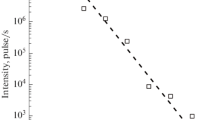
Abstract
The binary system Sb-Se was studied via laser ablation using antimony-selenium mixtures made from powdered elements in various ratios generating new SbmSen clusters. The results show that in addition to Sbm+ (m = 1–8) and Sen+ (n = 2–9) clusters, a series of SbmSen+ clusters such as SbSe1–8+, Sb2Se1–6+, Sb3Se1–5+, Sb4Se1–3+, and Sb5Se1,2+ is generated. In addition, some low intensity oxidized clusters like Se6O2+, Se7O2+, and SbSe2-6O5+ and partially hydroxylated clusters (SbSeO2H7+, SbSe5O4H+) are also formed. In total, 24 new antimony selenide clusters were generated. The knowledge gained can contribute to the elucidation of the structure of SbmSen glasses.

Graphical Abstract
Similar content being viewed by others
Introduction
Antimony forms several selenides. The structure of Sb2Se3 has been determined [1,2,3,4]. Recently, polycyclic polycations [Sb10Se10]2+, [Sb7Se8Br2]3+ and [Sb13Se16Br2]5+ [5, 6], Ba2Sb2Se5, and Ba6Sb7Se16 compounds [7] and a huge Sb12Se204− zintl anion [8] have been prepared and characterized. Antimony-selenium glasses are important members of the chalcogenide range of glasses, especially Ge-As(Te)-Sb-Se. Ge-Sb-Se moldable compounds are used in infrared optics [9,10,11].
In this work, the binary system Sb-Se was studied via laser ablation generating SbmSen clusters, in both positive and negative ion modes, using antimony-selenium powdered mixtures in various ratios as precursors. Laser ablation generation with quadrupole ion trap time-of-flight mass spectrometry (QIT-TOFMS) has already been shown to be an important and powerful methodology for studying the formation of clusters [12]; while the composition of SbmSen clusters was determined via computer simulation of the isotopic envelopes.
Experimental
Chemicals
1-Butyl-3-methylimidazolium chloride, antimony, and selenium powders were purchased from Sigma-Aldrich (St. Louis, Mo., USA). Glycerol and xylene were purchased from Lach-Ner (Neratovice, Czech Republic). Parafilm was purchased from Bemis NA (Neenah, Wis., USA). Acetonitrile and hydrochloric acid were purchased from Sigma-Aldrich (Steinheim, Germany). Red phosphorus was obtained from Riedel de Haën (Hannover, Germany) and was purified via sublimation in a nitrogen atmosphere. Water was double distilled using a quartz apparatus from Heraeus Quarzschmelze (Hanau, Germany). All other reagents were of analytical grade purity.
Mass Spectrometry
Mass spectra were recorded in both positive and negative ion modes using the mass spectrometer AXIMA Resonance from Kratos Analytical Ltd. (Manchester, UK) coupled with a quadrupole ion trap and time-of-flight detection. The instrument was equipped with a nitrogen laser (337 nm). The laser repetition rate was set to 5 Hz with a pulse time width of 3 ns.
Software and Computation
Stoichiometry of SbmSen clusters was determined via computer modeling of the isotopic envelopes using Launchpad software (Kompact version 2.9.3, 2011) from Kratos Analytical Ltd. (Manchester, UK).
Sample Preparation for Mass Spectrometry
The surface of solid antimony in pellets was cleaned by applying 0.1 M hydrochloric acid for 5 min. The pellets were then washed with double-distilled water and acetonitrile. Solid antimony was then powdered in an agate mortar. Finally, antimony, selenium, and antimony-selenium mixtures were suspended in acetonitrile (1 mg/ml). From these suspensions, 10 μL was deposited on a target and dried.
For laser ablation in glycerol a 10-μL sample of the Sb-Se suspension in acetonitrile was deposited on a target and dried as mentioned above. After adding 10-μL glycerol, the sample for laser ablation in glycerol was obtained.
