Abstract
Respiratory disease impacts significantly on health economics, and the burden of disease increases with age. A significant proportion of patients will present to acute hospital services with respiratory failure that requires prompt and appropriate management. Multiple options are available to deliver supplemental oxygenation from simple nasal prongs to non-invasive ventilation. The development of high-flow humidified oxygen systems and non-invasive ventilation has had a significant positive impact on the care of patients in respiratory failure. However, it is of crucial importance that individual patients are taken into account as some interventions may not be appropriate. Acceptable outcomes should be documented, and if necessary, a ceiling of care should be agreed so that futile therapies are not implemented.
Similar content being viewed by others
Introduction
Respiratory diseases incurs vast economic and health costs to western society, as demonstrated by the second edition of the Burden of Lung Disease publication [1•]. This showed that lung infections (pneumonia and tuberculosis) with COPD and lung cancer accounted for 9.5 million deaths worldwide and overall represents one tenth of the disability adjusted life years lost in 2008 alone.
In the same period, a department of health review in the UK demonstrated that the cost for respiratory care was nearly 4,300,000 pounds ($6,494,182,500). Respiratory diseases kill one in five people, more than ischaemic heart disease, and lung cancer kills more women than breast cancer. A high proportion of these diseases will manifest with respiratory failure.
Currently, approximately 20 % of the general population over the age of 70 years carry a diagnosis of COPD [2] and the incidence of pneumonia, pulmonary embolism, malignancy and interstitial lung disease (ILD) have all been shown to increase with age.
Respiratory failure can occur via several different mechanisms.
Pneumonia or atelectasis may result in a right to left shunt, wherein non-ventilated alveoli are perfused and there is a resultant drop in oxygen concentration in arterial blood. All individuals have a physiological ventilation/perfusion mismatch; however, conditions such as pulmonary emboli and obstructive lung disease will exaggerate this mismatch and result in worsening hypoxaemia. In the case of interstitial lung disease, there is a prolonged time needed for oxygen to diffuse across the alveolar wall and into the blood. Often, this does not cause significant problems at rest; however, in conditions when there is physiological stress, there is insufficient time for oxygenation to occur and hypoxaemia occurs. Hypoventilation, which can be related to obesity, iatrogenic neurological/neuromuscular in origin, causes a rise in partial pressure of carbon dioxide and therefore a decrease in partial pressure of oxygen. As many mechanisms can result in hypoxaemia, the first step in the management of the condition is to correctly identify the primary cause of the hypoxaemia. This allows therapies to be instigated that are most appropriate to correct it.
In this review article, we will discuss the different methods of supplemental oxygenation and ventilation available and their appropriate use as well as their limitations and possible adverse effects.
Nasal Cannula—Indications and Limitations
The first line method of oxygen (O2) delivery in acute respiratory failure is frequently via nasal cannula. This is a simple and well-tolerated method; however, it is limited in the accuracy and maximum concentration of FiO2 achieved. The formula is FiO2 = 20 % + (4 × oxygen litre flow); however, this is variable depending on the patient’s respiratory effort, rate of breathing and whether the patient is mouth or nose breathing. Delivery of >5 L/min of oxygen does not significantly increase the inspired concentration of O2 and may result in nosebleeds due to the lack of humidification [3].
Face Mask
If nasal cannula is not able to delivery enough O2 to maintain a SaO2 or arterial pO2 > 8 kPa and there are no concerns regarding type 2 respiratory failure (chronic hypoxic state such as occurs in COPD), then a simple face mask is a reasonable alternative. It can deliver a FiO2 of 24–60 % at a flow rate of 5–15 L/min. As with nasal cannula, the FiO2 is not constant and is influenced by respiratory rate and respiratory effort. [4] Use in the acute setting when sepsis is present, especially in a geriatric population, may worsen delirium as it can be uncomfortable and hinder communication and coughing.
