Abstract
Purpose of Review
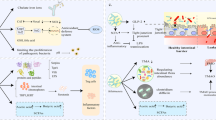
Global health concerns persist in the realm of cardiovascular diseases (CVDs), necessitating innovative strategies for both prevention and treatment. This narrative review aims to explore the potential of short-chain fatty acids (SCFAs)—namely, acetate, propionate, and butyrate—as agents in the realm of postbiotics for the management of CVDs.
Recent Findings
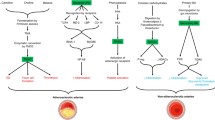
We commence our discussion by elucidating the concept of postbiotics and their pivotal significance in mitigating various aspects of cardiovascular diseases. This review centers on a comprehensive examination of diverse SCFAs and their associated receptors, notably GPR41, GPR43, and GPR109a. In addition, we delve into the intricate cellular and pharmacological mechanisms through which these receptors operate, providing insights into their specific roles in managing cardiovascular conditions such as hypertension, atherosclerosis, heart failure, and stroke.
Summary
The integration of current information in our analysis highlights the potential of both SCFAs and their receptors as a promising path for innovative therapeutic approaches in the field of cardiovascular health. The idea of postbiotics arises as an optimistic and inventive method, presenting new opportunities for preventing and treating cardiovascular diseases.






Similar content being viewed by others
Data Availability
There are no data associated with this manuscript.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol England. 2014;11:506–14.
Cuevas-González PF, Liceaga AM, Aguilar-Toalá JE. Postbiotics and paraprobiotics: from concepts to applications Food Res Int. Food Res Int Canada. 2020;136:109502.
Kianmehr S, Jahani M, Moazzen N, Ahanchian H, Khameneh B. The potential of probiotics for treating skin disorders: a concise review. Curr Pharm Biotechnol Netherlands. 2022;23:1851–63.
Zucko J, Starcevic A, Diminic J, Oros D, Mortazavian AM, Putnik P. Probiotic – friend or foe? Curr Opin Food Sci Elsevier Ltd. 2020;32:45–9. https://doi.org/10.1016/j.cofs.2020.01.007.
Vinderola G, Sanders ME, Salminen S, Szajewska H. Postbiotics: the concept and their use in healthy populations. Front Nutr. 2022;9:1–7.
Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol Springer, US. 2021;18:649–67.
Vinderola G, Sanders ME, Salminen S. The concept of postbiotics Foods. 2022;11:1–10.
Zhang M, Liang J, Yang Y, Liang H, Jia H, Li D. Current trends of targeted drug delivery for oral cancer therapy. Front Bioeng Biotechnol. 2020;8:1–11.
Shoaib M, Shehzad A, Omar M, Rakha A, Raza H, Sharif HR, et al. Inulin: properties, health benefits and food applications. Carbohydr Polym England. 2016;147:444–54.
Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr United States. 2014;54:938–56.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14.
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol England. 2017;14:491–502.
Palaria A, Johnson-Kanda I, O’Sullivan DJ. Effect of a synbiotic yogurt on levels of fecal bifidobacteria, clostridia, andenterobacteria. Appl Environ Microbiol. 2012;78:933–40.
Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med United States. 2019;25:1096–103.
Meier T, Gräfe K, Senn F, Sur P, Stangl GI, Dawczynski C, et al. Cardiovascular mortality attributable to dietary risk factors in 51 countries in the WHO European Region from 1990 to 2016: a systematic analysis of the Global Burden of Disease Study. Eur J Epidemiol. 2019;34:37–55.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol United States. 2020;76:2982–3021.
Banerjee A. Macroeconomics and cardiovascular risk factors: the same view through a differentlens? Circulation. 2013;217:1451–2.
Naseri P, Amiri P, Masihay-Akbar H, Jalali-Farahani S, Khalili D, Azizi F. Long-term incidence of cardiovascular outcomes in the middle-aged and elderly with different patterns of physical activity: Tehran lipid and glucose study. BMC Public Health BMC Public Health. 2020;20:1–10.
Keihanian F, Saeidinia A, Bagheri RK, Johnston TP, Sahebkar A. Curcumin, hemostasis, thrombosis, and coagulation. J Cell Physiol United States. 2018;233:4497–511.
Khameneh Bagheri R, Najafi MN, Ahmadi M, Saberi M, Maleki M, Baradaran RV. Investigation of the association between serum uric acid levels and HEART risk score in patients with acute coronary syndrome. Physiol Rep. 2022;10:2–9.
Saeidinia A, Keihanian F, Butler AE, Bagheri RK, Atkin SL, Sahebkar A. Curcumin in heart failure: a choice for complementary therapy? Pharmacol Res Netherlands. 2018;131:112–9.
Amini M, Zayeri F, Salehi M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: results from Global Burden of Disease Study 2017. BMC Public Health BMC Public Health. 2021;21:1–12.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol [Internet]. Elsevier Inc.; 2020 [cited 2023 Aug 23];76:2982–3021. Available from: https://www.jacc.org/doi/10.1016/j.jacc.2020.11.010?_ga=2.166900478.1713021033.1710932425-295567366.1710932425&_gl=1*z6wxdr*_ga*Mjk1NTY3MzY2LjE3MTA5MzI0MjU.*_ga_2V8VW4Y237*MTcxMDkzMjQyNC4xLjEuMTcxMDkzMjUwNC40Ny4wLjA.