For laser ablation generation in mixture of paraffines, 1 cm2 of parafilm was dissolved in 1 ml of xylene, and then the powdered mixture of antimony-selenium (1 mg) was suspended in this solution. Then, about 1 μL of the suspension was deposited on a target. After drying, the mass spectra were generated.
For laser ablation generation in ionic liquid, the powdered Sb-Se mixture was mixed with an ionic liquid (1-butyl-3-methyl imidazolium chloride), dissolved in acetonitrile, and ~ 1 μL of the suspension was deposited onto a target and dried.
Results
Antimony, selenium, and antimony-selenium clusters generated via laser ablation were studied. A reflectron mode was employed to record the mass spectra in both positive and negative ion modes. It was shown that many more clusters were generated in positive ion mode and the clusters produced in negative ion mode were of low intensity. The results will therefore be presented mainly for the positive ion mode. The majority of the clusters were formed in the m/z range of 200–800.
Antimony and Selenium
The overview of clusters generated from antimony and selenium is given in Table 1. Apart from Sbm+ (m = 1–6) and Sen+ (n = 2–9) clusters, several oxygen containing clusters were also detected.
Antimony Selenium Mixture (Molar Ratio Sb:Se = 1:1)
Examples of selected mass spectra are given in Figure 1, where identified clusters are also provided. For the ratio Sb:Se = 1:1, some Sen+ clusters (n = 4–9) and several SbSen+ (n = 3–7) were detected. In addition, two oxidized clusters of Se6O2+ and Se7O2+ were identified. A comparison of experimental and theoretical isotopic patterns for SbSe2+ is given in Fig. S1 A, and these are in good agreement.
Antimony Selenium Mixture (Molar Ratio Sb:Se = 10:1)
Here, the mass spectra recorded from the ratio Sb:Se = 10:1 are given in Figure 2a. In addition to several Sen+ (n = 4–9) and SbSen+ (n = 2–7) clusters, some other clusters such as SbSenO5+ (n = 2–6) were also detected, while the latter ones were always overlapped with SbSen+ clusters. Also, another two SbSe5O4H+ and Sb5O7+ clusters are shown in the inset spectrum. Comparison of experimental and theoretical isotopic patterns demonstrating the overlap of SbSe3+ and SbSe2O5+ clusters is shown in Fig. S1 B and there is good agreement between the experimental results and the theoretical model.
(a) Mass spectra recorded from LDI of Sb:Se mixture with the ratio of element equal to 10:1. Inset shows magnification of spectra in m/z range 550–800. Conditions: positive ion mode, laser energy 130 a.u. (b) Mass spectra recorded from LDI of Sb:Se mixture with the ratio of elements equal to 1:10. Inset shows magnification of spectra in m/z range 700–800. Conditions: positive ion mode, laser energy 180 a.u
Antimony Selenium Mixture (Molar Ratio Sb:Se = 1:10)
When selenium was in high excess over antimony, the mass spectra were different (Figure 2b). In this case, SbSen+ (n = 2–8), Sb2Sen+ (n = 1–6), Sb3Sen+ (n = 1–5), Sb4Sen+ (n = 1–3) and Sb5Sen+ (n = 1, 2) clusters were detected. Apart from these clusters, Sen+ (n = 4–9) clusters were also observed. Comparison of theoretical isotopic patterns corresponding to overlapping peaks of Se8+, Sb2Se5+, and Sb4Se2+ with the experimental spectrum is given in Fig. S1 C. Good agreement between the theoretical models and the experimental results was observed.
The Effect of Laser Energy (Molar Ratio Sb:Se = 1:1, Negative Ion Mode)
Spectra in the negative ion mode (Fig. S2) are different when compared to the positive ion mode. The most intensive peak is Se4− which contrasts with positive ion mode where Se5+ was the most intensive peak (cf. Figure 1). The effect of laser energy shown in this figure demonstrates that the intensity of clusters increases when laser energy goes from 90 to 100 a.u., but the mass spectra intensities decrease when the laser energy is increased to 110 a.u. A similar trend was observed for the Se4− cluster. Except for Se4−, the intensity of spectra grows as the laser energy increases. It is interesting to note that in both positive and negative ion modes, SbSeO2H7+/− clusters were detected (Fig. S3).
