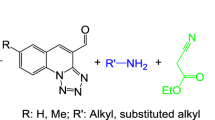
Abstract
An efficient and sustainable method was developed for the electrocatalytic synthesis of nano-sized 4H-pyran derivatives via a green, one-pot, three-component condensations of cyclic-1,3-diketones [including 1H-indene-1,3(2H)-dione, cyclohexane-1,3-dione, cyclopentane-1,3-dione, pyrimidine-2,4,6(1H,3H,5H)-trione], malononitrile or ethyl cyanoacetate, and isatins in an undivided electrochemically cell applying potassium bromide as the electrolyte in the alcoholic media. This electrosynthesis was introduced as a reliable and cost-effective approach for the high-yielding synthesis (76–92%) of the target compounds. The synthesized 4H-pyran and chromene nanoparticles could open an effective way for production of nano-sized drugs.
Graphical abstract

Similar content being viewed by others
Introduction
Nowadays the invention of fast, environmentally friendly, and inexpensive synthetic protocols have become a main part of research in organic and medicinal chemistry which is due to the facile preparation of compounds with biologically activities [1, 2]. The electrocatalytic multicomponent reaction has been known as an important approach to address this issue is, in which three or more starting materials are combined in an electrochemical cell with the appropriate electrolyte and working electrodes to generate the target products [3, 4]. Regarding to the recent growing interest of researchers in applying electrochemistry in organic compound synthesis, the electrosynthesis has been known as one of the most viable protocols in modern organic chemistry which offers a novel and promising versatile synthetic method for organochemists [5]. The electrochemical procedure permits the valuable synthesis of organic compounds for the large-scale processes due to its catalytic nature and also use of available, inexpensive, and environmentally-friendly chemical compounds such as electrolyte [6, 7]. The electrosynthesis of heterocyclic compounds at mild conditions such as ambient temperature and pressure make it highlight in organic synthesis. Additionally, the catalytic amount of an electrogenerated base can efficiently induce the catalytic chain transformation of organic reactants into target components. All of these aspects find in good agreement with the important principles in green chemistry [8].
4H-Pyran derivatives have been as a valuable class of heterocyclic compounds, which are extensively distributed in nature [9]. The fused pyran ring skeleton is considered as a famous heterocyclic compound and also as a significant framework of many natural products. In recent years, pyran and fused pyran derivatives have received the most attention owing to their association with the various types of biological and pharmaceutical characteristics [10]. The Pyran derivatives reveal interesting medicinal properties, including antimicrobial [11], antifungal [12], antitumor [13], anticoagulant, and anti-anaphylactic activities [14]. Moreover, these heterocyclic compounds exhibit potentially positive effects in the treatment of the neurodegenerative diseases such as Parkinson, Huntington, and Alzheimer, as well as AIDS-associated dementia [15]. These heterocyclic compounds are also applied in preparation of cosmetics, pigments and photoactive materials [16]. The drug structures with high surface-volume ratio display substantial improvement of solubility which cause stronger therapeutic effect. So, nano- or micro-sized drugs due to their high surface-volume ratio cause increasing of the drug adsorption and improving the curative characteristic. Accordingly, the development of several new methods to synthesize the nano-sized drugs is an significant challenge for both chemist and pharmacist. Several methods, including micronization, modification of polymorphic configuration, expansion of oil-based solutions, smart application of co-solvents, application of stabilizing agents, microemulsions, and creation of solid dispersions have been offered for the synthesis of nano-sized drug compounds.
Following our previous efforts to develop the new procedures for synthesis of the biologically active heterocyclic compounds from inexpensive and available chemical reagents [17,18,19], herein, a new, green, and convenient protocol was described for the synthesis of pyran derivatives based on the electrocatalytic multicomponent reaction. In this study, electrochemically induced catalytic three-component condensation of cyclic-1,3-diketones, malononitrile/ethyl cyanoacetate with isatins in KBr-contained ethanol were applied to synthesize the nanoparticles of pyran derivatives (Scheme 1).