Iso H. Cardiovascular disease, a major global burden: Epidemiology of stroke andischemic heart disease in Japan. Glob Heal Med. 2021;3:358–64.
Daviglus ML, Talavera GA, Avilés-Santa ML, Allison M, Cai J, Criqui MH, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA United States. 2012;308:1775–84.
Münzel T, Hahad O, Sørensen M, Lelieveld J, Duerr GD, Nieuwenhuijsen M, et al. Environmental risk factors and cardiovascular diseases: a comprehensive expert review. Cardiovasc Res. 2022;118:2880–902.
Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation United States. 2001;104:2746–53.
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update a report from the American Heart Association. Circulation Lippincott Williams and Wilkins. 2020;141:E139–596.
Elstrott B, Khan L, Olson S, Raghunathan V, DeLoughery T, Shatzel JJ. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol England. 2020;104:153–61.
Samadian F, Dalili N, Jamalian A. Lifestyle modifications to prevent and control hypertension. Iran J Kidney Dis Iran. 2016;10:237–63.
Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Coker TR, et al. Vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer: US Preventive Services Task Force Recommendation Statement. JAMA United States. 2022;327:2326–33.
Wierzejska R. Caffeine—common ingredient in a diet and its influence on human health. Rocz Panstw Zakl Hig Poland. 2012;63:141–7.
McCarty MF. Nutraceutical, dietary, and lifestyle options for prevention and treatment of ventricular hypertrophy and heart failure. Int J Mol Sci. 2021;22:1–47.
Romero M, Duarte J. Probiotics and prebiotics in cardiovascular diseases. Nutrients. 2023;15:3–6.
Divella R, Daniele A, Savino E, Paradiso A. Anticancer effects of nutraceuticals in the Mediterranean diet: an epigenetic diet model. Cancer Genomics Proteomics Greece. 2020;17:335–50.
Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol United States. 2019;73:2089–105.
Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res United States. 2017;120:1183–96.
Cheng HM, Koutsidis G, Lodge JK, Ashor A, Siervo M, Lara J. Tomato and lycopene supplementation and cardiovascular risk factors: a systematic review and meta-analysis. Atherosclerosis Ireland. 2017;257:100–8.
Pahumunto N, Duangnumsawang Y, Teanpaisan R. Effects of potential probiotics on the expression of cytokines and human β-defensins in human gingival epithelial cells and in vivo efficacy in a dog model. Arch Oral Bio England. 2022;142:105513.
Xu T, He X, Chen T. Editorial: The role of probiotics, postbiotics, and microbial metabolites in preventing and treating chronic diseases. Front Cell Infect Microbiol. 2023;13:1–2.
Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics-a step beyond pre- and probiotics. Nutrients. 2020;12:1–17.
Uebanso T, Shimohata T, Mawatari K, Takahashi A. Functional roles of B-vitamins in the gut and gut microbiome Mol Nutr Food Res. Mol Nutr Food Res Germany. 2020;64:e2000426.
Mosegaard S, Dipace G, Bross P, Carlsen J, Gregersen N, Olsen RKJ. Riboflavin deficiency—implications for general human health and inborn errors of metabolism. Int J Mol Sci. 2020;21:1–26.
Anhê FF, Jensen BAH, Perazza LR, Tchernof A, Schertzer JD, Marette A. Bacterial postbiotics as promising tools to mitigate cardiometabolic diseases. J Lipid Atheroscler. 2021;10:123–9.
•• Lu Y, Zhang Y, Zhao X, Shang C, Xiang M, Li L, et al. Microbiota-derived short-chain fatty acids: implications for cardiovascular and metabolic disease. Front Cardiovasc Med. 2022;9:1–17. The article explores the potential advantages of short-chain fatty acids (SCFAs) derived from the microbiota in the prevention and treatment of cardiovascular and metabolic diseases. Different strategies for influencing SCFAs, such as dietary adjustments, fecal microbiota transplantation, prebiotics, probiotics, and traditional Chinese medicine, show promise for upcoming therapeutic interventions.
Markowiak-Kope P, Slizewska K. The trend in the amount of SCFAs found in feces is more closely related to nutrition, environmental variables, and intestinal microbiome dysbiosis. Nutrients. 2020;12:1–23.
Nakkarach A, Foo HL, Song AAL, Mutalib NEA, Nitisinprasert S, Withayagiat U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota Microb Cell Fact. BioMed Central. 2021;20:1–17. https://doi.org/10.1186/s12934-020-01477-z.
Angelin J, Kavitha M. Exopolysaccharides from probiotic bacteria and their health potential. Int J Biol Macromol Netherlands. 2020;162:853–65.
