Abstract
Advances in surgical and anesthetic techniques have led to steady reductions in perioperative complications. At the same time, improvements in public health and medical care have increased quality of life and life expectancy. As a result, an expanding population of elderly patients with chronic medical diseases is undergoing surgical procedures. Thorough risk assessment and management is required to assure optimal perioperative care for geriatric patients. This includes a full pulmonary evaluation, which is often over-shadowed by cardiovascular concerns. Recent literature has provided improved clarity in preoperative pulmonary risk assessment and potential options for risk management interventions. In this paper, we detail the current evidence for perioperative pulmonary care of elderly patients.
Similar content being viewed by others
Introduction
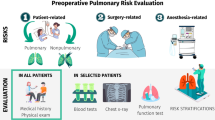
As the surgical population continues to age and increase in medical complexity, the potential for significant complications similarly rises. Although perioperative risk management often focuses on the cardiovascular system, pulmonary complications account for a larger proportion of adverse surgical outcomes and are the leading cause of postoperative mortality [1, 2]. Postoperative pulmonary complications (PPCs), including respiratory failure (prolonged mechanical ventilation or unplanned reintubation), pneumonia, chronic obstructive pulmonary disease (COPD) exacerbation, and atelectasis, also contribute substantially to hospital costs and readmissions [3, 4]. The elderly population is especially prone to these complications as evidenced by the correlation between PPCs and advanced age, cognitive impairment, and functional dependence [2, 5, 6••, 7••]. Newer risk prediction tools may improve identification of patients at risk for PPCs, and recent studies demonstrate the utility of several risk reduction strategies.
Risk Assessment
Established Risk Factors for PPCs
Risk factors for PPCs can be categorized into patient-related and procedure-related issues. Among patient-related risk factors, the most important is advancing age, with risk increasing after age 50 [8]. The odds ratio (OR) of PPCs in patients aged 50–59 is 1.50 and increases by decade with an OR for PPCs of 5.63 in patients over age 79 [8]. The American Society of Anesthesiologists (ASA) Physical Status Classification System is a graduated scoring system of patient comorbidity and also correlates with postoperative complications [9]. Higher scores, even a score of two (mild or controlled systemic disease) or greater, are associated with a substantial increase in the risk for PPCs [6••, 7••, 8]. Congestive heart failure (CHF), functional dependency, chronic lung disease, and impaired sensorium are also recognized risk factors for PPCs common in the elderly population [2, 10]. A number of other common conditions which increase the risk of respiratory complications are listed in Table 1.
The surgical site is the most significant procedure-specific predictor of PPCs [6••, 7, 8]. Due to their effect on pulmonary physiology, procedures closest to the diaphragm carry the greatest risk for PPCs. Vital capacity, residual volume, and functional residual capacity are reduced in thoracic and upper abdominal surgeries [11]. Patients who undergo abdominal surgery may develop postoperative diaphragmatic dysfunction that contributes to these changes [12].
Additional procedure-related risk factors include a surgical duration longer than 3–4 h and emergency surgery. There is still some debate about the risk of anesthetic technique. However, general anesthesia appears to confer a greater risk of PPCs and mortality compared to regional anesthesia [8, 13]. In addition, residual neuromuscular blockade is related to critical respiratory events immediately after surgery [14]; this is due to hypoventilation and decreased lung volumes in the immediate postoperative period. The use of shorter-acting agents may improve outcomes.
Emerging Risk Factors for PPCs
Although earlier studies did not identify pulmonary hypertension (PH) and obstructive sleep apnea (OSA) as significant risk factors for PPCs, more recent literature has shed light on the impact of these conditions in relation to perioperative morbidity and mortality.
Obstructive Sleep Apnea
Obesity has become more prevalent in our society; an estimated 35 % of the population is obese [15]. Given the clear relationship between obesity and OSA, it is estimated that a large number of patients with moderate to severe OSA are undiagnosed [16] and may potentially proceed to surgery without treatment. Indeed, studies of elective surgery patients screened for OSA with the Berlin and STOP (snoring, daytime tiredness, observed apnea during sleep, hypertension) questionnaires reveal that approximately 24–27 % of the elective, non-cardiothoracic surgical population is at risk for OSA [17, 18]. The surgical period poses additional risk for patients with OSA because anesthetics and other common perioperative medications can decrease respiratory drive and impair arousal, leading to higher rates of apnea and hypopnea. Recent studies have confirmed that OSA is a risk factor for perioperative pulmonary and cardiac complications as well as mortality. A study by Hwang et al. [19] demonstrated that patients with at least two out of four signs of sleep apnea (snoring, witnessed apneas, daytime somnolence, crowded oropharynx) and nocturnal desaturations had increased rates of pulmonary, cardiac, and bleeding complications. In additional studies, preoperative OSA is associated with overall complications [20, 21], postoperative respiratory failure and reintubation [20–22], cardiac complications [21, 23], ICU transfers [20, 23], and prolonged length of stay [21, 23].