Experimental
Materials and instruments
All consumed chemical materials and solvents were acquired from the Merck and Sigma-Aldrich companies. The melting points of products were evaluated by a melting point apparatus of IA 9100. Controlled-current coulometry and preparative electrolysis were performed via a SAMA potentiostat/galvanostat (Isfahan-Iran). The platinum cathode (5 cm2), graphite anode (5 cm2), as well as iron cathode, and magnesium anode were used as working electrodes. The infrared spectra (IR) were procured using the Bruker Vector 22 FTIR in KBr disks. The scanning electron microscopy images were acquired using the Hitachi S-4160 machine, which was made in Japan. The 1H NMR spectrums were achieved in DMSO-d6 using the Bruker-Arance AQS 500 MHz and the 13C NMR spectrums were recorded in DMSO-d6 employing a Bruker-Avance spectrometer 125 MHz. In addition, the mass spectrums were determined by Agilent Technology (HP) mass spectrometer operating at an ionization potential of 70 eV. Resulting products refined using thin layer chromatography (TLC) on silica gel PolyGram SILG-UV 254 plates for more purification.
General procedure for the synthesis of the pyran-derivatives nanoparticles
A mixture of cyclic 1,3-diketones (1a–d) (1 mmol), malononitrile (2a) or ethyl cyanoacetate (2b) (1 mmol), isatins (3a–e) (1 mmol), and potassium bromide (0.5 mmol) in alcohol (20 ml) was electrolyzed in an undivided cell supplied with a magnetic stirrer, iron cathode (5 cm2), magnesium anode (5 cm2) at the temperature of 40 °C and using catalytic quantity at the constant current density (20 mA cm−2, I = 100 mA) and passing the 0.1 F mol−1 of electricity through the system (time: 50 min). The reaction progression was controlled by TLC analysis (ethyl acetate/n-hexane 1/3). After the reaction was complete, the reaction mixture become cold to the ambient temperature, then it was condensed under the reduced pressure to one-fifth of initial volume.
The remaining solid was separated through filtration, next it was washed with cold ether and dried at room temperature. The resulting nano-sized products were determined through several techniques.
Data of representative examples
2-amino-2′,5-dioxo-5H-spiro(indeno(1,2-b)pyran-4,3′-indoline)-3-carbonitrile (4a)
White solid, (yield 92%), m.p 247–249 °C, IR (KBr) (νmax/cm−1) 3337 (NH2), 2904 (NH), 2223 (CN), 1712 (C=O), 1623 (C=O); 1HNMR (500 MHz, DMSO-d6): δ (ppm): 6.88–7.16 (m, 4H, Ar), 7.23 (s, 2H, NH2), 7.43–7.66 (m, 4H, Ar),10.43 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ (ppm): 45.2–56.9–103.4–117.8–121.7–125.2–126.9–127.8–127.9–128.5–129.7–129.9–134.5–136.3–137.8–141.7–160.1–168.1–171.4–193.1.
2-amino-2′,5-dioxi-5,6,7,8-tetrahydrospiro(chromene-4,3′-indoline)-3-carbonitrile (4b)
White solid, (yield 90%), m.p 302–304 °C, IR (KBr) (νmax/cm−1) 3347 (NH2), 3160 (NH), 2196 (CN), 1717 (C=O), 1658 (C=O); 1HNMR (500 MHz, DMSO-d6): δ (ppm): 1.58 (m, 2H, CH2), 1.96 (t, 2H, CH2), 2.83 (t, 2H, CH2), 6.88–7.42 (m, 4H, Ar), 7.31 (s, 2H, NH2), 10.24 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ (ppm): 19.2–28.6–36.5–45.4–58.8–107.8–116.9–122.0–125.2–127.7–127.9–130.1–141.0–155.1–160.1–168.7–200.3
2-amino-2′,5-dioxo-5H-spiro(cyclopenta(b)pyran-4,3′-indoline)-3-carbonitrile (4c)
White solid, (yield 89%), m.p 303–305 °C, IR (KBr) (νmax/cm−1) 3339 (NH2), 3142 (NH), 2183 (CN), 1711 (C=O), 1641 (C=O); 1HNMR (500 MHz, DMSO-d6): δ (ppm): 1.87 (t, 2H, CH2), 3.01 (t, 2H, CH2),6.93-7.41 (m, 4H, Ar), 7.36 (s, 2H, NH2), 10.44 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ (ppm): 28.3–32.2–46.9–58.3–108.7–117.6–122.6–125.1–127.8–128.2–130.0–142.4–159.1–160.4–169.3–203.7
2-amino-1′-methyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitrile (4m)
White solid, (yield 92%), m.p 252–254 °C, 1HNMR (500 MHz, DMSO-d6): δ (ppm): 1.91 (t, 2H, CH2), 2.18 (t, 2H, CH2), 2.66 (t, 2H, CH2), 3.14 (s, 3H, CH3), 6.96–7.24 (m, 4H, Ar),7.28 (s, 2H, NH2); 13C-NMR (125 MHz, DMSO-d6): δ (ppm): 20.20–26.80–27.18–36.77–46.99–57.27–108.53–112.28–117.67–122,83–123.39–128.81–134,13–149.96–159.20–166.60–177.09–195.45.
