0.3 and 5.5 cases/1000 patients.
Risk Factors
Known risk factors for CDI include: co- and previously administered broad-spectrum antibiotics; age and comorbidities; poor infection-control practices; GI tract surgery; and gastric-acid suppressing agents [13, 34,35,36]. Thirteen studies in our search, including a large database study representing 40% of all adult-care hospitalizations, identified risk factors for CDI in Japanese patients, which included older age, higher comorbidity index; gastric acid-suppressing proton pump inhibitors (PPIs); and a longer pre-operative LOS before GI surgery [19, 20, 24,25,26, 29, 31, 32, 36,37,38,39,40] (Table 1). Malignant disease and intensive care unit (ICU) stay were linked with increased risk for CDI recurrence in in- and outpatients [20]. Six articles reported on the number of days spent in hospital prior to surgery or CDI diagnosis, indicating a wide variation in inpatient stay before diagnosis [18, 20, 29, 38, 41, 42]. The only study to formally compare pre-operative number of days in hospital for patients who did and did not develop CDI found no difference between the two patient groups, with a median (interquartile range) of 6 (3–14) days and 5 (3–8) days, respectively (p < 0.001) [29].
Patients undergoing HSCT are recognized to be at particular risk of infection. Allogeneic HSCT, conditioning for HSCT, acute leukemia and prolonged neutropenia in the first 30 days after HSCT may all confer an increased risk for CDI as reported in a single-center study [24]. In contrast, it was noted that among allogeneic HSCT patients, treatment with total body irradiation may reduce post-transplant risk of CDI [25].
A study of pediatric patients reported that tube feeding was significantly associated with higher colonization rates by toxin-positive C. difficile [32].
Antibiotic use was a risk factor for C. difficile diarrhea in a number of studies [19, 31, 37, 38]; however, in a study of liver transplant patients, the intensity of antibiotic use (measured as use of preoperative antibiotics or the number of antibiotics used postoperatively) was not a predictor for C. difficile diarrhea [26]. Among hospitalized pediatric patients with cancer, use of a wide variety of antibiotics (the study specified at least four different types) in the 60 days prior to CDI diagnosis was a significant risk factor for the development of CDI [31].
Specific Strains Responsible for CDI
A review of CDI in Asia in 2013 reported that PCR ribotypes 027 and 078 were rare, while variant toxin A−/toxin B+ strains of ribotype 017 were common. Furthermore, in Japan, common ribotypes include 014, 002 and 001, and ribotype smz/018 has been implicated in widespread disease [3]. The review noted that a variety of typing techniques has been used in Japan, including tcdA and tcdB detection, pulsed field gel electrophoresis (PFGE), PCR ribotyping and slpA typing. Although molecular typing had identified toxigenic A−B+ strains, the authors did not comment on binary (CDT) toxin assessment or C. difficile surveillance in Japan [3].
In our literature search, 16 papers provided further details of testing methods used in Japan and described reports on the isolates and strains associated with CDI in Japanese cohorts (Table 2). The methods used to detect CDI and/or detect, isolate and type C. difficile included stool culture and C DIFF QUIK CHEK COMPLETE®; stool culture, PCR ribotyping and slpA sequence typing; stool culture, PCR and PFGE; and rRNA-targeted RT-qPCR and multiplex PCR for toxin gene profiling. Reported methods of testing for C. difficile toxins included: toxin A testing by Uniquick (in 2004) [43]; PCR assessment for toxin A, B and CDT genes [44]; TOX A/B QUIK CHEK® [45]; and multiplex PCR [46]. Rapid detection methods evaluated and reported in Japan include detection of GDH and toxin A/B in feces (using Immunocard® and TOX A/B, respectively) [47]; and detection of GDH and toxin A/B simultaneously using C DIFF QUIK CHEK COMPLETE® [41]. Although mass spectrometry methods have also been reported, these were not considered suitable for typing C. difficile [48].