Pulmonary Hypertension
Similar to OSA, previous database studies of PPC predictors did not identify PH as an independent risk factor. However, multiple recent publications have identified PH as a risk factor for postoperative morbidity and mortality after major non-cardiac surgery [24, 25, 26, 27•, 28, 29]. PH may be exacerbated by increased peripheral vascular resistance caused by common perioperative conditions, such as hypoxia, acidosis, hypercapnia, and positive pressure ventilation. Orthopedic surgery involves potential intraprocedural embolization of bone marrow, bone debris, and cement, which also increase pulmonary pressures [26•]. Fluid shifts and pharmacologic agents (including many anesthetics and sympathomimetics) may also contribute to perioperative complications in the patient with PH [27•].
In non-cardiac surgeries, patients with PH have 30-day mortality rates ranging from 3.5 to 7 % and up to 15 % for emergent cases [24, 28]. In a large sample of hip and knee arthroplasty patients with an average age above 65 years, mortality was at least four times greater in patients with PH than among case-matched controls [22]. Additionally, respiratory failure rates are estimated at 21–28 % in PH patients, and the incidence of heart failure, readmissions, prolonged ICU stay, and sepsis are also increased compared to patients without PH [25, 29].
Because of the potential for poor outcomes in these patients, the preoperative assessment of the patient with PH includes a careful risk-benefit analysis of the need for surgery and the risk for postoperative morbidity and mortality. If surgical intervention is undertaken, careful optimization in specialized centers with expertise in the management of patients with PH may reduce risk [30•].
Risk Prediction Tools
Multiple surgical risk calculators have been developed to predict perioperative mortality and morbidity, including pulmonary complications [6••, 7••, 10, 31, 32]. Risk calculators can be valuable tools during the preoperative evaluation to provide a more concrete estimate of risk and a basis for counseling patients and other physicians. Previous risk assessment tools for PPCs have been challenging to use or focused on specific patient populations [10, 31], necessitating updated tools applicable to clinical practice.
Two newer perioperative pulmonary risk calculators by Gupta and colleagues were developed using data from the National Surgical Quality Improvement Project database to identify the risk of postoperative respiratory failure and pneumonia [6••, 7••]. Risk factors used in the postoperative respiratory failure model included procedure type and urgency, ASA classification, dependent functional status, and preoperative sepsis [7••]. For postoperative pneumonia age, ASA classification, COPD, dependent functional status, preoperative sepsis, smoking status, and the type of operation were the most significant risk predictors and were included in the model [6••]. For both models, the authors created easy-to-use spreadsheet calculators (online versions available at http://www.surgicalriskcalculator.com/) that provide an estimation of postoperative respiratory failure and pneumonia risk. Although the database used to create these models lacked some potentially important variables, such as OSA and PH, the risk models were validated with additional data sets and have excellent predictive performance.
The Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) index is another newer calculator that uses seven independent risk factors to assess the individual risk of PPCs [32]. These risk factors include advancing age, low preoperative arterial oxygen saturation, acute respiratory infection during the previous month, preoperative anemia, upper abdominal or intrathoracic surgery, surgical duration of at least 2 h, and emergency surgery. Using a weighted scoring system, the risk of PPCs is stratified as low, intermediate, or high risk with an estimation of the incidence of PPCs for each of these categories. Benefits of the ARISCAT index include the ease of use without the assistance of electronic devices, the inclusion of readily available clinical criteria, and its validation in a separate patient population [33•]. The calculator has the disadvantage of including minor complications (e.g., bronchospasm) in the compiled definition of PPCs [32].