Ethyl-2-amino-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carboxylate (4n)
White solid, (yield 92%), m.p 247–249 °C, 1HNMR (500 MHz, DMSO-d6): δ (ppm): 0.79 (t, 3H, CH3), 1.88 (t, 2H, CH2), 2.19 (m, 2H, CH2),2.64 (t, 2H, CH2), 3.70 (q, 2H, CH2), 6.66–7.05 (m, 4H, Ar), 7.86 (s, 2H, NH2), 10.15 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ (ppm): 13.75–20.11–27.39–38.56–59.27–108.48–114.70–120.96–122.88–127.57–136.57–144.49–159.45–164.64–168.12–180.33–195.24.
Ethyl-2-amino-1′-methyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carboxylate (4o)
White solid, (yield 92%), m.p 236–238 °C, 1HNMR (500 MHz, DMSO-d6): δ (ppm): 0.73 (t, 3H, CH3), 1.88 (t, 2H, CH2), 2.17 (m, 2H, CH2),2.65 (t, 2H, CH2), 3.11 (s, 3H, CH3), 3.66 (q, 2H, CH2), 6.83–7.17 (m, 4H, Ar), 7.89 (s, 2H, NH2); 13C-NMR (125 MHz, DMSO-d6): δ (ppm): 13.95–20.08–26.55–27.36–37.46–59.18–107.22–114.61–121.76–122.69–127.87–135.62–145.71–159.61–164.85–167.97–178.70–195.32.
7′-amino-2,2′,4′-trioxo-1′,2′,3′,4′-tetrahydrospiro[indoline-3,5′-pyrano[2,3-d]pyrimidine]-6′-carbonitrile (4p)
Yellow solid, (yield 92%), m.p 282-285 °C; 1HNMR (500 MHz, DMSO-d6): δ (ppm): 6.78–7.17 (m, 4H, Ar), 7.34 (s, 2H, NH2), 10.46 (s, 1H, NH), 11.06(s, 1H, NH),12.20 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ (ppm): 46/96–58.26–86.77–109.36–117.32–122.29–123.95–128.61–133.88–142.57–149.84–153.84–158.79–162.11–178.07.
Ethyl-7′-amino-2,2′,4′-trioxo-1′,2′,3′,4′-tetrahydrospiro[indoline-3,5′-pyrano[2,3-d]pyrimidine]-6′-carboxylate (4q)
White solid, (yield 92%), m.p 229–232 °C; 1HNMR (500 MHz, DMSO-d6): δ (ppm): 0.78 (s, 3H, CH3), 3.71 (q, 2H, CH2), 6.67–7.08 (m, 4H, Ar), 7.97 (s, 2H, NH2), 10.03 (s, 1H, NH),12.18 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ (ppm): 13.30–22.58–44.19–58.78–89.19–108.38–121.09–123.05–127.64–135.32–149.61–152.25–158.41–161.88–167.68–179.57.
7′-amino-1-methyl-2,2′,4′-trioxo-1′,2′,3′,4′-tetrahydrospiro[indoline-3,5′-pyrano[2,3-d]pyrimidine]-6′-carbonitrile (4r)
White solid, (yield 92%), m.p 308–311 °C; 1HNMR (500 MHz, DMSO-d6): δ (ppm): 3.14 (s,3H,CH3), 6.90–7.29 (m, 4H, Ar), 7.40 (s, 2H, NH2), 11.08 (s, 1H, NH),12.26 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ (ppm): 26.72–87.05–108.05–110.71–122.82–123.78–125.93–129.03–133.10-138.04–144.04–149.75–154.01–158.95–161.77–176.60.