Venturi Mask
The Venturi effect is the resultant drop in pressure when a gas or liquid passes through a narrow space. Venturi masks have fenestrations allowing for entrainment of room air, therefore diluting the oxygen concentration to a set figure, depending on the size of the fenestration and rate of O2 flow (Table 1). When there is concern regarding hypercapnoea, the use of a Venturi mask is appropriate as the concentration of oxygen does not change with the patient’s rate or depth of breathing. This allows for controlled oxygen delivery and maintenance of SaO2 88–92 %.
One Hundred Percent Non-rebreather Masks
In an emergency setting, when a patient is hypoxic and oxygen requirements are high, a face mask with a non-rebreather chamber is commonly used. This allows for delivery of up to 90 % FiO2. The disadvantages are similar to that of a normal face mask. When this method of oxygen delivery is employed, an ABG should be obtained as soon as possible to assess oxygenation and monitor for carbon dioxide retention.
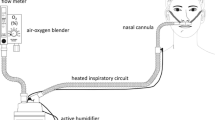
High-Flow Humidified Oxygen—e.g. Airvo™
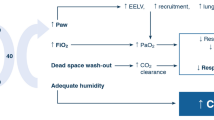
High-flow oxygen therapy is primarily administered via the nasal route; however, both tracheostomy and face mask attachments are available. It allows for the delivery of up to 60 L/min with adjustable FiO2 and humidification. High flow has a number of proposed physiological effects including:
-
i)
Reduction of CO2 in the anatomical dead space [5, 6]
In an animal study, it was shown that as the rate of flow increased, the PaCO2 decreased. This effect was more profound when the patient exhaled through an open mouth. Moller et al. in 2015 used anatomical upper airway with nasal high flow. The levels of clearance in the nasal cavities increased by 1.8 mL/s for every 1.0 L/min increase in flow. This study demonstrated the rapid clearance of the nasal cavities by nasal high-flow therapy. This in turn reduces the amount of dead space rebreathing.
-
ii)
PEEP effect
Due to the constant rate of high flow, there is a resultant increase in end expiratory pressure. This is variable, and there are multiple factors which affect the PEEP achieved including flow rate, BMI, gender and breathing with an open or closed mouth [7••]. Parke et al. demonstrated that with the mouth closed, breathing and a flow rate of 80 L/min can generate a maximum PEEP of 4.5 cm H2O.
-
iii)
Humidification
Humidification of the inspired O2 allows for better tolerance of oxygen at high-flow rates and may also assist with clearance of secretions.
High-flow humidified oxygen is primarily used in patients with type 1 respiratory failure and is particularly useful when there is a ceiling of care established due to its tolerability versus full face mask and non-invasive ventilation. There is some evidence that there may be a role for use in type 2 respiratory failure; however, non-invasive ventilation in the form of bilevel positive airway pressure (BPAP) should be used in preference.
Heliox
Heliox is a mixture of 79 % helium and 21 % oxygen and has a gas density nearly six times less than that of atmospheric air. This lower density results in increased laminar flow, decreased turbulence and therefore lower airflow resistance. Heliox is not currently used in routine clinical practice; however, there is evidence supporting its use in upper airway obstruction and, to a lesser extent, obstructive lung disease (asthma, COPD). Whilst the physiologic effect is less in smaller airways [8], in the setting of COPD, a reduction in respiratory rate reduces the effects of dynamic hyperinflation with significant subjective improvement of dyspnoea.
Non-invasive Ventilation (NIV)—Acute and Long Term
The use of non-invasive ventilation, which can be continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), has significantly increased since the mid 1990s. They both provide positive end expiratory pressure, assisting oxygenation and can be entrained with supplemental oxygen. CPAP is set at a fixed pressure and is primarily used in type 1 respiratory failure. This is in contrast to BiPAP which has a set inspiratory pressure and different expiratory pressure. The expiratory positive airway pressure (EPAP) provides assisted oxygenation, and the inspiratory positive airway pressure (IPAP) allows for better ventilation and therefore reduction in carbon dioxide. The advent of NIV has seen a significant decrease in mortality and intubation rates as well as offering another modality of treatment for patients with a do-not-resuscitate order. There are some contraindications and risks with NIV which are listed in Table 2.