•• Park M, Joung M, Park JH, Ha SK, Park HY. Role of postbiotics in diet-induced metabolic disorders. Nutrients. 2022;14:1–14. The article explores the potential of postbiotics, which are substances generated or released through the metabolic activities of microorganisms, in the prevention, relief, and treatment of metabolic disorders induced by diet. Postbiotics exhibit diverse positive effects, including anti-obesity, anti-diabetic, and anti-hypertensive properties, offering potential assistance in managing metabolic disorders. Nevertheless, additional research is necessary to ascertain their effectiveness and safety.
Hussain A, Zia KM, Tabasum S, Noreen A, Ali M, Iqbal R, et al. Blends and composites of exopolysaccharides; properties and applications: a review. Int J Biol Macromol Netherlands. 2017;94:10–27.
Patel S, Majumder A, Goyal A. Potentials of exopolysaccharides from lactic acid bacteria. Indian J Microbiol. 2012;52:3–12.
Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, et al. Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol Netherlands. 2002;72:215–24.
Kim HS, Chae HS, Jeong SG, Ham JS, Im SK, Ahn CN, et al. In vitro antioxidative properties of lactobacilli. Asian-Australasian J Anim Sci. 2006;19:262–5.
Izuddin WI, Humam AM, Loh TC, Foo HL, Samsudin AA. Dietary postbiotic Lactobacillus plantarum improves serum and ruminal antioxidant activity and upregulates hepatic antioxidant enzymes and ruminal barrier function in post-weaning lambs. Antioxidants. 2020;9:1–13.
Chorawala MR, Chauhan S, Patel R, Shah G. Cell wall contents of probiotics (Lactobacillus species) protect against lipopolysaccharide (LPS)-induced murine colitis by limiting immuno-inflammation and oxidative stress. Probiotics Antimicrob Proteins United States. 2021;13:1005–17.
Duncker SC, Wang L, Hols P, Bienenstock J. The D-alanine content of lipoteichoic acid is crucial for Lactobacillus plantarum-mediated protection from visceral pain perception in a rat colorectal distension model. Neurogastroenterol Motil England. 2008;20:843–50.
Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol United States. 2008;122:261–6.
Wang Z, MacLeod DT, Di Nardo A. Commensal bacteria lipoteichoic acid increases skin mast cell antimicrobial activity against vaccinia viruses. J Immunol United States. 2012;189:1551–8.
Thakur K, Tomar SK. Invitro study of riboflavin producing lactobacilli as potential probiotic. Lwt Elsevier Ltd. 2016;68:570–8. https://doi.org/10.1016/j.lwt.2015.12.059.
LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol Elsevier Ltd. 2013;24:160–8. https://doi.org/10.1016/j.copbio.2012.08.005.
Morowitz MJ, Carlisle EM, Alverdy JC. Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. Surg Clin North Am. 2011;91:771–85.
Moon EC, Park MS, Lim T, Kim RH, Ji GE, Kim SY, et al. Antibacterial effect of cell-free supernatant fraction from Lactobacillus paracasei CH88 against Gardnerella vaginalis. Sci Rep Nature Publishing Group UK. 2022;12:1–10. https://doi.org/10.1038/s41598-022-08808-7.
Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol United States. 2012;303:G32–41.
Osman A, El-Gazzar N, Almanaa TN, El-Hadary A, Sitohy M. Lipolytic postbiotic from Lactobacillus paracasei manages metabolic syndrome in Albino Wistar rats. Molecules. 2021;26:1–22.
Hsu CN, Hou CY, Hsu WH, Tain YL. Cardiovascular diseases of developmental origins: preventive aspects of gut microbiota-targeted therapy. Nutrients. 2021;13:1–16.
Mosca A, Abreu Y, Abreu AT, Gwee KA, Ianiro G, Tack J, Nguyen TVH, et al. The clinical evidence for postbiotics as microbial therapeutics. Gut Microbes. 2022;14:1–14.
Peng M, Tabashsum Z, Anderson M, Truong A, Houser AK, Padilla J, et al. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr Rev food Sci food Saf United States. 2020;19:1908–33.
Marques FZ, Jama HA, Tsyganov K, Gill PA, Rhys-Jones D, Muralitharan RR, et al. Guidelines for transparency on gut microbiome studies in essential and experimental hypertension Hypertens (Dallas, Tex 1979) United States. 2019;74:1279–93.
Gill PA, van Zelm MC, Muir JG, Gibson PR. Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. 2018;48:15–34.
Tan JK, McKenzie C, Mariño E, Macia L, Mackay CR. Metabolite-sensing G protein-coupled receptors-facilitators of diet-related immune regulation. Annu Rev Immunol United States. 2017;35:371–402.
Keshaviah PR. The role of acetate in the etiology of symptomatic hypotension. Artif Organs United States. 1982;6:378–87.
Hakim RM, Pontzer MA, Tilton D, Lazarus JM, Gottlieb MN. Effects of acetate and bicarbonate dialysate in stable chronic dialysis patients. Kidney Int United States. 1985;28:535–40.
Cen J, Sargsyan E, Bergsten P. Fatty acids stimulate insulin secretion from human pancreatic islets at fasting glucose concentrations via mitochondria-dependent and -independent mechanisms. Nutr Metab. 2016;13:1–9.
Kaye DM, Shihata WA, Jama HA, Tsyganov K, Ziemann M, Kiriazis H, et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation United States. 2020;141:1393–403.