Ethyl-7′-amino-1-methyl-2,2′,4′-trioxo-1′,2′,3′,4′-tetrahydrospiro[indoline-3,5′-pyrano[2,3-d]pyrimidine]-6′-carboxylate (4s)
White solid, (yield 92%), m.p 236–238 °C; 1HNMR (500 MHz, DMSO-d6): δ (ppm): 0.72 (s, 3H, CH3), 3.02 (s, 3H, CH3), 3.60 (q, 2H, CH2), 6.85–7.19 (m, 4H, Ar), 7.97 (s, 2H, NH2), 10.03 (s, 1H, NH),12.18 (s, 1H, NH); 13C-NMR (125 MHz, DMSO-d6): δ (ppm): 13.49–56.22–61.56–76.46–89.79–108.68–120.64–123.27–127.64–135.53–144.19–145.84–149.54–152.85–158.29–161.80–167.75–179.71.
Results and discussion
In order to continue our previous attempts for electrosynthesis of heterocyclic compounds [20], in this work, a new strategy based on the electrocatalytic three-component transformation of cyclic-1,3-diketones (1a–d), malononitrile (2a) or ethyl cyanoacetate (2b), and isatins (3a-e) was reported for the synthesis of nano-sized pyran derivatives. The procedure was performed using constant-current electrolysis in an undivided cell with KBr as the supporting electrolyte. First, to optimize the reaction conditions, the three-component reactants, including cyclic-1,3-diketones (2H-indene-1,3-dione) (1a), malononitrile (2a), and isatin (3a) were followed in alcoholic media inside of an undivided cell as a pattern reaction.
The reaction was examined in different conditions such as different temperatures, several solvents (ethanol, methanol, and n-propanol) and various applied current. The platinum cathode along with the graphite anode in ethanol could upgrade the higher yields synthesis of pyran derivatives at shorter time with the current density of 20 mA cm−2 (I = 100 mA, electrode surface = 5 cm2). Ethanol was preferred as an alcoholic solvent at the temperature of 40 °C, instead of propanol and methanol. Electrolysis of cyclic-1,3-diketones (1a–d), malononitrile (2a) or ethyl cyanoacetate (2b), and isatins (3a–e) in ethanol at the constant current produced the nano-sized pyran derivatives (4a–s) (Table 1). In the next step, magnesium and iron were chosen as the anode and the cathode electrodes respectively in the different alcoholic electrolytes (Table 2). Then, the obtained products were defined using scanning electron microscopy (SEM) which verified the nano-sized 4H-pyran derivative synthesis in the form of tunable nanoparticles (Fig. 1). It is suggested that the application of magnesium as the anode electrode and iron as the cathode electrode might be causing the production of the nano-sized pyran derivatives. The optimized current density and temperature had no significant changes compared to the previous works, but the synthesis duration of 4a decreased from 65 min in previous woks to 50 min in this work (Table 2). In this research, to expand the area of the reaction, a comprehensive study was carried out on a wide range of pyran derivatives and almost all of the pyran derivatives were synthesized at the nano-sized scale. The electrolysis of the starting materials with magnesium as the anode and iron as the cathode in ethanol at the constant current (20 mA cm−2), were led to nano-sized pyran derivatives (4a–s) with 76–92% yields (Tables 1, 3).
At the first step, deprotonation of ethanol occurred in cathode which led to the formation of an ethoxide anion (Scheme 2). The next reaction occurred between the ethoxide anion and malononitrile, producing the malononitrile anion. Then, Knoevenagel condensation of the malononitrile anion with isatins (3a) took places through the removal of a hydroxide anion and formation of 2-(2-oxoindolin-3-ylidene)malononitrile (Scheme 3). The following hydroxide-promoted Michael addition of cyclic-1,3-diketones such as 1,3-Indandione (1a) resulted in the intermediate (5) which was followed by intramolecular cyclization and production of the corresponding 4H-pyran derivatives 4 through the regeneration of the ethoxide anion as the cyclic starting of the reaction with malononitrile.