Review of SbmSen Clusters
An overview of the SbmSen clusters generated from Sb:Se = 1:1, Sb:Se = 1:10, and Sb:Se = 10:1 is given in Table 1 and Fig. S4. Interestingly, all SbmSen clusters produced when using Sb:Se = 1:1 are also observed when Sb:Se was equal to 10:1.
Some Special Approaches to Measurement
In addition to laser ablation of the Sb-Se mixtures, some special approaches were examined.
Laser Ablation Generation in the “Hole”
When the laser was fired on one point (Fig. S5) with a power from 80 a.u. to 180 a.u., there is a formation of a “hole” in the deposited sample material (Sb:Se = 1:1). The generation goes on in the reaction “crucible” (hole formed by the laser) and the clusters were observed at 140 a.u. (trace point). Although the intensities of clusters are low, some new Sb2Se+, Sb2Se2+, Sb3Se+, and Sb3+ clusters are formed (compare to Figure 1). As the laser energy increased, the proportion of Sb2Se2+ overlaying Se5+ and Sb2Se2+ increased.
Laser Ablation Generation of SbmSen Clusters in Glycerol, Parafilm, and in Ionic Liquid
When a mixture of Sb:Se = 1:1 was prepared in glycerol, laser ablation of this mixture showed the same clusters (compare with Figure 1), except for SbSeO2H7+, Se6O2+, and Se7O2+ clusters (Fig. S6), but the spectra were of lower intensity.
When the mixture of Sb:Se = 1:1 was prepared in parafilm, the clusters produced in m/z range 300–600 are the same as those observed using just a 1:1 mixture (Figure 1), but they are different in m/z range 600–900 (Figure 3), where four clusters Sb3Sen+ (n = 3–6) were generated.
When a mixture of antimony-selenium (1:1) was prepared in an ionic liquid, in comparison to Figure 1, SbSe3+ and two new clusters Sb2Se2+ and Sb3Se+ were identified in the mass spectrum (Fig. S7). Some clusters were of complex overlay and were not identified.
Laser Ablation of a Commercial Sb2Se3 Compound
Laser desorption ionization of commercial crystalline Sb2Se3 shows the formation of SbSe2+, SbSe3+, Sb2Se2–4+, Sb2Se3–4+, and Sb3Se1–5+ clusters, the same as when mixtures of Sb:Se = 1:10 were ablated. The only exceptions are Sen+ clusters. Missing Sen+ clusters is natural, as Sen+ structural fragments with Se-Se bounds are not part of Sb2Se3 crystal structure (Figure 4). In negative ion mode, only SbSe2−, Sb2Se−, SbSe3−, and Sb2Se2− clusters were detected.
Structure of SbmSen Clusters
Structures of some unary clusters were recently determined for selenium [12,13,14,15] and antimony [16, 17] clusters. However, structures of generated binary clusters are not known. The determination of the structures from mass spectra is not possible and quantum chemistry computation is outside the scope of this work.
Discussion
In order to understand the structures of Sb-Se chalcogenide glasses, we studied the formation of clusters generated from a mixture of Sb-Se in three different ratios and in different media. Comparing the results obtained, the clusters formed when using the ratio Sb:Se = 1:1 and 10:1 was quite similar. Contamination of SbmSen+ clusters with oxidized SbmSenO5+ species was not observed for Sb:Se = 1:1 samples. This is probably because antimony is easily oxidized and thus oxidized specie was detected from samples with high excess of antimony. Therefore, when antimony was in high excess, some oxidized species like SbmSenO5+ could be produced in greater extend. The situation was different when Sb:Se = 1:10, many new SbmSen+ clusters were observed which were not generated in Sb:Se = 1:1. The reason could be that selenium has a smaller atomic diameter and lower melting temperature than antimony. Therefore, when selenium is in molar excess, the selenium surrounds the antimony, and under the influence of the laser, it will melt first and combine with the antimony around it to form various clusters.