Our literature review (Table 2) suggests that most C. difficile isolates associated with infection in Japan produce both toxins A and B. Available studies suggest a low prevalence of binary toxin-positive (CDT+) strains—between 0 and 6.8% reported across studies [19, 44, 46, 49,50,51]. A small study from 2015 found that outbreak and non-outbreak isolates were predominantly smz/ysmz (by slpA typing) and that of five binary toxin-positive strains, one was ribotype 027 and one 078 [51]. Individual papers appear to support the conclusion that ribotypes 027 and 078 are rare in Japan [44, 49, 50], and that smz/018, yok, 002, 014 and 369 (trf by slpA typing) are common [3, 42,43,44, 46, 49,50,51]. A recently published substudy of a global RCT that isolated 54 strains of toxigenic C. difficile (by EIA/PCR assays) from the stool cultures of 93 hospitalized patients at 35 sites in Japan appeared to corroborate this finding [52]. The most common PCR ribotypes were 052 (28% of isolates), 018 (19% of isolates), 002 (15% of isolates) and 369 (9% of isolates), and 052 was considered to be an ‘established’ strain, as it was widely distributed across Japan. Ribotypes 027 and 078 were not isolated in the substudy [52].
Recurrence
Twelve publications reported on CDI recurrence (Table 3) [18, 21, 24,25,26, 31, 39,40,41,42, 44], with rates in studies that included specific definitions of recurrence ranging from 3.3% in 30 HSCT patients [24] to 27.3% in a cohort of 11 liver transplant recipients with CDI [26]. The time period over which recurrence was defined or assessed varied widely from 14 to 365 days after the initial CDI episode [18, 21, 24, 25, 31, 44]. One retrospective chart review of 242 liver transplant recipients, 11 of whom developed CDI, reported recurrence in 2 of 8 patients who received vancomycin [26]; no analysis of risk was made in relation to the choice of treatment for CDI. A retrospective cohort study based on chart reviews of hospital-onset CDI cases at four teaching hospitals found that neither the severity of CDI nor adherence to clinical practice guidelines affected the risk of CDI recurrence [21]. In a retrospective study, multivariate analysis identified malignant disease (p = 0.03) and ICU hospitalization (p = 0.049) as risk factors for CDI recurrence within 8 weeks of the previous CDI episode [20]. A systematic review and meta-analysis of published studies reported that use of PPIs was significantly associated with recurrent CDI (pooled OR 1.73, 95% CI 1.39–2.15; p = 0.02) (Table 3) [40].
Healthcare Utilization
A large retrospective chart review of 22,863 patients by Honda et al. reported a median LOS among 126 CDI cases of 41.5 days (17.5 days before and 18 days post-CDI diagnosis) [18]. The sparsity of available LOS data did not allow any analysis of trends for CDI-related LOS over the years examined. The adjusted attributable LOS and costs related to CDI were reported from one large database study of 143,652 hospitalized patients [29]. There were 409 cases of CDI with the infection contributing to a LOS increase of 12.4 days (95% CI 9.7–15.0; p < 0.001) and a cost increase of US$6576 (95% CI 3753–9398; p < 0.001) compared with control patients who did not develop CDI. However, of note, this study included patients who had undergone specific surgical procedures and had CDI identified using diagnostic codes rather than by diagnostic tests [29], which could potentially lead to inaccurate identification of CDI.
Cases of CDI may require patient transfer to the ICU. The study by Honda et al. found that 9.5% of 126 CDI cases needed ICU admission [18]. There were 65 (51.6%) patients who were classified as having severe CDI, using the severity assessment score developed by Zar et al. [53]; three patients (2.4%) underwent CDI-related colectomy/diverting loop ileostomy because of critical illness or failure of medical therapy. Another retrospective chart review based on stool samples from in- and outpatients at a single hospital identified 76 patients with hospital-onset CDI, with three (3.9%) cases requiring ICU care [20].