Conclusion
The introduced nanoparticles of pyran derivatives were synthesized using a simple and effective electrosynthesis approach under the mild, rapid, and selective conditions. Besides the excellent yields of target compounds, the electrocatalytic procedure offers a reasonable combination of conventional three-component reactions with convenient ecological advantages and facile electrochemical methods. This method could be evaluated as a noteworthy alternative approach due to the mentioned advantages. The produced nanoparticles of 4H-pyran derivatives could be very effective especially in pharmacy and medicine.
References
Banerjee, S., Horn, A., Khatri, H., Sereda, G.: A green one-pot multicomponent synthesis of 4H-pyrans and polysubstituted aniline derivatives of biological, pharmacological, and optical applications using silica nanoparticles as reusable catalyst. Tetrahedron Lett. 52, 1878–1881 (2011)
Kumar, K.A., Renuka, N., Kumar, G.V., Lokeshwari, D.M.: The developments, exploration of new methodologies and varied biological activities of pyran analogs
Chem, J.: Pharm. Res. 7(11), 693–700 (2015)
Elinson, M.N., Dorofeev, A.S., Miloserdov, F.M., Nikishin, G.I.: Electrocatalytic multicomponent assembling of isatins, 3-methyl-2-pyrazolin-5-ones and malononitrile: facile and convenient way to functionalized spirocyclic [indole-3,4-pyrano[2,3-c]pyrazole] system. Mol. Divers. 13, 47–52 (2009)
Elinson, M.N., Ilovsaiky, A.I., Dorofeev, A.S., Merkulova, V.M., Stepanov, N.O., Miloserdov, F.M., Ogibin, Y.N., Nikishin, G.I.: Electrocatalytic multicomponent transformation of cyclic 1,3-diketones, isatins, and malononitrile: facile and convenient way to functionalized spirocyclic (5,6,7,8-tetrahydro-4Hchromene)-4,30-oxindole system. Tetrahedron 63, 10543–10548 (2007)
Wang, L., Gao, J., Wan, L., Wang, Y., Yao, C.: Electrocatalytic multicomponent transformation of cyclopentane-1,3-dione, aldehydes and malononitrile: an efficient way to cyclopenta[b]pyran derivatives. Res. Chem. Intermed. 41, 2775–2785 (2015)
Elinson, M.N., Dorofeev, A.S., Miloserdov, F.M., Ilovaisky, A.I., Feducovich, S.K., Belyakov, P.A., Nikishin, G.I.: Catalysis of salicylaldehydes and two different C–H acids with electricity: first example of an efficient multicomponent approach to the design of functionalized medicinally privileged 2-amino-4H-chromene scaffold. Adv. Synth. Catal. 350, 591–601 (2008)
Makarem, S., Fakhari, A.R., Mohammadi, A.A.: Electro-organic synthesis of nanosized particles of 2-amino-pyranes. Ind. Eng. Chem. Res. 51, 2200–2204 (2012)
Elinson, M.N., Llovaisky, A.I., Merkulova, V.M., Demchuk, D.V., Belyakov, P.A., Ogibin, Y.N., Nikishin, G.I.: The electrocatalytic cascade assembling of isatins, malononitrile and N-alkyl barbiturates: an efficient multicomponent approach to the spiro[indole-3,5-pyrano[2,3-d]pyrimidine] framework. Electrochim. Acta 53, 8346–8350 (2008)
Chattapadhyay, T.K., Dureja, P.: Antifungal activity of 4-methyl-6-alkyl-2H-pyran-2-ones. J. Agric. Food Chem. 54, 2129–2133 (2006)
Wang, T., Liu, J., Zhong, H., Chen, H., Lv, Z., Zhang, Y., Zhang, M., Geng, D., Niu, C., Li, Y., Li, K.: Synthesis and anti-tumor activity of novel ethyl 3-aryl-4-oxo-3,3a,4,6-tetrahydro-1H-furo[3,4-c]pyran-3a-carboxylates. Bioorg. Med. Chem. Lett. 21, 3381–3383 (2011)
Heravi, M.M., Baghernejad, B., Oskooie, H.A.: A novel and efficient catalyst to one-pot synthesis of 2-amino-4H-chromenes by methanesulfonic acid. J. Chin. Chem. Soc. 55, 659–662 (2008)