Marques FZ, Nelson E, Chu P-Y, Horlock D, Fiedler A, Ziemann M, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation United States. 2017;135:964–77.
Keshaviah P, Shapiro FL. A critical examination of dialysis-induced hypotension. Am J Kidney Dis [Internet]. 1982;2:290–301. Available from: https://www.sciencedirect.com/science/article/pii/S0272638682800784.
Poll BG, Xu J, Jun S, Sanchez J, Zaidman NA, He X, et al. Acetate, a short-chain fatty acid, acutely lowers heart rate and cardiac contractility along with blood pressure. J Pharmacol Exp Ther. 2021;377:39–50.
Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics United States. 2016;48:826–34.
Onyszkiewicz M, Gawrys-Kopczynska M, Konopelski P, Aleksandrowicz M, Sawicka A, Koźniewska E, et al. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch Germany. 2019;471:1441–53.
Maeda K, Shinzato T, Nakai S, Takai I, Kobayakawa H. Mechanism of dialysis-induced hypotension. Nagoya J Med Sci. 1992;54:1–10.
Suokas A, Kupari M, Heikkilä J, Lindros K, Ylikahri R. Acute cardiovascular and metabolic effects of acetate in men. Alcohol Clin Exp Res England. 1988;12:52–8.
• Jiang X, Zhang Y, Zhang H, Zhang X, Yin X, Yuan F, et al. Acetate suppresses myocardial contraction via the short-chain fatty acid receptor GPR43. Front Physiol. 2022;13:1–11. This study examines the influence of acetate, a short-chain fatty acid, on myocardial contraction in rat ventricular myocytes. The research reveals that acetate temporarily hinders contraction by interacting with the short-chain fatty acid receptor GPR43 in cardiomyocytes. Various techniques, including echocardiography, Langendorff heart perfusion, and isolated cardiomyocyte measurements, were employed to assess the effects of acetate on cardiac function and calcium handling.
Dang G, Wu W, Zhang H, Everaert N. A new paradigm for a new simple chemical: butyrate & immune regulation. Food Funct England. 2021;12:12181–93.
Stoeva MK, Garcia-So J, Justice N, Myers J, Tyagi S, Nemchek M, et al. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. 2021;13:1–28.
Salvi PS, Cowles RA. Butyrate and the intestinal epithelium: modulation of proliferation and inflammation in homeostasis and disease. Cells. 2021;10:1–13.
Liu H, Wang J, He T, Becker S, Zhang G, Li D, et al. Butyrate: a double-edged sword for health? Am Soc Nutr. 2018;9:21–9.
Amiri P, Hosseini SA, Ghaffari S, Tutunchi H, Ghaffari S, Mosharkesh E, et al. Role of butyrate, a gut microbiota derived metabolite, in cardiovascular diseases: a comprehensive narrative review. Front Pharmacol. 2022;12:1–12.
Hodgkinson K, El Abbar F, Dobranowski P, Manoogian J, Butcher J, Figeys D, et al. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin Nutr [Internet]. 2023;42:61–75. Available from: https://www.sciencedirect.com/science/article/pii/S0261561422003843.
Kasahara K, Krautkramer KA, Org E, Romano KA, Kerby RL, Vivas EI, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol England. 2018;3:1461–71.
Aguilar EC, da Silva JF, Navia-Pelaez JM, Leonel AJ, Lopes LG, Menezes-Garcia Z, et al. Sodium butyrate modulates adipocyte expansion, adipogenesis, and insulin receptor signaling by upregulation of PPAR-γ in obese Apo E knockout mice. Nutrition United States. 2018;47:75–82.
Du Y, Li X, Su C, Xi M, Zhang X, Jiang Z, et al. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br J Pharmacol England. 2020;177:1754–72.
Wang Y, Xu Y, Yang M, Zhang M, Xiao M, Li X. Butyrate mitigates TNF-α-induced attachment of monocytes to endothelial cells. J Bioenerg Biomembr United States. 2020;52:247–56.
Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol Japan. 2017;52:1–8.
Ahmed RM, Mohammed AK. Amelioration of hepatotoxicity by sodium butyrate administration in rats. World’s Vet J. 2022;12:323–9.
Ranganna K, Mathew OP, Yatsu FM, Yousefipour Z, Hayes BE, Milton SG. Involvement of glutathione/glutathione S-transferase antioxidant system in butyrate-inhibited vascular smooth muscle cell proliferation. FEBS J England. 2007;274:5962–78.
Are A, Aronsson L, Wang S, Greicius G, Yuan KL, Gustafsson JÅ, et al. Enterococcus faecalis from newborn babies regulate endogenous PPARγ activity and IL-10 levels in colonic epithelial cells. Proc Natl Acad Sci USA. 2008;105:1943–8.
den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes United States. 2015;64:2398–408.
Rigano D, Sirignano C, Taglialatela-Scafati O. The potential of natural products for targeting PPARα. Acta Pharm Sin B [Internet]. 2017;7:427–38. Available from: https://www.sciencedirect.com/science/article/pii/S2211383517301624.