Acute Indications for NIV
Type 1 Respiratory Failure
When a patient is hypoxaemic and not responding to supplemental oxygen therapy, such as occurs with pneumonia or pulmonary oedema, a trial of non-invasive ventilation in conjunction with the primary therapy is an option. The evidence regarding this is somewhat contradictory [9–11].
NIV is especially useful when a ceiling of care has been set and the patient is not for intubation. If intubation is appropriate, we would suggest that NIV should only be used in the interim whilst admission to the intensive care unit is obtained. The patient will need close monitoring to assess for failure or non-compliance. Common troubleshooting problems include intolerance due to poor fit or claustrophobia and excessive leak. These are often readily remedied with a different mask and “ramping” the pressure to allow time to acclimatise.
Type 2 Respiratory Failure with Acidosis
BiPAP should be used as first-line management in a patient with type 2 respiratory failure with acidosis. Initial settings should be EPAP 4–6 and IPAP 10–12, and a repeat arterial blood gas should be performed within 30–60 min to assess for response. The aim is PaO2 > 8 kPa, a reduction in PaCO2 and a trend towards normalisation of pH. If the PaCO2 remains high, the pH remains low, but the PaO2 is >8 kPa, the IPAP should be increased alone. If the PaO2 is also low, both the IPAP and EPAP should be increased but disproportionately as if the pressure difference does not alter, it is unlikely to result in an improvement of the pH. If the patient is not tolerating BiPAP or there is no improvement in the ABG after 1–2 h, early consideration should be given to intubation and mechanical ventilation as delay is associated with increased morbidity and mortality.
Long-Term NIV
In stable COPD, nocturnal NIV may be used to provide respiratory support. There is again conflicting evidence regarding this with studies showing variable effects on mortality and quality of life compared to treatment with standard inhaled therapy [12, 13 14••].
Patients for whom domiciliary nocturnal NIV is prescribed include the following:
-
Patients established on BiPAP in the setting of an acute exacerbation who have not successfully weaned
-
Patients with nocturnal desaturation despite supplemental oxygen
-
Patients with recurrent hypercapnia with acidosis
Obstructive sleep apnoea (OSA) and obesity hypoventilation syndrome (OHS) are being increasingly recognised, thanks to increased awareness of these conditions in recent years. Whilst the increasingly obese population mostly referenced is children and young adults, this epidemic is also affecting the geriatric population. In America, an estimated 40 % of the population between 60 and 70 years old have a BMI of greater than 30 and 30 % of people aged 70–79 are obese [15]. As a result, there is an associated increase in incidence of these sleep-related disorders.
OSA is diagnosed following a sleep study when the apnoea/hypopnoea index (AHI) >5, although therapy with CPAP is often not instituted until the AHI is >15, or in cases where the patient is excessively somnolent and there is an immediate risk to the patient or society, such as for long-distance drivers.
Following a diagnostic sleep study, the patient should undergo a repeated test with autotitration of CPAP to establish the pressure required to suppress the AHI to <5.
OHS is diagnosed when an obese patient has type 2 respiratory failure without an alternative cause (neurological, pulmonary, cardiac, etc.) The primary treatment is weight loss; however, BiPAP ± supplemental oxygen should be commenced whilst waiting for significant weight loss/bariatric surgery as untreated OHS conveys a significant mortality. Supplemental oxygen without positive pressure ventilation is contraindicated as it may worsen hypercapnia and acidosis and is unlikely to convey significant oxygenation.
Invasive Ventilation
Ventilating the elderly has a number of important differences versus ventilating a younger patient. Common differences include decreased lung and chest wall compliance, increased ventilation/perfusion mismatch, poor cough and poor nutrition.
Indications for ventilation via an endotracheal tube (ET) include failure of NIV; severe acidosis (<7.25); inability of the patient to protect their own airway—e.g. decreased level of consciousness; inability to cope with respiratory secretions; and mechanical issues such as facial trauma and central masses resulting in stridor.
There are numerous complications of invasive ventilation.