Despite evidence that it confers risk for poor outcomes, OSA remains unrepresented in available pulmonary risk indices. The STOP-BANG questionnaire (STOP plus BMI >35 kg/m2, age >50 years, neck circumference >40 cm, and male gender) is easy to use and identifies patients at risk for OSA and PPCs [34, 35••]. Lockhart and co-authors evaluated the impact of preoperative screening for OSA and PPCs by screening patients for OSA with the Berlin, STOP, Flemons, and STOP-BANG tools [36•]. The different tools had poor concordance in estimating OSA risk, and after multivariate analysis, none predicted 30-day or 1-year mortality. However, STOP-BANG predicted rates of postoperative complications. In another study, surgical patients whose STOP-BANG scores were ≥5 had a high probability of moderate to severe OSA [35••]. While this method is sensitive for OSA, its specificity is relatively poor [37•]. A recent study demonstrated that a serum bicarbonate level of ≥28 mmol/L significantly improves the specificity of predicting moderate to severe OSA [37•]. The authors of this study proposed a screening process in which patients with STOP-BANG scores ≥3 and bicarbonate levels ≥28 mmol/L are considered at a higher risk for moderate to severe OSA, as are those with STOP-BANG scores of ≥6 [37•]. Preoperative screening for OSA using high sensitivity tools such as STOP-BANG is recommended by the ASA Task Force, and diagnostic testing with polysomnography may be indicated in patients with high screening scores [38••].
Diagnostic Testing
The most important aspect of the preoperative pulmonary evaluation is the history and physical exam, which should elucidate known or undiagnosed lung disease as well as other known non-pulmonary risk factors. Laboratory and other diagnostic testing are targeted in selected patients based on the findings of the history and exam. Routine screening diagnostic studies are rarely indicated.
As opposed to lung resection surgery, there is no consensus regarding the role of pulmonary function tests (PFTs) in non-thoracic surgeries, and no spirometry thresholds have been identified as a contraindication to surgery. Moreover, PFTs are probably no better than clinical assessment in estimating the risk of PPCs [8].
Abnormal chest x-rays are common in the elderly population, but there is little evidence that routine chest radiographs add meaningful information to the preoperative evaluation. In one meta-analysis, <1 % of preoperative chest x-rays had unexpected abnormalities and <0.1 % had an abnormality that influenced management [39]. Chest x-rays are indicated in symptomatic patients and those with suspected but undiagnosed pulmonary disease. Preoperative chest radiography may also be reasonable in patients undergoing higher risk non-thoracic procedures, such as abdominal aortic aneurysm repair [8].
Although some data suggest that albumin levels <3.5 g/dL and BUN levels ≥21 mg/dL are predictive of postoperative pulmonary outcomes, newer studies have not identified these as significant independent risk factors for PPCs. Current literature also does not support the routine use of arterial blood gas testing for preoperative pulmonary risk stratification [8], but measurement of serum bicarbonate may be useful in risk stratification of patients at risk for OSA [37•].
Risk Reduction Strategies
Many risk factors for PPCs are non-modifiable, but identification of risk is still important to raise perioperative caretakers’ vigilance and surveillance for respiratory problems. In addition to preoperative optimization of chronic cardiopulmonary disease, patients with increased pulmonary risk may also be candidates for evidence-based risk reduction strategies (Table 2). Although some methods are outside the purview of internists and geriatricians, knowledge of intraoperative risk reduction techniques is useful so that medical providers can inform surgical teams of patients who are high risk and may benefit from them.
Lung Expansion Methods
Lung expansion techniques (deep breathing maneuvers, incentive spirometry, chest physiotherapy, intermittent positive pressure breathing, or positive airway pressure (PAP)) have long been regarded as the mainstay of prophylaxis against pulmonary complications. Despite this, a recent Cochrane review found no evidence that incentive spirometry reduced PPCs after upper abdominal surgery [40]. A Cochrane review of continuous positive airway pressure (CPAP) after abdominal surgery reported weak evidence that postoperative CPAP reduced PPCs but not mortality [41]. Significant heterogeneity in studies of lung expansion modalities is a large contributor to conflicting data regarding the effectiveness of these interventions. In their systematic review of PPC prevention, Qaseem et al. concluded that for patients with risk factors for PPCs, some form of lung expansion was better than no therapy, but combining different methods offered no added benefit [42].
Selective Nasogastric Intubation
Prophylactic nasogastric decompression after abdominal surgery does not improve surgical outcomes and may increase the risk of oropharyngeal aspiration. Restriction of nasogastric intubation for clear indications (e.g., symptomatic gastric distention, intractable vomiting) results in decreased PPCs [42, 43].