Safari, J., Zarnegar, Z., Heydarian, M.: Magnetic Fe3O4 nanoparticles as efficient and reusable catalyst for the green synthesis of 2-amino-4H-chromene in aqueous media. Bull. Chem. Soc. Jpn 85, 1332–1338 (2012)
Konkoy, C., Fick, D., Cai, S., Lan, N., Keana, J.F.: Substituted 5-oxo-5-6,7,8-tetrahydro-4H-1-benzopyrans and benzothiopyrans and the use thereof as potentiators Of AMPA, US Patent 6680332B1 (2001)
Armesto, D., Horspool, W.M., Martin, N., Ramos, A., Seaone, C.: Synthesis of cyclobutenes by the novel photochemical ring contraction of 4-substituted 2-amino-3,5-dicyano-6-phenyl-4H-pyrans. J. Org. Chem. 54, 3069–3072 (1989)
Balalaie, S., Bararjanian, M., Sheikh-Ahmadi, M., Hekmat, S., Salehi, P.: Diammonium hydrogen phosphate: an efficient and versatile catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran derivatives in aqueous media. Synth. Commun. 37, 1097–1108 (2007)
Montazeri, N.: An efficient catalyst for the multi-component synthesis of Pyrano [2, 3-d] Pyrimidinone derivatives. Int. J. Nano Dimens. 6(3), 283–287 (2015)
Mirza, B., Mirzazadeh, R., Zeeb, M.: A new and efficient synthesis of 2-aminobenzothiazoles derivatives from o-nitroaniline. J. Chem. Res. 37, 778–779 (2013)
Mirza, B., Zeeb, M.: CuBr-catalyzed oxidation/coupling: an efficient and applicable strategy for the synthesis of 2-aryl benzimidazoles from 1-fluoro-2-nitrobenzene and benzylamines. Synth. Commun. 45, 524–530 (2015)
Mirza, B.: An efficient method for the one-pot tandem synthesis of 3,5-disubstituted1,2,4-oxadiazoles from benzyl halides. J. Chem. Res. 39, 373–375 (2015)
Mohammad Darvish, Z., Mirza, B., Makarem, S.: Electrocatalytic multicomponent reaction for synthesis of nanoparticles of spirooxindole derivatives from isatins, malononitrile, and dimedone. J. Heterocycl. Chem. 54, 1763–1766 (2017)
Dandia, A., Singh, R., Singh, D.: Facile one-pot synthesis of new annulated hexacyclic ring system indeno-pyrano-furo-indoles and spiro indenopyran-indoles under microwave irradiation. Indian J. Chem. 48, 1001–1005 (2009)
Hari, G.S., Lee, Y.R.: Efficient one-pot synthesis of spirooxindole derivatives by ethylenediamine diacetate catalyzed reactions in water. Synthesis 3, 453–464 (2010)
Javanshir, S., Pourshiri, N.S., Dolatkhah, Z., Farhadnia, M.: Caspian Isinglass, a versatile and sustainable biocatalyst for domino synthesis of spirooxindoles and spiroacenaphthylenes in water. Monatsh. Chem. 148, 703–710 (2017)
Wu, C., Shen, R., Shen, J., Hu, C.: An efficient method for multicomponent synthesis of spiro[4H-pyran-oxindole] derivatives catalyzed by magnesium perchlorate. Bull. Korean Chem. Soc. 34, 2431–2435 (2013)
Li, Y., Chen, H., Shi, Ch., Shi, D., Ji, S.: Efficient one-pot synthesis of spirooxindole derivatives catalyzed by l-proline in aqueous medium. ACS Comb. Sci. 12, 231–237 (2010)
Elwarraky, L.M., Abdel-Fatah, M.A., Gary, B.D., Piazza, G.A., Abadi, A.H.: an efficient and green one-pot synthesis of novel spirooxindole derivatives with potential anti-tumor activity in an aqueous solvent. Chem. Rapid Commun. 2, 33–40 (2014)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Taheri, M., Mirza, B. & Zeeb, M. Electrosynthesis of nano-sized pyran and chromene derivatives by one-pot reaction between cyclic-1,3-diketons, malononitrile/ethyl cyanoacetate, and isatins. J Nanostruct Chem 8, 421–429 (2018). https://doi.org/10.1007/s40097-018-0282-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-018-0282-5