When the laser acts on one point, a “hole” will be created. Although low laser energy is not enough to form ions, chemical reactions actually took place between the elements.
Conclusions
Laser desorption ionization with quadrupole ion trap time-of-flight mass spectrometry (QIT-TOFMS) can be used as a type of efficient device to generate various SbmSen clusters. The clusters Sbm, Sen, and SbmSen were generated from mixtures of the elements antimony and selenium. In total, 24 new antimony selenide clusters were observed. The results obtained contribute to a deeper understanding of the preparation and structure of SbmSen materials, glasses, or various phase-change products.
References
Tideswell, N.W., Kruse, F.H., McCullough, J.D.: The crystal structure of antimony selenide, Sb2Se3. Acta Cryst. 10, 99–102 (1957)
Voutsas, G.P., Papazoglou, A.G., Rentzeperis, P.J., Siapkas, D.: The crystal structure of antimony selenide, Sb2Se3. Z. Kristallogr. Cryst. Mater. 171, 261–268 (1985)
Vadapoo, R., Krishnan, S., Yilmaz, H., Marin, C.: Electronic structure of antimony selenide (Sb2Se3) from GW calculations. Phys. Status Solidi B. 3, 700–705 (2011)
Deringer, V.L., Stoffel, R.P., Wuttig, M., Dronskowski, R.: Vibrational properties and bonding nature of Sb2Se3 and their implications for chalcogenide materials. Chem. Sci. 6, 5255–5262 (2015)
Ahmed, E., Isaeva, A., Fiedler, A., Haft, M., Ruck, M.: [Sb10Se10]2+, a heteronuclear polycyclic polycation from a room-temperature ionic liquid. Chem. Eur. J. 17, 6847–6852 (2011)
Ahmed, E., Breternitz, J., Groh, M.F., Isaeva, A., Ruck, M.: [Sb7Se8Br2]3+ and [Sb13Se16Br2]5+- double and quadruple spiro cubanes from ionic liquids. Eur. J. Inorg. Chem. 19, 3037–3042 (2014)
Wang, J., Lee, K., Kovnir, K.: Synthesis, crystal structure, and thermoelectric properties of two new barium antimony selenides: Ba2Sb2Se5 and Ba6Sb7Se16. J. Mater. Chem. C. 3, 9811–9818 (2015)
Martin, T.M., Wood, P.T., Kolis, J.W.: Synthesis and structure of an [Sb12Se20]4− salt: the largest molecular zintl ion. Inorg. Chem. 33, 1587–1588 (1994)
Ko, J.B., Myung, T.-S.: Structural and thermal properties of Ge-Sb-Se chalcogenide glasses for an application in infrared optical product design and manufacture. J. Ceram. Process. Res. 12, 132–134 (2011)
Choi, J.H., Cha, D.-H., Kim, J.-H., Kim, H.-J.: Development of thermally stable and moldable chalcogenide glass for flexible infrared lenses. J. Mater. Res. 31, 1674–1680 (2016)
Parnell, H., Butterworth, J.H., Sakr, H., Tang, Z., Furniss, D., Benson, T.M., Scotchford, C., Seddon, A.B.: Ge-Sb-Se glass fiber-optics for in-vivo mid-infrared optical biopsy. Proc. SPIE. 9703, 970309 (2016)
Prokeš, L., Kubáček, P., Peña-Méndez, E.M., Amato, F., Conde, J.E., Alberti, M., Havel, J.: Laser ablation synthesis of gold selenides by using a mass spectrometer as a synthesizer: laser desorption ionization time-of-flight mass spectrometry. Chem. Eur. J. 22, 11261–11268 (2016)
Hohl, D., Jones, R.O., Car, R., Parrinello, M.: The structure of selenium clusters - Se3 to Se8. Chem. Phys. Lett. 139, 540–545 (1987)