Shorter-Acting Neuromuscular Blockade
Compared to intermediate-acting medications (atracurium and vecuronium), the use of a long-acting neuromuscular blocking agent (pancuronium) was associated with a higher incidence of residual neuromuscular blockade and a higher incidence of PPCs among patients with residual block [42]. In a more recent study by Grosse-Sundrup et al., intermediate-acting neuromuscular blockade use and routine reversal with cholinergic agents was also associated with increased respiratory complications, while quantitative (versus qualitative) neuromuscular blockade monitoring was associated with lower pulmonary risk [44]. Thus, it is reasonable to use shorter-acting neuromuscular blockade with quantitative monitoring and to avoid routine pharmacologic reversal in patients at increased risk for PPCs.
Laparoscopic/Thoracoscopic Surgical Approach
Early studies of variable quality and design suggested improved pulmonary outcomes for patients undergoing laparoscopic versus open abdominal procedures, but firm evidence of benefit was lacking [42]. Subsequently, studies of bariatric surgery [45] and esophagectomy [46, 47] patients have provided stronger evidence that laparoscopic and thoracoscopic surgical approaches have a lower risk of PPCs than open procedures. Multiple different factors influence the selection of surgical approach, and the benefits of laparoscopy and thoracoscopy are dependent on the surgeon’s experience. For this reason, clinicians performing preoperative evaluations should notify surgeons of patients’ increased pulmonary risk and inquire about the possibility of risk-reducing surgical approaches rather than recommending a particular type of procedure.
Regional/Neuraxial Anesthesia
Anesthesia type as a potential source of pulmonary risk or PPC prevention has been vigorously debated. Although some studies offer conflicting data, a 2014 Cochrane systematic review found that neuraxial anesthesia either alone or when added to general anesthesia significantly reduced the risk of postoperative pneumonia [48]. In one study of elderly patients, regional anesthesia was not beneficial in hip fracture repair [49], but a recent systematic review of regional techniques as part of a multimodal anesthetic approach concluded they may reduce pulmonary complications in the geriatric population [50]. For patients who are at increased risk for PPCs, clinicians should alert the surgical team (surgeon and anesthesiologist) so that the patient’s respiratory status can be factored into the selection of anesthesia type.
Epidural or Intravenous Patient-Controlled Analgesia
Epidural anesthesia has the potential for reducing PPCs by limiting hypoventilation and respiratory dysfunction from pain or use of systemic opioids and by enhancing the ability to take breaths after surgery without pain. Much like the literature for neuraxial and regional anesthesia, studies investigating the impact of epidural analgesia on PPCs have been heterogeneous and conflicting. Meta-analyses of postoperative thoracic epidural analgesia have shown reduction in the risk of PPCs in abdominal aortic and coronary revascularization surgery [51, 52]. For other types of surgery and lumbar epidural analgesia, data for efficacy is very limited. Patient-controlled intravenous (IV) opioids also offer the potential for limiting opioid-induced hypoventilation compared to conventional IV opioid administration (i.e., injected by nurse on request). Walder et al. confirmed that PPCs are less frequent for patients who received patient-controlled analgesia (PCA) compared to conventional systemic opioids [53]. In their systematic review of PPC prevention strategies, Qaseem and colleagues determined that epidural analgesia and patient-controlled analgesia are superior to conventional IV administration of opioids [42]. Postoperative analgesia is usually managed by surgeons and anesthesiologists, but communication of pulmonary risk by the preoperative clinician assures that this is factored into the surgical team’s decision making.
Preoperative Cardiopulmonary Physiotherapy
Preoperative cardiopulmonary physiotherapy has the potential to improve patients’ inspiratory muscle function and overall physical conditioning thereby reducing the risk of postoperative respiratory problems. In a randomized controlled trial of patients undergoing coronary artery bypass grafting (CABG), Hulzebos et al. demonstrated that 20 min of daily lung expansion for 2 weeks prior to surgery (e.g., inspiratory muscle training) reduced PPCs by 50 % [54]. Herdy and colleagues also found that cardiopulmonary rehabilitation begun at least 5 days prior to CABG led to fewer days of mechanical ventilation and lower rates of PPCs [55]. Thus, when feasible, preoperative initiation of lung expansion or physical conditioning exercises is a potentially useful way to reduce PPCs.