-
Barotrauma refers to alveolar rupture due to excessive pressure and can manifest as a pneumothorax, subcutaneous emphysema and rarely pneumomediastinum.
-
Ventilator-associated pneumonia is defined as a pneumonia which occurs 48 h post intubation. It has a variable incidence, depending on the indication for ventilation (9–27 %) [16]
-
Ventilator-associated lung injury (VALI) occurs in up to one in four mechanically ventilated patients. It is difficult to clinically distinguish between VALI and acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). It is thought to be caused by a combination of volutrauma (alveolar overdistension) and atelectotrauma (recruitment/derecruitment causing injury). It manifests as pulmonary oedema, pulmonary haemorrhage and loss of functional surfactant causing collapse.
-
Atrophy of diaphragm and respiratory muscles occurs in prolonged ventilation and can present significant problems when attempts are made to wean from invasive ventilation.
If a prolonged need for mechanical ventilation is expected or there is difficulty weaning the patient from mechanical ventilation, a tracheostomy is often performed.
A tracheostomy conveys multiple benefits over an ET tube such as increased comfort, ease of suctioning and allows for the patient to be transferred out of the intensive care unit. A tracheostomy also allows for easier patient communication. There is a possible increased risk of pneumonia however, there can be complications with the stoma site, and decannalisation prior to tract formation may be fatal.
Multiple different types of tracheostomy catheters, including cuffed, uncuffed and fenestrated are available, all of which serve different purposes and are used in different circumstances—acute versus chronic use, depending on the patient’s cough, etc. These can be used as a conduit for simple oxygen therapy to mechanical ventilation.
If a tracheostomy is no longer required for mechanical ventilation, an attempt should be made to wean and remove. However, this is a complex decision and should be made by a multi-disciplinary team including the physician/surgeon, anaesthetist, speech therapists and physiotherapy after discussion and informed consent from the patient. Weaning can be a prolonged process and is not without associated risk. Patients should be informed of symptoms they can expect to experience, such as an unusual sensation on inspiration, and counselled when to call for help—dyspnoea, inability to clear secretions. The tracheostomy should be deflated for a period of 24 h without incident prior to decannulation. The tube should also be capped for a period of 24 h to ensure that the patient is comfortable with oral breathing again and that they can manage their secretions. Once they have tolerated capping for 24 h, the tracheostomy can be removed safely.
High-Flow Transtracheal Catheters
When there is persistent hypoxaemia <7.3 kPa despite maximal therapy in a stable patient or when the PaO2 is <7.8 kPa with evidence of pulmonary hypertension, secondary polycythaemia or cor pulmonale, long-term oxygen therapy (LTOT) should be started.
The standard for delivering oxygen in LTOT is via nasal cannulae; however, many patients dislike wearing nasal cannulae and this results in decreased compliance. An alternative, whilst not yet in common practice in Europe, is LTOT via a transtracheal catheter.
There are several contraindications to transtracheal oxygen therapy which include the following:
-
1)
Abnormal upper airway anatomy
-
2)
Severe anxiety
-
3)
Prior non-compliance with therapy
-
4)
Acute respiratory failure
-
5)
Comorbidities that would limit maintenance of catheter
Relative contraindications include the following:
-
1)
Difficulty controlling secretions
-
2)
Coagulopathy
-
3)
Immunocompromised and poor healing
-
4)
High oxygen requirements and poor physiological reserve
Once patients have been selected and educated regarding transtracheal oxygen, a tracheocutaneous fistula is created. This can be a surgical (Lipkin procedure) or percutaneous fistula via a modified Seldinger technique.
Following the creation of the tract, a catheter is inserted into the immature fistula and secured via a necklace device. In the initial stages, care must be taken regarding maintenance of the catheter in order to prevent symptomatic mucus plugs, monitor for subcutaneous emphysema and avoid tract loss.