Xu, W., Bai, W.: The selenium clusters Sen (n = 1–5) and their anions: structures and electron affinities. J. Mol. Struc.-THEOCHEM. 854, 89–105 (2008)
Alparone, A.: Density functional theory Raman spectra of cyclic selenium clusters Sen (n = 5–12). Comput. Theor. Chem. 988, 81–85 (2012)
Geusic, M.E., Freeman, R.R., Duncan, M.A.: Neutral and ionic clusters of antimony and bismuth: a comparison of magic numbers. J. Chem. Phys. 89, 223–229 (1988)
Zhai, H.-J., Wang, L.-S., Kuznetsov, A.E., Boldyrev, A.I.: Probing the electronic structure and aromaticity of pentapnictogen cluster anions Pn 5− (Pn = P, As, Sb, and Bi) using photoelectron spectroscopy and ab initio calculations. J. Phys. Chem. A. 106, 5600–5606 (2002)
Acknowledgements
This work was funded with support from the Grant Agency of the Czech Republic (Projects No. GA18-03823S). This research has been also supported by CEPLANT, the project R&D center for low-cost plasma and nanotechnology surface modification, and CZ.1.05/2.1.00/03.0086 funding by the European Regional Development Fund and the Project CZ.1.07/2.3.00/30.0058 of the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Figure S1 A.
Comparison of experimental and theoretical isotopic pattern for SbSe2+. Conditions: positive ion mode, laser energy 130 a.u. (PNG 125 kb)
Figure S1 B.
Comparison of experimental and theoretical isotopic patterns for SbSe2O5+ and SbSe3+ clusters. Conditions: positive ion mode, laser energy 130 a.u. (PNG 202 kb)
Figure S1 C.
Comparison of experimental and theoretical isotopic patterns for Se8+, Sb2Se5+, and Sb4Se2+. Conditions: positive ion mode, laser energy 180 a.u. (PNG 296 kb)
Figure S2.
Mass spectra recorded from LDI of Sb:Se mixture with the ratio of elements equal to 1:1 in the m/z range 200-600. The effect of laser energy. Conditions: negative ion mode, laser energy 90-110 a.u.; * means the isotopic pattern is too complex or spectra of low intensity to identify clusters. (PNG 157 kb)
Figure S3.
Comparison of experimental and theoretical isotopic pattern for SbSeO2H7+. Conditions: positive ion mode, laser energy 130 a.u. (PNG 226 kb)
Figure S4.
Overview of stoichiometry of SbmSen clusters generated from Sb:Se = 1:1 and Sb:Se = 1:10. (PNG 121 kb)
Figure S5.
Mass spectra recorded from LDI of Sb:Se mixture with the ratio of elements equal to 1:1 in m/z range 250-500 (synthesis in the “hole”). The effect of laser energy. Conditions: laser acts on one point, positive ion mode, laser energy 140-180 a.u. (PNG 124 kb)
Figure S6.
Mass spectra recorded from LDI of Sb:Se mixture (1:1) in the glycerol. Conditions: positive ion mode, laser energy 130 a.u. (PNG 130 kb)
Figure S7.
Mass spectra recorded from LDI of Sb:Se mixture (1:1) in the ionic liquid. Conditions: positive ion mode, laser energy 130 a.u. (PNG 115 kb)
Rights and permissions
About this article
Cite this article
Huang, F., Prokeš, L. & Havel, J. Laser Ablation Generation of Antimony Selenide Clusters: Laser Desorption Ionization (LDI) Quadrupole Ion Trap Time of Flight Mass Spectrometry. J. Am. Soc. Mass Spectrom. 30, 634–638 (2019). https://doi.org/10.1007/s13361-018-2119-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-018-2119-3