Lung-Protective Mechanical Ventilation
A burgeoning area of perioperative pulmonary risk management is the use of protective lung ventilation, which entails the use of low tidal volumes, positive end-expiratory pressure (PEEP), and lung recruitment maneuvers (application of high airway pressures to open collapsed lung areas) [56]. For years, this method has been employed in intensive care units for the reduction of ventilator-associated lung injury in patients with acute respiratory distress syndrome. Recently, a number of investigators have conducted small studies of lung-protective ventilation in surgical patients. In a recent meta-analysis, the use of low tidal volumes (6 ml/kg for most studies) resulted in risk ratios of 0.4 (95 % confidence interval [CI], 0.22–0.70) for lung injury and 0.64 (95 % CI, 0.43–0.97) for pulmonary infection [57••]. Utilization of higher PEEP (5 cm of water for most studies) similarly improved outcomes; risk ratios were 0.29 (95 % CI, 0.14–0.60) for lung injury and 0.62 (95 % CI, 0.40–0.96) for pulmonary infection. Although individual patient characteristics may make use of these strategies less favorable, lung-protective mechanical ventilation should be strongly considered in any patient at increased risk for PPCs.
Smoking Cessation
The detrimental effects of smoking are myriad, and smoking cessation advocacy is a universally accepted responsibility of healthcare providers. However, the relative importance and potential benefits of smoking abstinence in the perioperative period remain controversial. Smoking clearly impairs normal respiratory functions. Airway hyperreactivity, increased mucus production, and disrupted clearance mechanisms (impaired epithelial cilia) increase the risk for compromised oxygenation and pulmonary infections [58]. The risk of these problems is even higher for smokers in the postoperative period [59]. One would assume that preoperative abstinence from smoking would always improve postoperative outcomes, but the evidence has been conflicting.
Several previous studies have shown that smoking cessation before surgery was associated with lower incidence PPCs, but others found no difference in outcomes between smokers and non-smokers [60••]. One study has even been interpreted as demonstrating a possible increase in the risk of PPCs among patients who quit smoking shortly before surgery [61]. More recently, meta-analyses have clarified these discordant data. Myers and colleagues’ meta-analysis of preoperative smoking cessation found a non-statistically significant increase in relative risk for PPCs in patients who stopped smoking <8 weeks before surgery [62]. Another meta-analysis by Wong et al. corroborated these findings, showing that <4 weeks of smoking abstinence did not significantly increase the risk of pulmonary complications compared to those who continued smoking [60••]. For those who quit >4 weeks before surgery, the relative risk of PPCs was 0.77 (95 % CI, 0.61–0.96), and for those who quit >8 weeks before surgery, the relative risk of PPCs was the same as non-smokers.
From these studies, one can conclude that smoking cessation at least 4–8 weeks before surgery will reduce the risks of PPCs. Unfortunately, the heterogeneity and small numbers of patients make the benefit for abstinence <4 weeks before surgery less certain. A number of studies have attempted to overcome this problem through randomized clinical trials of smoking cessation interventions. In a recent Cochrane review, interventions initiated ≥4 weeks before surgery were beneficial at achieving long-term cessation and reducing the incidence of complications [63•]. However, none of the included studies demonstrated a significant impact on PPCs. No studies of preoperative smoking cessation interventions reported adverse outcomes.
Rather than focusing on the exact minimum time needed for cessation, it is advisable that clinicians take a more holistic approach to the problem of tobacco use. For any patient undergoing elective surgery, general health should be fully optimized, including stopping smoking as far in advance as possible. The preoperative evaluation provides an opportunity for a “teachable moment” regarding the importance of smoking cessation [64, 65].
OSA Management
Recent studies have evaluated management interventions for patients at high risk for or with known OSA. In one retrospective study of patients who were screened as high risk for OSA, patients either underwent a risk management program (based on ASA guidelines) intended to decrease OSA complications or had preoperative polysomnography with PAP initiation if indicated. Other than recovery unit length of stay, there was no difference in outcomes between the two groups [66]. This corroborates the findings of Lockhart and colleagues who found that patients who screened high risk for OSA and received empiric risk reduction measures (including non-supine positioning and close monitoring of respiratory status) have rates of postoperative mortality that are similar to those for patients screened as low OSA risk [36•].
In another study using the Flemons tool to identify patients at high risk for OSA, investigators found that patients who received auto-titrating CPAP postoperatively had no difference in outcomes than those who did not [67]. The utility of preoperative CPAP initiation was explored in a retrospective study by Mutter and colleagues who evaluated non-cardiac surgery patients separated into three OSA groups: those who had surgery and were diagnosed with OSA within 5 years after their surgical date (suggesting OSA was present but untreated during the perioperative period); those who were diagnosed and being treated for OSA at the time of surgery; and matched controls screened as low risk for OSA. In multivariate analyses, the risk of PPCs was increased for both untreated and treated OSA, but those who had treated OSA had fewer postoperative cardiovascular complications than untreated patients, suggesting that preoperative initiation of PAP in OSA patients may lower complications [68]. Considered together, these two studies suggest PAP may improve outcomes in OSA patients, but only when initiated prior to the postoperative setting.