Mortality
All-cause mortality within 30 days ranged from 3.4 to 15.1% [18, 20, 21, 29, 39]. Honda et al. reported that mortality was associated with an increased Zar severity score [18]. Of note, the Zar severity criteria will score a patient 1 point (2 points are defined as severe CDI) based only on age 60 years or older [53], and the median age of patients in the Honda et al. study was 78 years [18]. A multicenter retrospective cohort study of 160 hospitalized patients with CDI reported 30-day all-cause mortality of 13%, and found no significant difference between the mortality rate among patients with severe and non-severe CDI, or among those whose treatment adhered and did not adhere to clinical practice guidelines [21]. At 90 days, all-cause mortality among CDI cases was reported as 14.5% in one retrospective chart review [20]. Hosokawa et al. [25] concluded that, among patients who underwent unrelated cord blood transplantation, overall survival at 2 years was no different for those who developed CDI than for those who did not (42 vs. 46%, respectively; p = 0.77). One database study demonstrated that inpatient mortality was significantly higher in CDI patients than in those without CDI (3.4 vs. 1.6%; p = 0.008) [29], although the results of this study should be viewed with caution owing to the reliance on recorded diagnoses of CDI from administrative databases (which are less well validated than those in prospective cohorts or registries, for example), the inclusion of only patients undergoing GI surgery, and the loss of many patients from the propensity score-matched analysis. The use of vancomycin was associated with reduced mortality (OR 0.43; 95% CI 0.25–0.75) in a multicenter, case–control and cohort study of 1026 CDI patients [39].
Discussion
This systematic literature review identified 55 papers providing insights into the rates of CDI, patient groups affected and impact of CDI in Japan. Most of the studies were retrospective data reviews, and many focused on patients with suspected CDI, and the rates and prevalence of CDI in those groups. Fewer studies reported overall rates of hospital- and community-acquired CDI. Nevertheless, the current literature suggests that hospital-onset CDI in Japan occurs at an incidence of 0.8–4.7 cases/10,000 patient-days; lower than that reported in Europe for winter 2012–2013 (country range 0.7–28.7/10,000 patient bed-days), and similar to the US for hospital-onset CDI in 2013 (6.0/10,000 patient-days for laboratory-identified CDI and 4.4/10,000 patient-days for traditional surveillance-detected CDI) [54, 55]. However, direct comparisons between studies are difficult owing to differences in design, population size and detection methods used. The prevalence of CDI in Japan (0.3–5.5/1000 patient admissions) was lower than that in a US Veterans Health Administration (VHA) report from 2014, which recorded that from October 2010 to June 2012, CDI prevalence at admission was between 5.3 and 6.9/1000 admissions, in settings where 50% of hospitals used nucleic acid amplification tests [56]. An analysis of US VHA data for February 2012 to January 2014 reported a pooled CDI admission prevalence rate of 0.38/100 admissions [57].
CDI in Japan appears to have similar epidemiology to that in South Korea, although distinct from regions of South Asia [3]. There are a number of factors that contribute to the unique epidemiology of CDI in Japan. First, if testing for CDI is not conducted, this makes data-gathering on both the rate of testing and test results difficult. While there is a national surveillance program for infectious diseases in Japan, there is no national C. difficile screening program. Methods of testing are also an important consideration. Indeed, datasets from CDI surveillance programs highlight that country-to-country variations in CDI incidence largely reflect differences in surveillance methods and how rigorously CDI is investigated in testing strategies [54]. This literature review highlights that testing, typing and laboratory methods for assessment and diagnosis of CDI have evolved in Japan in recent years, with new methods continuing to be evaluated against established methods. However, some authors have noted that many reports of CDI epidemiology in Japan have relied on insensitive testing methods, such as EIA toxin tests [16]. While more sensitive nucleic acid amplification tests are recommended in clinical practice guidelines for the detection of C. difficile [7], these are not yet subject to reimbursement in Japan and are therefore not widely used, thus reliance on EIA remains common in clinical practice. It could be argued that a low detection frequency, as seen for CDI toxin tests in stool samples, may in fact reflect a low disease burden, as demonstrated by Shimizu et al., who reported that patients with positive EIA toxin tests in stools had more severe CDI than those patients with negative stool toxin tests who then had positive cultures [41]. It is recognized that CDI is under-diagnosed in many regions and countries because of a combination of absence of clinical suspicion and suboptimal laboratory diagnostics [54]. This may also be the case in Japan, although no papers in this search specifically focused on reporting under-diagnosed or missed CDI cases.