Conclusion
Given that the methods of administering respiratory support are so variable in accuracy, efficacy and levels of invasion, it is vitally important that the patient’s physiological reserve, prognosis and wishes are taken into account prior to proceeding. This is especially true when ventilating via an endotracheal tube, and whilst age is never an absolute contraindication to intubation, it needs to be a carefully considered decision between the physician and patient/family.
Unfortunately, an appropriate ceiling of care is often not established until emergency management is needed, at which stage it is too late to have an informed and rational conversation regarding outcomes. It is essential that patient dignity is always maintained. There is a large degree of variation in the incidence of advanced directives—in the USA, it can be as high as 70 %; however, in Europe, it is often less than 10 % [17, 18]. The provision of oxygenation and ventilator support is complex and associated with ethical and medicolegal dilemmas.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• ERS whitebook—burden of lung disease. The white book presents a rigorous examination of lung health and disease in Europe as it stands and an informed analysis of future trends.
Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–50.
Tiep BL. Continuous flow oxygen therapy. In: Tiep BL, editor. Portable oxygen therapy: including oxygen conserving methodology. Mt. Kisco, Futura Publishing Co. p. 205–220
Burton GG, Hodgkin JE, Ward JJ. Respiratory care—a guide to clinical practice. 4th ed. Philidelphia: Lippincott-Raven Pub co; 1997. p. 381–95.
Frizzola M, Miller TL, Rodriguez ME, Zhu Y, Rojas J, Hesek A, et al. High-flow nasal cannula: impact on oxygenation and ventilation in an acute lung model. Pediatr Pulmonol. 2011;46:67–74.
Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care. 2005;50(5):604–9.
•• Ritchie JE, Williams AB, Gerard C, Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care. 2011;39:1103–10. This paper demonstrated that high gas flow enabled an accurate inspired oxygen fraction to be delivered. The positive mean airway pressure created by the high flow may serve as a bridge to formal positive pressure systems.
Colebourn CL, Barber V, Young JD. Use of helium-oxygen mixture in adult patients presenting with exacerbations of asthma and chronic obstructive pulmonary disease: a systematic review. Anaesthesia. 2007;62:34–42.
Schettino G, Altobelli N, Kacmarek RM. Noninvasive positive-pressure ventilation in acute respiratory failure outside clinical trials: experience at the Massachusetts General Hospital. Crit Care Med. 2008;36(2):441.
Delclaux C, L’Her E, Alberti C, Mancebo J, Abroug F, Conti G, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000;284(18):2352.
Keenan SP, Sinuff T, Cook DJ, Hill NS. Does noninvasive positive pressure ventilation improve outcome in acute hypoxemic respiratory failure? a systematic review. Crit Care Med. 2004;32(12):2516.
McEvoy RD, Pierce RJ, Hillman D, Esterman A, Ellis EE, Catcheside PG, et al. Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009;64(7):561.
Casanova C, Celli BR, Tost L, Soriano E, Abreu J, Velasco V, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest. 2000;118(6):1582.
•• Köhnlein T, Windisch W, Köhler D, Drabik A, Geiseler J, Hartl S, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698. This large multicentre study demonstrated that the addition of long-term NPPV to standard treatment improves survival of patients with hypercapnic, stable COPD when NPPV is targeted to greatly reduce hypercapnia.
Gallagher Camden S, Gates J. Obesity: changing the face of geriatric care. Ostomy Wound Manage. 2006;52(10):36–8. 40–4.
Hunter JD. Ventilator associated pneumonia. BMJ. 2012;344:e3325.
Silveira MJ, Kim SYH, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362:1211–8.
Aw D, Hayhoe B, Smajdor A, Bowker LK, Conroy SP, Myint PK. Advance care planning and the older patient. QJM. 2012;105(3):225–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Niki Byrne, Tara Cahill, Vincent Brennan and David Breen declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pulmonology and Respiratory Care
Rights and permissions
About this article
Cite this article
Brennan, V., Cahill, T., Byrne, N. et al. Oxygen Therapy in the Elderly: When Nasal Cannula Is Not Enough. Curr Geri Rep 5, 283–288 (2016). https://doi.org/10.1007/s13670-016-0192-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13670-016-0192-7