Currently, the most comprehensive recommendations for postoperative management of patients at risk for or with known OSA are from the ASA task force [38••]. Among these guidelines are recommendations to consider preoperative initiation of PAP particularly if OSA is severe. Preoperative weight loss should also be considered when feasible. Much of the ASA task force guideline addresses postoperative pain management. Recommendations include the use of regional analgesic techniques to reduce or eliminate the requirement for systemic opioids and avoidance of continuous opioid infusions. The guideline also advises caution for concurrent administration of sedative agents due to the increased risk of respiratory depression and airway obstruction. Adjunctive analgesics (e.g., non-steroidal anti-inflammatory medications) are a strategy to reduce opioid requirements. Patients on CPAP or non-invasive positive pressure ventilation (NIPPV) therapy prior to surgery should use their home devices postoperatively. The authors also recommend non-supine positioning and continuous pulse oximetry monitoring until the risk of respiratory depression has abated. For untreated patients who screen high risk for OSA options include empiric treatment with CPAP or NIPPV, or if time allows, a formal preoperative sleep study. The location of surgery (ambulatory vs inpatient) should also be carefully considered for such patients. Those undergoing deep sedation or likely to receive significant postoperative opioid treatment may be more appropriate for surgery in a hospital setting.
Conclusions
Improvements in technology and practice have made surgical treatments available to an increasing number of older patients. Still, the perioperative setting confers significant risks, especially for the elderly. Postoperative pulmonary complications are among the most common of these potential adverse outcomes. With the use of available risk prediction models, clinicians can estimate and counsel patients on the risk of PPCs. OSA and PH remain underrepresented in these available tools but definitely increase the risk of respiratory complications. Therefore, special care is required to screen for and treat these conditions in the perioperative period. For patients who screen high risk for PPCs, several risk reduction strategies offer potential mitigation strategies. Although few risk reduction interventions should be employed routinely, all should be considered for their utility in a particular clinical situation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rahmanian PB, Kröner A, Langebartels G, et al. Impact of major noncardiac complications on outcome following cardiac surgery procedures: logistic regression analysis in a very recent patient cohort. Interact Cardiovasc Thorac Surg. 2013;17:319–27.
Qaseem A, Snow V, Fitterman N, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing non-cardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144:575–80.
Lawson EH, Hall BL, Louie R, et al. Association between occurrence of a postoperative complication and readmission. Ann Surg. 2013;258:10–8.
Shander A, Fleisher LA, Barie PS, et al. Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med. 2011;39:2163–72.
Aykut K, Albayrak G, Guzeloglu M, Baysak A, Hazan E. Preoperative mild cognitive dysfunction predicts pulmonary complications after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2013;27(6):1267–70.
Gupta H, Gupta PK, Schuller D, et al. Development and validation of a risk calculator for predicting postoperative pneumonia. Mayo Clin Proc. 2013;88(11):1241–9. Well-designed study utilizing large, comprehensive surgical database that determined strongest predictors of postoperative pneumonia and derived a user-friendly calculator for estimating risk.
Gupta H, Gupta PK, Fang X, et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest. 2011;140:1207–15.
Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581–95.
American Society of Anesthesiologists. ASA Physical Status Classification System https://www.asahq.org/clinical/physicalstatus.htm Accessed 10/30/2014
Johnson RG, Arozullah A, Neumayer L, et al. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204(6):1188–98.
Meyers JR, Lembeck L, O’Kane H, Baue AE. Changes in functional residual capacity of the lung after operation. Arch Surg. 1975;110(5):576–83.
Ford GT, Whitelaw WA, Rosenal TW, Cruse PJ, Guenter CA. Diaphragm function after upper abdominal surgery in humans. Am Rev Respir Dis. 1983;127(4):431–6.
Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321(7275):1493.
Murphy GS, Szokol JW, Marymont JH, et al. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesth Analg. 2008;107(1):130–7.
Health, United States 2013. http://www.cdc.gov/nchs/data/hus/hus13.pdf#064. Last accessed Oct 31, 2014.
Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5.
Chung F, Ward B, Ho J, et al. Preoperative identification of sleep apnea risk in elective surgical patients, using the Berlin questionnaire. J Clin Anesth. 2007;19(2):130–4.
Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;5:812–21.
Hwang D, Shakir N, Limann B, et al. Association of sleep disordered breathing with postoperative complications. Chest. 2008;133:1128–34.
Kaw R, Pasupuleti V, Walker E, Ramaswamy A, Foldvary-Schafer N. Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141(2):436–41.
Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76(9):897–905.
Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112(1):113–21.
Kaw R, Chung F, Pasupuleti V, et al. Meta-analysis of the association between obstructive sleep apnea and postoperative outcome. Br J Anaesth. 2012;109(6):897–906.
Ramakrishna G, Sprung J, Ravi BS, et al. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol. 2005;45:1691–9.
Lai HC, Lai HC, Wang KY, et al. Severe pulmonary hypertension complicates postoperative outcomes of noncardiac surgery. Br J Anaesth. 2007;99:184–90.
Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111(5):1110–6. Large retrospective study utilizing the National Inpatient Sample and multivariate analysis to determine the impact of pulmonary hypertension on morbidity and mortality in one of the most common types of surgery in the elderly.
Minai OA, Yared YP, Kaw R, Subramanian K, Hill NS. Perioperative risk and management in patients with pulmonary hypertension. Chest. 2013;144(1):329–40. Well-written narrative summary of the potential complications and risk reduction strategies in patients with pulmonary hypertension.
Meyer S, Mclaughlin VV, Seyfarth HJ, et al. Outcomes of noncardiac, nonobstetric surgery in patients with PAH: an international prospective survey. Eur Respir J. 2013;41:1302–7.
Kaw R, Pasupuleti V, Deshpande A, et al. Pulmonary hypertension: an important predictor of outcomes in patients undergoing non-cardiac surgery. Respir Med. 2011;105(4):619–24.
Fleisher LA, Fleischmann KE, Auerbach AD, et al. ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014. doi:10.1016/j.jacc.2014.07.944. Focused on perioperative cardiovascular disease, this guideline includes pulmonary hypertension and provides risk recommendations on its perioperative management.
Arozullah AM, Khuri SF, Henderson WG, Daley J. Participants in the National Veterans Affairs Surgical Quality Improvement Program. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major non-cardiac surgery. Ann Int Med. 2001;135(10):847–57.
Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–50.
Mazo V, Sabaté S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121(2):219–31. Provided valuable external validation of a previously developed pulmonary risk prediction tool (ARISCAT).
Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21.
Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnea. Br J Anaesth. 2012;108(5):768–75. Authored by one of the leading experts in perioperative OSA management, this study demonstrated the strong sensitivity and improved specificity of the STOP-Bang score for predicting OSA.
Lockhart EM, Willingham MD, Abdallah AB, et al. Obstructive sleep apnea screening and postoperative mortality in a large surgical cohort. Sleep Med. 2013;14:407–15.
Chung F, Chau E, Yang Y, et al. Serum bicarbonate level improves specificity of stop-bang screening for obstructive sleep apnea. Chest. 2013;143(5):1284–93.
American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2014;120(2):268–86. Well-written and thoroughly researched guidelines for the management of OSA patient in the perioperative setting.
Archer C, Levy AR, McGregor M. Value of routine preoperative chest x-rays: a meta-analysis. Can J Anaesth. 1993;40:1022–7.
do Nascimento Junior P, Módolo NS, Andrade S, et al. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database Syst Rev. 2014 Feb 8;2:CD006058. doi: 10.1002/14651858.CD006058.pub3
Ireland CJ, Chapman TM, Mathew SF, Herbison GP, Zacharias M. Continuous positive airway pressure (CPAP) during the postoperative period for prevention of postoperative morbidity and mortality following major abdominal surgery. Cochrane Database Syst Rev. 2014 Aug 1;8:CD008930. doi: 10.1002/14651858.CD008930.pub2.
Lawrence VA, Cornell JE, Smetana GW. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):596–608.
Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev. 2007;18(3):CD004929.
Grosse-Sundrup M, Henneman JP, Sandberg WS, et al. Intermediate acting nondepolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. Br Med J. 2012;345:e6329.
Weller WE, Rosati C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann Surg. 2008;248:10–5.
Briez N, Piessen G, Torres F, et al. Effects of hybrid minimally invasive oesophagectomy on major postoperative pulmonary complications. Br J Surg. 2012;99:1547–53.