Increased LOS is both a risk factor for, and an outcome of, CDI [29]. A notable factor in Japan is the general tendency for a longer LOS compared with many other countries, which may have an impact on, and thus affect, CDI risk and rates [58]. Incidence of CDI is typically presented as per patient-days (i.e. patient-bed days or inpatient days) and it is important to understand the differences in overall LOS in different geographic regions when comparing incidence data. National statistics from Japan show that in 2014 mean LOS was 31.9 days for all patients and 41.7 days in those ≥ 65 years [59], whereas the comparable 2014 statistics from the USA were 4.6 days for all patients (not restricted to those with CDI), 5.2 days for patients aged 65–74 years and 5.3 days for those aged 75 years and over [60]. This suggests that if follow up was similar between nations, roughly eight times more patients would be included in the US study compared with a Japanese study to yield 10,000 patient-days as the denominator. Given the lengthy LOS in Japan, as in studies from other countries, rates of, and risks for, CDI in Japanese patients are increased by factors such as older age, presence of comorbidities, certain clinical/surgical procedures and exposure to drug therapies, including antibiotics [1]. We found that CDI recurrence similarly depended on a variety of factors, including clinical circumstances and settings, ranging from 3.3% in HSCT patients [24] to 27.3% in liver transplant patients [26]. We found very few data on risk factors for CDI recurrence, such as the choice of treatment for initial CDI. One study identified malignant disease and ICU hospitalization as risk factors for CDI recurrence [20], although the broad CIs cited for both (Table 3) suggest that analysis of risk factors in a larger population is required. As previously established [1], one systematic review and meta-analysis identified in our review revealed a strong association between PPI use and the risk for initial and recurrent CDI in both adults and children [40].
Most CDI cases in Japan are caused by strains of C. difficile that produce both toxins A and B. Recent reviews of CDI in Asia, which are supported by our literature review, showed that ribotype smz/18 (or 018) predominates in Japan [3] and that ribotype trf/369 is gaining prominence in the country [61]. Several of the studies we reviewed confirmed a low prevalence of ribotypes 027 and 078 in Japan. It has been postulated that the low prevalence of these highly virulent strains that cause sporadic outbreaks in Western countries may account for the low general incidence of CDI in Japan [21]. Further, we found that recent papers reporting on CDI strain and ribotype increasingly included a binary toxin assessment, although CDT+ C. difficile was rare in Japan [42, 46].
There are a number of limitations to this review. Most reports on CDI epidemiology in Japan included here were based on hospital cohort data, and were often (in 32 of 55 studies) single-site reports concerning either inpatients or patients discharged from hospital to the community. We did not find papers reporting on CDI rates in long-term care facilities, although, given the prolonged LOS reported in studies in our review, some hospitals may ‘replace’ long-term care facilities in some instances. There was a large degree of heterogeneity in relation to the definition of initial and recurrent CDI in the studies included in our review. This hampered our ability to evaluate the epidemiological data in a consistent manner and reduced the potential to draw robust conclusions. Although the papers reviewed spanned more than a 10-year period, there were no data describing local infection changes over time. However, we did find sporadic snapshots of CDI in certain patient subgroups and cohorts. Our review was further limited by a lack of information relating to the rate of CDI testing and no data on community-associated CDI.
Future studies and surveillance are necessary to gather data on the numbers and types of patients affected by, or at risk of, CDI in Japan. Furthermore, data on the clinical impact of CDI are important for management and resource planning, and to ensure optimal patient care and outcomes.
Conclusion
The current literature offers some insights into the evolving epidemiology of CDI in Japan, yet highlights a number of unresolved questions. Notably, heterogeneity in the CDI definitions in the studies we reviewed limited our ability to draw robust conclusions. With the availability of newer diagnostic tools and release of clinical practice guidelines for CDI, there is a need to undertake more comprehensive and coordinated studies and surveillance of both CDI cases and C. difficile isolates to map current trends, review the impact of infection control measures, increase knowledge of risk factors, and more fully understand the extent and impact of hospital-onset CDI in Japanese patient populations today.
References
Chakra CNA, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS ONE. 2014;9:e107420.
Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–36.
Collins DA, Hawkey PM, Riley TV. Epidemiology of Clostridium difficile infection in Asia. Antimicrob Resist Infect Control [Internet]. 2013;2:21. http://aricjournal.biomedcentral.com/articles/10.1186/2047-2994-2-21.