Xiao Y, Guo Z, Li Z, Ling H, Song C. Role and mechanism of action of butyrate in atherosclerotic diseases: a review. J Appl Microbiol. 2021;131:543–52.
Li G, Yao W, Jiang H. Short-chain fatty acids enhance adipocyte differentiation in the stromal vascular fraction of porcine adipose tissue. J Nutr United States. 2014;144:1887–95.
Yan H, Ajuwon KM. Mechanism of butyrate stimulation of triglyceride storage and adipokine expression during adipogenic differentiation of porcine stromovascular cells. PLoS ONE. 2015;10:1–20.
Ciura J, Jagodziński PP. Butyrate increases the formation of anti-angiogenic vascular endothelial growth factor variants in human lung microvascular endothelial cells. Mol Biol Rep Netherlands. 2010;37:3729–34.
Cookson TA. Bacterial-induced blood pressure reduction: mechanisms for the treatment of hypertension via the gut. Front Cardiovasc Med. 2021;8:1–13.
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. Nature Publishing Group. 2013;504:446–50. https://doi.org/10.1038/nature12721.
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science United States. 2013;341:569–73.
Park J-S, Lee E-J, Lee J-C, Kim W-K, Kim H-S. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int Immunopharmacol Netherlands. 2007;7:70–7.
Yang T, Magee KL, Colon-Perez LM, Larkin R, Liao Y-S, Balazic E, et al. Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats Acta Physiol (Oxf). Acta Physiol (Oxf) England. 2019;226:e13256.
Toral M, Robles-Vera I, de la Visitación N, Romero M, Yang T, Sánchez M, et al. Critical role of the interaction gut microbiota - sympathetic nervous system in the regulation of blood pressure. Front Physiol Switzerland. 2019;10:231.
Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–25.
Ermer T, Eckardt K-U, Aronson PS, Knauf F. Oxalate, inflammasome, and progression of kidney disease. Curr Opin Nephrol Hypertens England. 2016;25:363–71.
Kittanamongkolchai W, Mara KC, Mehta RA, Vaughan LE, Denic A, Knoedler JJ, et al. Risk of hypertension among first-time symptomatic kidney stone formers. Clin J Am Soc Nephrol United States. 2017;12:476–82.
Wang L, Zhu Q, Lu A, Liu X, Zhang L, Xu C, et al. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. J Hypertens Netherlands. 2017;35:1899–908.
Hsu C-N, Yu H-R, Lin I-C, Tiao M-M, Huang L-T, Hou C-Y, et al. Sodium butyrate modulates blood pressure and gut microbiota in maternal tryptophan-free diet-induced hypertension rat offspring. J Nutr Biochem. 2022;108:1–9.
Ichimura A, Hasegawa S, Kasubuchi M, Kimura I. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front Pharmacol. 2014;5:1–6.
El-Azzouny M, Evans CR, Treutelaar MK, Kennedy RT, Burant CF. Increased glucose metabolism and glycerolipid formation by fatty acids and GPR40 receptor signaling underlies the fatty acid potentiation of insulin secretion*. J Biol Chem. 2014;289:13575–88.
Secor JD, Fligor SC, Tsikis ST, Yu LJ, Puder M. Free fatty acid receptors as mediators and therapeutic targets in liver disease. Front Physiol. 2021;12:1–9.
•• Grundmann M, Bender E, Schamberger J, Eitner F. Pharmacology of free fatty acid receptors and their allosteric modulators. Int J Mol Sci. 2021;22:1–38. Scientists have identified numerous ligands that target allosteric sites on FFARs, aiming to create drugs for diverse diseases. Allosteric ligands for GPCRs are appealing due to their pharmacological profiles in comparison to orthosteric ligands. Despite this, the utilization of allosteric mechanisms in GPCR biology for medical purposes remains limited, with only a small number of allosteric ligands currently receiving approval. The review delves into the biology of FFARs, the molecular pharmacology of allosteric ligands targeting FFARs, and the opportunities and challenges associated with drug discovery in this context.
Schlatterer K, Peschel A, Kretschmer D. Short-chain fatty acid and FFAR2 activation – a new option for treating infections? Front Cell Infect Microbiol. 2021;11:1–9.
Mishra SP, Karunakar P, Taraphder S, Yadav H. Free fatty acid receptors 2 and 3 as microbial metabolite sensors to shape host health: pharmacophysiological view. Biomedicines. 2020;8:1–45.
Mohammad S. Role of free fatty acid receptor 2 (FFAR2) in the regulation of metabolic homeostasis. Curr Drug Targets United Arab Emirates. 2015;16:771–5.
Iván J, Major E, Sipos A, Kovács K, Horváth D, Tamás I, et al. The short-chain fatty acid propionate inhibits adipogenic differentiation of human chorion-derived mesenchymal stem cells through the free fatty acid receptor 2. Stem Cells Dev. 2017;26:1724–33.
He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21:1–16.
Nooromid M, Chen EB, Xiong L, Shapiro K, Jiang Q, Demsas F, et al. Microbe-derived butyrate and its receptor, free fatty acid receptor 3, but not free fatty acid receptor 2, mitigate neointimal hyperplasia susceptibility after arterial injury. J Am Heart Assoc. 2020;9:1–12.