Tsujimoto H, Takahata R, Nomura S, et al. Video-assisted thoracoscopic surgery for esophageal cancer attenuates postoperative systemic responses and pulmonary complications. Surgery. 2012;151:667–73.
Guay J, Choi P, Suresh S, et al. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2014 Jan 25;1:CD010108. doi: 10.1002/14651858.CD010108.pub2.
Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948–56.
Nordquist D, Halaszynski TM. Perioperative multimodal anesthesia using regional techniques in the aging surgical patient. Pain Res Treat. 2014;2014:902174. doi: 10.1155/2014/902174. Epub 2014 Jan 20.
Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg. 2007;104:689–702.
Nishimori M, Low JH, Zheng H, et al. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev. 2012;7:CD005059.
Walder B, Schafer M, Henzi I, Tramèr MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol Scand. 2001;45:795–804.
Hulzebos EH, Helders PJ, Favié NJ, et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. 2006;296:1851–7.
Herdy AH, Marcchi PLB, Vila A, et al. Pre and postoperative cardiopulmonary rehabilitation in hospitalized patients undergoing coronary artery bypass surgery: a randomized controlled trial. Am J Phys Med Rehabil. 2008;87:714–9.
Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–36.
Hemmes SNT, Neto AS, Schultz MJ. Intraoperative ventilator strategies to prevent postoperative pulmonary complications: a meta-analysis. Curr Opin Anaesthesiol. 2013;26(2):126–33. Well-designed meta-analysis demonstrating the benefits of lung-protective ventilation in perioperative patients.
Bluman LG, Mosca M, Newman N, Simon DG. Preoperative smoking habits and postoperative pulmonary complications. Chest. 1998;113(4):883–9.
Nakagawa M, Tanaka H, Tsukuma H, Kishi Y. Relationship between the duration of the preoperative smoke-free period and the incidence of postoperative pulmonary complications after pulmonary surgery. Chest. 2001;120(3):705–10.
Wong J, Lam DP, Abrishami A, et al. Short-term preoperative smoking cessation and postoperative complications: a systematic review and meta-analysis. Can J Anaesth. 2012;59:268.
Warner MA, Offord KP, Warner ME, et al. Role of preoperative cessation of smoking and other factors in postoperative pulmonary complications: a blinded prospective study of coronary artery bypass patients. Mayo Clin Proc. 1989;64(6):609–16.
Myers K, Hajek P, Hinds C, McRobbie H. Stopping smoking shortly before surgery and postoperative complications: a systematic review and meta-analysis. Arch Intern Med. 2011;171(11):983–9.
Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev. 2014 Mar 27;3:CD002294. doi:10.1002/14651858.CD002294.pub4. Comprehensive review of the perioperative complications of smoking and interventions to promote tobacco cessation.
Lee SM, Landry J, Jones PM, Buhrmann O, Morley-Forster P. The effectiveness of a perioperative smoking cessation program: a randomized clinical trial. Anesth Analg. 2013;117(3):605–13.
Shi Y, Warner DO. Surgery as a teachable moment for smoking cessation. Anesthesiology. 2010;112(1):102–7.
Chong CT, Tey J, Leow SL, Low W, Kwan KM, Wong YL, et al. Management plan to reduce risks in perioperative care of patients with obstructive sleep apnoea averts the need for presurgical polysomnography. Ann Acad Med Singap. 2013;42(3):110–9.
O’Gorman SM, Gay PC, Morgenthaler TI. Does autotitrating positive airway pressure therapy improve postoperative outcome in patients at risk for obstructive sleep apnea syndrome? a randomized controlled clinical trial. Chest. 2013;144:72–8.
Mutter TC, Chateau D, Moffatt M, Ramsey C, Roos LL, Kryger M. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology. 2014;121(4):707–18.
Compliance with Ethics Guidelines
Conflict of Interest
Kurt J. Pfeifer declares that he has no conflict of interest.
Barbara A. Slawski has received compensation from Marathon Pharmaceuticals for the service on an advisory board.
Gerald W. Smetana declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pulmonology and Respiratory Care
Rights and permissions
About this article
Cite this article
Pfeifer, K.J., Slawski, B.A. & Smetana, G.W. Perioperative Pulmonary Management of the Elderly Patient. Curr Geri Rep 4, 183–191 (2015). https://doi.org/10.1007/s13670-014-0116-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13670-014-0116-3