Yamagishi Y, Mikamo H. Recent epidemiology of Clostridium difficile infection in Japan. Jpn J Antibiot. 2015;68:345–58.
Shionogi & Co. Antitrichomonal agent, Flagyl®—approval for additional indication for amoebic dysentery, giardiasis, anaerobic infection and infectious enteritis [Internet]. 2012 [cited 2017 Jun 7]. http://www.shionogi.co.jp/en/company/news/2012/pmrltj0000000wim-att/e_120810-2.pdf.
Pfizer Inc. Acquired manufacturing and marketing approval for anaerobic bacterial infection treatment agent “Anemetro® IV infusion solution 500 mg” [Internet]. 2014 [cited 2017 Jun 7]. http://www.pfizer.co.jp/pfizer/company/press/2014/2014_07_04.html.
Onishi K, Somoda Y, Imamura A, Iwabuchi C, Okuda MNT. JAID/JSC infection treatment guideline 2015. Intestinal infection. Jpn J Chemother. 2016;64:31–65.
CEBM. Oxford Centre for Evidence-based Medicine—Levels of Evidence (March 2009) [Internet]. Cent. Evidence-Based Med. 2009 [cited 2017 Feb 23]. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/.
Cairns MD, Preston MD, Hall CL, Gerding DN, Hawkey PM, Kato H, et al. Correction for Cairns et al., “Comparative genome analysis and global phylogeny of the toxin variant Clostridium difficile PCR ribotype 017 reveals the evolution of two independent sublineages”. J Clin Microbiol. 2017;55:1971.
Cairns MD, Preston MD, Hall CL, Gerding DN, Hawkey PM, Kato H, et al. Comparative genome analysis and global phylogeny of the toxin variant Clostridium difficile PCR ribotype 017 reveals the evolution of two independent sublineages. J Clin Microbiol. 2017;55:865–76.
Nomura K, Fujimoto Y, Yamashita M, Morimoto Y, Ohshiro M, Sato K, et al. Absence of pseudomembranes in Clostridium difficile-associated diarrhea in patients using immunosuppression agents. Scand J Gastroenterol. 2009;44:74–8.
Sasahara T, Ae R, Watanabe M, Kimura Y, Yonekawa C, Hayashi S, et al. Contamination of healthcare workers’ hands with bacterial spores. J Infect Chemother. 2016;22:521–5.
Fujimori S. What are the effects of proton pump inhibitors on the small intestine? World J Gastroenterol. 2015;21:6817–9.
Senoh M, Kato H, Murase T, Hagiya H, Tagashira Y, Fukuda T, et al. Reverse transcription polymerase chain reaction-based method for selectively detecting vegetative cells of toxigenic Clostridium difficile. Microbiol Immunol. 2014;58:615–20.
Tojo M, Nagamatsu M, Hayakawa K, Mezaki K, Kirikae T, Ohmagari N. Evaluation of an automated rapid diagnostic test for detection of Clostridium difficile. PLoS ONE. 2014;9:e106102.
Honda H, Dubberke ER. Clostridium difficile infection in solid organ transplant recipients. Curr Opin Infect Dis. 2014;27:336–41.
Arimoto J, Horita N, Kato S. Diagnostic test accuracy of glutamate dehydrogenase for Clostridium difficile: systematic review and meta-analysis. Sci Rep. 2016;6:29754.
Honda H, Yamazaki A, Sato Y, Dubberke ER. Incidence and mortality associated with Clostridium difficile infection at a Japanese tertiary care center. Anaerobe. 2014;25:5–10.
Mori N, Aoki Y. Clinical characteristics and risk factors for community-acquired Clostridium difficile infection: a retrospective, case-control study in a tertiary care hospital in Japan. J Infect Chemother. 2015;21:864–7.
Hikone M, Ainoda Y, Tago S, Fujita T, Hirai Y, Takeuchi K, et al. Risk factors for recurrent hospital-acquired Clostridium difficile infection in a Japanese university hospital. Clin Exp Gastroenterol. 2015;8:191–6.