Kennedy RL, Vangaveti V, Jarrod G, Baune BT. Free fatty acid receptors: emerging targets for treatment of diabetes and its complications. Ther Adv Endocrinol Metab. 2010;1:165–75.
Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology United States. 2013;154:3552–64.
Priyadarshini M, Layden BT. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets United States. 2015;7:e1045182.
Zamarbide M, Martinez-Pinilla E, Gil-Bea F, Yanagisawa M, Franco R, Perez-Mediavilla A. Genetic inactivation of free fatty acid receptor 3 impedes behavioral deficits and pathological hallmarks in the APPswe Alzheimer’s disease mouse model. Int J Mol Sci. 2022;23:1–19.
Jadeja RN, Jones MA, Fromal O, Powell FL, Khurana S, Singh N, et al. Loss of GPR109A/HCAR2 induces aging-associated hepatic steatosis. Aging (Albany NY). 2019;11:386–400.
Giri B, Belanger K, Seamon M, Bradley E, Purohit S, Chong R, et al. Niacin ameliorates neuro-inflammation in parkinson’s disease via GPR109A. Int J Mol Sci. 2019;20:1–14. https://www.proteinatlas.org/ENSG00000182782-HCAR2/tissue.
Abdelrahman AA, Powell FL, Jadeja RN, Jones MA, Thounaojam MC, Bartoli M, et al. Expression and activation of the ketone body receptor HCAR2/GPR109A promotes preservation of retinal endothelial cell barrier function Exp Eye Res England. 2022;221.
Yu J, Xiang JY, Xiang H, Xie Q. Cecal butyrate (not propionate) was connected with metabolism-related chemicals of mice, based on the different effects of the two Inonotus obliquus extracts on obesity and their mechanisms. ACS Omega. 2020;5:16690–700.
Lin HV, Frassetto A, Kowalik EJJ, Nawrocki AR, Lu MM, Kosinski JR, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:1–9.
Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N. Ghrelin, CCK, GLP-1, and PYY(3–36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev United States. 2017;97:411–63.
Eslick S, Williams EJ, Berthon BS, Wright T, Karihaloo C, Gately M, et al. Weight loss and short-chain fatty acids reduce systemic inflammation in monocytes and adipose tissue macrophages from obese subjects. Nutrients. 2022;14:1–17.
•• van Deuren T, Blaak EE, Canfora EE. Butyrate to combat obesity and obesity-associated metabolic disorders: current status and future implications for therapeutic use. Obes Rev. 2022;23:1–27. Butyrate, a short-chain fatty acid synthesized by gut bacteria, exhibits potential therapeutic benefits for obesity and associated metabolic disorders. Findings from animal studies indicate favorable effects on adipose tissue metabolism, inflammation, insulin sensitivity, and the regulation of body weight. Nevertheless, human research is restricted, and variations among individuals suggest that results might be influenced by personal characteristics. Subsequent research should explore factors affecting treatment outcomes and refine targeted interventions for optimization.
Mayorga-Ramos A, Barba-Ostria C, Simancas-Racines D, Guamán LP. Protective role of butyrate in obesity and diabetes: New insights. Front Nutr. 2022;9:1–9.
Zhu W, Peng K, Zhao Y, Xu C, Tao X, Liu Y, et al. Sodium butyrate attenuated diet-induced obesity, insulin resistance and inflammation partly by promoting fat thermogenesis via intro-adipose sympathetic innervation. Front Pharmacol. 2022;13:1–13.
Kushwaha V, Rai P, Varshney S, Gupta S, Khandelwal N, Kumar D, et al. Sodium butyrate reduces endoplasmic reticulum stress by modulating CHOP and empowers favorable anti-inflammatory adipose tissue immune-metabolism in HFD fed mice model of obesity. Food Chem Mol Sci. 2022;4:1–11.
Khan S, Jena G. Sodium butyrate reduces insulin-resistance, fat accumulation and dyslipidemia in type-2 diabetic rat: a comparative study with metformin. Chem Biol Interact Ireland. 2016;254:124–34.
Jia Y, Hong J, Li H, Hu Y, Jia L, Cai D, et al. Butyrate stimulates adipose lipolysis and mitochondrial oxidative phosphorylation through histone hyperacetylation-associated β(3)-adrenergic receptor activation in high-fat diet-induced obese mice. Exp Physiol England. 2017;102:273–81.
Jia X, Xu W, Zhang L, Li X, Wang R, Wu S. Impact of gut microbiota and microbiota-related metabolites on hyperlipidemia. Front Cell Infect Microbiol. 2021;11:1–14.
Oyabambi AO, Olaniyi KS. Sodium butyrate aggravates glucose dysregulation and dyslipidemia in high fat-fed Wistar rats Metab Open England. 2023;17:100226.
Hong J, Jia Y, Pan S, Jia L, Li H, Han Z, et al. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget United States. 2016;7:56071–82.
Karoor V, Strassheim D, Sullivan T, Verin A, Umapathy NS, Dempsey EC, et al. The short-chain fatty acid butyrate attenuates pulmonary vascular remodeling and inflammation in hypoxia-induced pulmonary hypertension. Int J Mol Sci. 2021;22:1–19.