Kobayashi K, Sekiya N, Ainoda Y, Kurai H, Imamura A. Adherence to clinical practice guidelines for the management of Clostridium difficile infection in Japan: a multicenter retrospective study. Eur J Clin Microbiol Infect Dis Germany. 2017;36:1947–53.
Komatsu S, Sakamoto E, Norimizu S, Shingu Y, Asahara T, Nomoto K, et al. Efficacy of perioperative synbiotics treatment for the prevention of surgical site infection after laparoscopic colorectal surgery: a randomized controlled trial. Surg Today. 2016;46:479–90.
Suzuki H, Senda J, Yamashita K, Tokuda Y, Kanesaka Y, Kotaki N, et al. Impact of intensive infection control team activities on the acquisition of methicillin-resistant Staphylococcus aureus, drug-resistant Pseudomonas aeruginosa and the incidence of Clostridium difficile-associated disease. J Infect Chemother. 2013;19:1047–52.
Akahoshi Y, Kimura S, Nakano H, Harada N, Kameda K, Ugai T, et al. Significance of a positive Clostridium difficile toxin test after hematopoietic stem cell transplantation. Clin Transplant. 2016;30:703–8.
Hosokawa K, Takami A, Tsuji M, Araoka H. Relative incidences and outcomes of Clostridium difficile infection following transplantation of unrelated cord blood, unrelated bone marrow, and related peripheral blood in adult patients: a single institute study. Transpl Infect Dis. 2014;16:412–20.
Hashimoto M, Sugawara Y, Tamura S, Kaneko J, Matsui Y, Togashi J, et al. Clostridium difficile-associated diarrhea after living donor liver transplantation. World J Gastroenterol. 2007;13:2072–6.
Iwamoto M, Kamimura T, Nagashima T, Kamata Y, Aoki Y, Onishi S, et al. Healthcare-associated infections in rheumatology in Japan. Rheumatol Int. 2012;32:801–4.
Sasabuchi Y, Matsui H, Lefor AK, Fushimi K, Yasunaga H. Risks and benefits of stress ulcer prophylaxis for patients with severe sepsis. Crit Care Med. 2016;44:e464–9.
Yasunaga H, Horiguchi H, Hashimoto H, Matsuda S, Fushimi K. The burden of Clostridium difficile-associated disease following digestive tract surgery in Japan. J Hosp Infect. 2012;82:175–80.
Kaneko T, Matsuda R, Taguri M, Inamori M, Ogura A, Miyajima E, et al. Clostridium difficile infection in patients with ulcerative colitis: investigations of risk factors and efficacy of antibiotics for steroid refractory patients. Clin Res Hepatol Gastroenterol. 2011;35:315–20.
Daida A, Yoshihara H, Inai I, Hasegawa D, Ishida Y, Urayama KY, et al. Risk factors for hospital-acquired Clostridium difficile infection among pediatric patients with cancer. J Pediatr Hematol Oncol. 2017;39:e167–72.
Furuichi M, Imajo E, Sato Y, Tanno S, Kawada M, Sato S. Characteristics of Clostridium difficile colonization in Japanese children. J Infect Chemother. 2014;20:307–11.
Schutze GE, Willoughby RE. Clostridium difficile infection in infants and children. Pediatrics. 2013;131:196–200.
Matsumoto K, Kanazawa N, Shigemi A. Factors affecting treatment and recurrence of Clostridium difficile infections. Biol Pharm Bull. 2014;37:1811–5.
Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001–10.
Roughead EE, Chan EW, Choi N-K, Griffiths J, Jin X-M, Lee J, et al. Proton pump inhibitors and risk of Clostridium difficile infection: a multi-country study using sequence symmetry analysis. Expert Opin Drug Saf Engl. 2016;15:1589–95.
Mizui T, Teramachi H, Tachi T, Tamura K, Shiga H, Komada N, et al. Risk factors for Clostridium difficile-associated diarrhea and the effectiveness of prophylactic probiotic therapy. Pharmazie. 2013;68:706–10.
Ogami N, Yoshida J, Ishimaru T, Kikuchi T, Matsubara N, Ueno T, et al. Is Clostridium difficile infection influenced by antimicrobial use density in wards? Jpn J Antibiot. 2013;66:87–95.
Takahashi M, Mori N, Bito S. Multi-institution case-control and cohort study of risk factors for the development and mortality of Clostridium difficile infections in Japan. BMJ Open. 2014;4:e005665.
Oshima T, Wu L, Li M, Fukui H, Watari J, Miwa H. Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis. J Gastroenterol Jpn. 2018;53:84–94.
Shimizu H, Mori M, Yoshimoto N. Clostridium difficile infection is more severe when toxin is detected in the stool than when detected only by a toxigenic culture. Intern Med. 2015;54:2155–9.
Mori N, Yoshizawa S, Saga T, Ishii Y, Murakami H, Iwata M, et al. Incorrect diagnosis of Clostridium difficile infection in a university hospital in Japan. J Infect Chemother. 2015;21:718–22.
Kato H, Kato H, Nakamura M, Iwashima Y, Nakamura A, Ueda R. Rapid analysis of Clostridium difficile strains recovered from hospitalized patients by using the slpA sequence typing system. J Infect Chemother. 2009;15:199–202.
Iwashima Y, Nakamura A, Kato HH, Kato HH, Wakimoto Y, Wakiyama N, et al. A retrospective study of the epidemiology of Clostridium difficile infection at a University Hospital in Japan: genotypic features of the isolates and clinical characteristics of the patients. J Infect Chemother. 2010;16:329–33.
Kobayashi M, Sako A, Ogami T, Nishimura S, Asayama N, Yada T, et al. Validation of the 3-day rule for stool bacterial tests in Japan. Intern Med. 2014;53:533–9.
Kuwata Y, Tanimoto S, Sawabe E. Molecular epidemiology and antimicrobial susceptibility of Clostridium difficile isolated from a university teaching hospital in Japan. Eur J Clin Microbiol Infect Dis. 2015;34:763–72.
Kawada M, Annaka M, Kato H. Evaluation of a simultaneous detection kit for the glutamate dehydrogenase antigen and toxin A/B in feces for diagnosis of Clostridium difficile infection. J Infect Chemother. 2011;17:807–11.
Kiyosuke M, Kibe Y, Oho M. Comparison of two types of matrix-assisted laser desorption/ionization time-of-flight mass spectrometer for the identification and typing of Clostridium difficile. J Med Microbiol. 2015;64:1144–50.
Kato H, Kato H, Ito Y, Akahane T, Izumida S, Yokoyama T, et al. Typing of Clostridium difficile isolates endemic in Japan by sequencing of slpA and its application to direct typing. J Med Microbiol. 2010;59:556–62.
Sawabe E, Kato H, Osawa K, Chida T, Tojo N, Arakawa Y, et al. Molecular analysis of Clostridium difficile at a university teaching hospital in Japan: a shift in the predominant type over a five-year period. Eur J Clin Microbiol Infect Dis. 2007;26:695–703.
Senoh M, Kato H, Fukuda T, Niikawa A, Hori Y, Hagiya H, et al. Predominance of PCR-ribotypes, 018 (smz) and 369 (trf) of Clostridium difficile in Japan: a potential relationship with other global circulating strains? J Med Microbiol. 2015;64:1226–36.
Mikamo H, Aoyama N, Sawata M, Fujimoto G, Dorr MB, Yoshinari T. The effect of bezlotoxumab for prevention of recurrent Clostridium difficile infection (CDI) in Japanese patients. J Infect Chemother. 2018;24:123–9.
Zar FA, Bakkanagari SR, Moorthi KMLST, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis [Internet]. 2007;45:302–7. http://www.ncbi.nlm.nih.gov/pubmed/17599306.
Davies KA, Longshaw CM, Davis GL, Bouza E, Barbut F, Barna Z, et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect Dis. 2014;14:1208–19.
Durkin MJ, Baker AW, Dicks KV, Lewis SS, Chen LF, Anderson DJ, et al. A comparison between National Healthcare Safety Network laboratory-identified event reporting versus traditional surveillance for Clostridium difficile infection. Infect Control Hosp Epidemiol. 2015;36:125–31.