Panagia M, He H, Baka T, Pimentel DR, Croteau D, Bachschmid MM, et al. Increasing mitochondrial ATP synthesis with butyrate normalizes ADP and contractile function in metabolic heart disease. NMR Biomed. 2020;33:1–10.
Kirschner SK, Deutz NEP, Rijnaarts I, Smit TJ, Larsen DJ, Engelen MPKJ. Impaired intestinal function is associated with lower muscle and cognitive health and well-being in patients with congestive heart failure. JPEN J Parenter Enteral Nutr United States. 2022;46:660–70.
Tilves C, Yeh HC, Maruthur N, Juraschek SP, Miller E, White K, et al. Increases in circulating and fecal butyrate are associated with reduced blood pressure and hypertension: results from the SPIRIT trial. J Am Heart Assoc. 2022;11:1–9.
Dardi P, dos Santos-Eichler RA, de Oliveira S, Vinolo MAR, Câmara NOS, Rossoni LV. Reduced intestinal butyrate availability is associated with the vascular remodeling in resistance arteries of hypertensive rats. Front Physiol. 2022;13:1–12.
Zhang L, Deng M, Lu A, Chen Y, Chen Y, Wu C, et al. Sodium butyrate attenuates angiotensin II-induced cardiac hypertrophy by inhibiting COX2/PGE2 pathway via a HDAC5/HDAC6-dependent mechanism. J Cell Mol Med. 2019;23:8139–50.
Robles-Vera I, Toral M, de la Visitación N, Sánchez M, Gómez-Guzmán M, Romero M, et al. Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: role of short-chain fatty acids Mol Nutr Food Res Germany. 2020;64:e1900616.
Wang Y, Xu Y, Yang M, Zhang M, Xiao M, Li X. Butyrate mitigates TNF-α-induced attachment of monocytes to endothelial cells. J Bioenerg Biomembr Journal of Bioenergetics and Biomembranes. 2020;52:247–56.
Marcil V, Delvin E, Seidman E, Poitras L, Zoltowska M, Garofalo C, et al. Modulation of lipid synthesis, apolipoprotein biogenesis, and lipoprotein assembly by butyrate. Am J Physiol - Gastrointest Liver Physiol. 2002;283:340–6.
Barcelos RCS, de Mello-Sampayo C, Antoniazzi CTD, Segat HJ, Silva H, Veit JC, et al. Oral supplementation with fish oil reduces dryness and pruritus in the acetone-induced dry skin rat model. J Dermatol Sci Netherlands. 2015;79:298–304.
Ma H, Yang L, Liu Y, Yan R, Wang R, Zhang P, et al. Butyrate suppresses atherosclerotic inflammation by regulating macrophages and polarization via GPR43/HDAC-miRNAs axis in ApoE−/− mice. PLoS One. 2023;18:1–23. https://doi.org/10.1371/journal.pone.0282685.
Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation United States. 2019;139:1407–21.
Osto E. The promise of the gut metabolite propionate for a novel and personalized lipid-lowering treatment. Eur Heart J. 2022;43:534–7.
Pakhomov N, Baugh JA. The role of diet-derived short-chain fatty acids in regulating cardiac pressure overload. Am J Physiol - Hear Circ Physiol. 2021;320:H475–86.
Ward NC, Carnagarin R, Nolde JM, Lugo-Gavidia LM, Chan J, Jose A, et al. Circulating short-chain fatty acids in hypertension: a reflection of various hypertensive phenotypes. J Hypertens Netherlands. 2022;40:1589–96.
Tain Y, Hou C, Lin S, Tzeng H, Lee W, Wu KLH, et al. Reprogramming effects of postbiotic butyrate and propionate on maternal high fructose diet-induced offspring hypertension. Nurtients. 2023;15:1–15.
Kassan M, Kwon Y, Munkhsaikhan U, Sahyoun AM, Ishrat T, Galán M, et al. Protective role of short-chain fatty acids against Ang-II-induced mitochondrial dysfunction in brain endothelial cells: a potential role of heme oxygenase 2. Antioxidants. 2023;12:1–14.
Cismaru CA, Cismaru GL, Burz C, Nutu A, IBN. SARS-CoV-2 – the hidden agonist of the pressor arm of renin- angiotensin system : considerations for statins and propionate derivatives. J Med Radiat Oncol. 2021;1:131–8.
Chinnathambi V, Blesson CS, Vincent KL, Saade GR, Hankins GD, Yallampalli C, et al. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertens (Dallas, Tex 1979) United States; 2014;64:405–14.
Muralitharan RR, Marques FZ. Diet-related gut microbial metabolites and sensing in hypertension. J Hum Hypertens. 2021;35:162–9.
• Haghikia A, Zimmermann F, Schumann P, Jasina A, Roessler J, Schmidt D, et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur Heart J. 2022;43:518–33. The research investigates how propionic acid (PA), a byproduct originating from the gut microbiota, influences the metabolism of cholesterol in the intestines and the development of atherosclerosis. Furthermore, it assesses how PA affects the levels of factors related to glucose metabolism and lipid regulation in the plasma. The findings reveal that PA diminishes atherosclerosis through immune-dependent control of intestinal cholesterol metabolism, leading to a reduction in cholesteryl esters. Moreover, a clinical trial illustrates the impact of PA on LDL and overall cholesterol levels in individuals dealing with hypercholesterolemia.
Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol England. 2017;19:29–41.
Yi C, Sun W, Ding L, Yan M, Sun C, Qiu C, et al. Short-chain fatty acids weaken ox-LDL-induced cell inflammatory injury by inhibiting the NLRP3/caspase-1 pathway and affecting cellular metabolism in THP-1 cells. Molecules. 2022;27:1–15.
Hou Y-F, Shan C, Zhuang S-Y, Zhuang Q-Q, Ghosh A, Zhu K-C, et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease Microbiome England. 2021;9:34.
Yan J, Pan Y, Shao W, Wang C, Wang R, He Y, et al. Beneficial effect of the short-chain fatty acid propionate on vascular calcification through intestinal microbiota remodelling. Microbiome. BioMed Central. 2022;10:1–30. https://doi.org/10.1186/s40168-022-01390-0.
Ang Z, Ding JL. GPR41 and GPR43 in obesity and inflammation - protective or causative? Front Immunol. 2016;7:1–5.
Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki S-I, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol an Off J Polish Physiol Soc Poland. 2008;59(Suppl 2):251–62.
Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1–12.
Bellahcene M, O’Dowd JF, Wargent ET, Zaibi MS, Hislop DC, Ngala RA, et al. Male mice that lack the G-protein-coupled receptor GPR41 have low energy expenditure and increased body fat content. Br J Nutr. 2013;109:1755–64.
Carbone AM, Borges JI, Suster MS, Sizova A, Cora N, Desimine VL, et al. Regulator of G-protein signaling-4 attenuates cardiac adverse remodeling and neuronal norepinephrine release-promoting free fatty acid receptor FFAR3 signaling. Int J Mol Sci. 2022;23:1–13.
Xu J, Moore BN, Pluznick JL. Short-chain fatty acid receptors and blood pressure regulation: Council on Hypertension Mid-Career Award for Research Excellence. 2021 Hypertension. American Heart Association. 2022;79:2127–37. https://doi.org/10.1161/HYPERTENSIONAHA.122.18558.
Park BO, Kim SH, Kim JH, Kim SY, Park BC, Han SB, et al. The short-chain fatty acid receptor gpr43 modulates yap/taz via rhoa. Mol Cells. 2021;44:458–67.
Nakajima A, Nakatani A, Hasegawa S, Irie J, Ozawa K, Tsujimoto G, et al. The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS ONE. 2017;12:1–18.
Bros M, Haas K, Moll L, Grabbe S. RhoA as a key regulator of innate and adaptive immunity. Cells. 2019;8:1–30.
Mosaddad SA, Salari Y, Amookhteh S, Soufdoost RS, Seifalian A, Bonakdar S, et al. Response to mechanical cues by interplay of YAP/TAZ transcription factors and key mechanical checkpoints of the cell: a comprehensive review. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol Germany. 2021;55:33–60.
Yoo H, Singh A, Li H, Strat AN, Bagué T, Ganapathy PS, et al. Simvastatin attenuates glucocorticoid-induced human trabecular meshwork cell dysfunction via YAP/TAZ inactivation. Curr Eye Res England. 2023;48:736–49.
Kobayashi M, Mikami D, Kimura H, Kamiyama K, Morikawa Y, Yokoi S, et al. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun United States. 2017;486:499–505.
Luo QJ, Sun MX, Guo YW, Tan SW, Wu XY, Abassa KK, et al. Sodium butyrate protects against lipopolysaccharide-induced liver injury partially via the GPR43/β-arrestin-2/NF-κB network. Gastroenterol Rep. 2021;9:154–65.
Chen L, Yuan J, Li H, Ding Y, Yang X, Yuan Z, et al. Trans-cinnamaldehyde attenuates renal ischemia/reperfusion injury through suppressing inflammation via JNK/p38 MAPK signaling pathway. Int Immunopharmacol Netherlands. 2023;118:110088.
Zhang X, Liu Z, Li W, Kang Y, Xu Z, Li X, et al. MAPKs/AP-1, not NF-κB, is responsible for MCP-1 production in TNF-α-activated adipocytes. Adipocyte (Taylor & Francis). 2022;11:477–86. https://doi.org/10.1080/21623945.2022.2107786.
Funding
None.
Author information
Authors and Affiliations
Contributions
Seyed Sadeq Mousavi Ghahfarrokhi and Mohamadsadegh Mohamadzadeh: writing—original draft. Nasrin Samadi, Mohammad-Reza Fazeli, Sara Khaki: visualization, review, and editing. Bahman Khameneh and Ramin Khameneh Bagheri: conceptualization and supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mousavi Ghahfarrokhi, S., Mohamadzadeh, M., Samadi, N. et al. Management of Cardiovascular Diseases by Short-Chain Fatty Acid Postbiotics. Curr Nutr Rep (2024). https://doi.org/10.1007/s13668-024-00531-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s13668-024-00531-1