Evans ME, Simbartl LA, Kralovic SM, Jain R, Roselle GA. Clostridium difficile infections in Veterans Health Administration acute care facilities. Infect Control Hosp Epidemiol. 2014;35:1037–42.
Reeves JS, Evans ME, Simbartl LA, Kralovic SM, Kelly AA, Jain R, et al. Clostridium difficile infections in veterans health administration long-term care facilities. Infect Control Hosp Epidemiol. [Internet]. 2016 [cited 2017 Feb 23];37:295–300. http://www.ncbi.nlm.nih.gov/pubmed/26686361.
Organisation for Economic Co-operation and Development. Length of hospital stay [Internet]. 2016 [cited 2017 Jun 26]. https://data.oecd.org/healthcare/length-of-hospital-stay.htm.
Ministry of Health Labour and Welfare. Summary of patient survey 2014. [Internet]. 2015 [cited 2017 Jul 24]. http://www.mhlw.go.jp/english/database/db-hss/dl/sps_2014_03.pdf.
Agency for Healthcare Research and Quality (AHRQ). HCUP fast stats—trends in inpatient stays 2005-2014 [Internet]. 2014 [cited 2017 Jul 24]. https://www.hcup-us.ahrq.gov/faststats/NationalTrendsServlet.
Collins DA. Epidemiology of Clostridium difficile infection in the Asia-Pacific region [Internet]. The University of Western Australia; 2016. http://research-repository.uwa.edu.au/files/11692621/THESIS_DOCTOR_OF_PHILOSOPHY_COLLINS_Deirdre_Anne_2016.pdf.
Hata H, Yamaguchi T, Hasegawa S, Nomura A, Hida K. Oral and parenteral versus parenteral antibiotic prophylaxis in elective laparoscopic colorectal surgery (JMTO PREV 07-01): a phase 3, multicenter, open-label. Random Trial Ann Surg. 2016;263:1085–91.
Sadahiro S, Suzuki T, Tanaka A, Okada K, Kamata H, Ozaki T, et al. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery. 2014;155:493–503.
Watanabe M, Hitomi S, Sawahata T. Nosocomial diarrhea caused by Clostridium perfringens in the Tsukuba-Tsuchiura district. Jpn J Infect Chemother. 2008;14:228–31.
Kikkawa H, Hitomi S, Watanabe M. Prevalence of toxin A-nonproducing/toxin-B-producing Clostridium difficile in the Tsukuba-Tsuchiura district. Jpn J Infect Chemother. 2007;13:35–8.
Kunishima H, Chiba J, Saito M, Honda Y, Kaku M. Antimicrobial susceptibilities of Clostridium difficile isolated in Japan. J Infect Chemother. 2013;19:360–2.
Oka K, Osaki T, Hanawa T, Kurata S, Okazaki M, Manzoku T. Molecular and microbiological characterization of Clostridium difficile isolates from single, relapse, and reinfection cases. J Clin Microbiol. 2012;50:915–21.
Yuhashi K, Yagihara Y, Misawa Y, Sato T, Saito R, Okugawa S, et al. Diagnosing Clostridium difficile-associated diarrhea using enzyme immunoassay: the clinical significance of toxin negativity in glutamate dehydrogenase-positive patients. Infect Drug Resist. 2016;27:93–9.
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431–55.
Acknowledgements
Funding
This analysis was initiated by Astellas Pharma, Inc. The article processing charges were funded by Astellas Pharma, Inc.
Editorial Assistance
Editorial support to the authors was provided by Rhian Harper Owen, Winnie McFadzean and Iona Easthope for Cello Health MedErgy (Europe), funded by Astellas Pharma, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. Fidaxomicin, an Astellas product, is available in some geographical regions for the treatment of CDI. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Thomas V. Riley has received grants from Cepheid, Merck, Sanofi and Otsuka. Tomomi Kimura is a full-time employee of Astellas Pharma, Inc. (Japan).
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by either of the authors.
Data Availability
The results of the literature searches analysed during the current review are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/104B4F6021959470.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Riley, T.V., Kimura, T. The Epidemiology of Clostridium difficile Infection in Japan: A Systematic Review. Infect Dis Ther 7, 39–70 (2018). https://doi.org/10.1007/s40121-018-0186-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-018-0186-1