Abstract
In order to meet a growing need for fieldable mass spectrometer systems for precise elemental and isotopic analyses, the liquid sampling-atmospheric pressure glow discharge (LS-APGD) has a number of very promising characteristics. One key set of attributes that await validation deals with the performance characteristics relative to isotope ratio precision and accuracy. Owing to its availability and prior experience with this research team, the initial evaluation of isotope ratio (IR) performance was performed on a Thermo Scientific Exactive Orbitrap instrument. While the mass accuracy and resolution performance for Orbitrap analyzers are well-documented, no detailed evaluations of the IR performance have been published. Efforts described here involve two variables: the inherent IR precision and accuracy delivered by the LS-APGD microplasma and the inherent IR measurement qualities of Orbitrap analyzers. Important to the IR performance, the various operating parameters of the Orbitrap sampling interface, high-energy collisional dissociation (HCD) stage, and ion injection/data acquisition have been evaluated. The IR performance for a range of other elements, including natural, depleted, and enriched uranium isotopes was determined. In all cases, the precision and accuracy are degraded when measuring low abundance (<0.1% isotope fractions). In the best case, IR precision on the order of 0.1% RSD can be achieved, with values of 1%–3% RSD observed for low-abundance species. The results suggest that the LS-APGD is a promising candidate for field deployable MS analysis and that the high resolving powers of the Orbitrap may be complemented with a here-to-fore unknown capacity to deliver high-precision IRs.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An area of continuing intense interest has been the development of portable or fieldable (man-portable, luggable, or transportable) analytical instrumentation. One specific set of drivers for portable or fieldable analytical instrumentation are the needs of the nuclear safeguards community for versatile, easy-to-use, in-field systems for determining the 235U/238U IRs in UF6 at enrichment facilities [1,2]. A potential future issue arises from new, stricter shipping regulations concerning gaseous UF6 samples [3]. An ideal mass spectrometer system would require minimal consumables, low operational overhead (power, cooling, water, etc.), produce little-to-no waste, require small sample amounts, be amenable to different sample forms (solids, liquids, gases), useable by personnel who are not mass spectrometry experts, and produce analytical results (accuracy and precision) similar to laboratory-based techniques.
There have been significant size reductions in diverse segments of separation sciences, mass spectrometry, optical spectroscopy, and sensor technologies [4–6]. One area that has not seen equivalent size reductions is the field of atomic spectroscopy, which still relies heavily on standard inductively coupled plasma (ICP) sources for optical emission spectroscopy and mass spectrometry. Despite the general activity in the field of microplasmas, including that of the electrolyte cathode discharge (ELCAD) presented by Cserfalvi and improved by Hieftje (explored as a source for optical emission analysis) [7–15], there has been no commercially accepted replacement of the ICP for use in the field of elemental analysis and none in the portable arena.
With the ultimate goal of developing a field deployable mass spectrometer, the liquid sampling-atmospheric pressure glow discharge (LS-APGDMS) makes significant strides in meeting the aforementioned goals. The normal operating parameters of the LS-APGD are gas flow rates of ≤1 L min–1, liquid flow rates of 5–40 μL min–1, and power consumption of only 50 W. Further, the LS-APGD is run in total consumption mode when operated correctly, which reduces waste streams to zero. Prior work out of the Marcus group using the LS-APGD for mass spectrometry shows promise as a miniature ion source [16–18]. While other elemental/atomic ion sources are not suitable to analyze molecular species due to excessive thermal dissociation, the LS-APGD source has also been shown to be a viable ion source for organics by simple changing the electrolytic solution makeup [19]. Conversely, the atmospheric pressure chemical ionization (APCI) source, which is itself a discharge-based device, is not applicable for elemental MS [20]. In the case of the APCI, the predominate ionization mechanism is a proton transfer reaction, whereas ionization of atomic species in the ICP or LS-APGD plasmas is via electron ionization. Furthermore, the LS-APGD has also been used as a secondary ion source for laser ablation samples and as a desorption ionization source for organic samples [21,22]. The work presented here seeks to demonstrate that a LS-APGD microplasma source is a viable option for isotope ratio mass spectrometry (IRMS). While the practical fields of use are still being developed for the LS-APGD, it is reasonable to question its potential applicability for IRMS, as applied in the laboratory and in the field. While initial work on the development of the LS-APGD has highlighted its versatility and defined its wide range of operating parameters for quantitative elemental analysis, there has been no work done on characterizing it as an ion source for IR analysis.
The initial demonstration of the LS-APGD as the first elemental microplasma ionization source was done using a Thermo Scientific Exactive Orbitrap instrument at EMSL/PNNL [16]. The LS-APGD was coupled to the instrument directly in the place of the supplied electrospray ionization (ESI) source, with no modifications to the ion sampling interface. In an effort to assess the IR performance of the microplasma source, it was logical to first test this using a standard commercial quadrupole inductively coupled plasma (ICP-MS) instrument. Unfortunately, the high pumping speed through the sampling orifice of the standard ICP-MS resulted in the microplasma being sucked into the interface, striking a discharge between the sampling and skimmer cones. As such, the derived mass spectra were inconsistent, and yielded no analytically relevant signals for solution-phase analytes. Ultimately, the skimmer cone was eroded, expanding (irregularly) by more than 50% of its initial diameter, after just an hour of operation. Thus, a benefit for the use of the LS-APGD is the sole ability to operate using standard AP interfaces found in organic MS systems, which are much smaller and lower overhead interfaces than those for ICP-MS. Unfortunately, this precluded the use of standard ICP-MS instruments to characterize the IR performance of the LS-APGD source, and so the efforts turned back to the use of Exactive Orbitrap instrument.
Orbitrap instruments are most widely applied in the area of biomolecular mass spectrometry [23,24], where they are used with ESI sources, to provide very high (m/Δm >100,000) mass resolving powers with high mass accuracy. As stated above the Orbitrap MS is not typically used for elemental IR analysis, but its standard configuration provides a flexible sampling interface, including the coupling of the LS-APGD. Of course, the high mass resolution provided by the Orbitrap is of interest for inter-element and molecular species interference reduction, as demonstrated previously. Unfortunately there has been a significant lack of research into using it as a mass analyzer for IRMS, and this is thus the basis for this work. This particular coupling involves two large unknowns as there have been no previous studies presented on the inherent capabilities of neither the microplasma nor the mass analyzer system in IRMS. As such, the study could not answer the independent questions explicitly, however the results that are presented show a promising level of accuracy and precision. The results are especially surprising given the fact that trapping mass spectrometers (of all forms) have largely been ignored in the field of isotope analysis and give hope that such instruments may successfully be used in the future, particularly in reduced footprint form.
Presented within are the results of a parametric optimization of the Orbitrap data acquisition paramters (scan range, capillary collision energy, collision cell energy, maximum ion injection time, and number of microscans per scan) and their roles in the spectral composition and IR statistics. Accuracy, bias, and precision for the IR analysis of uranium and other elements are reported. Uranium IR characteristics were determined for natural-abundance, depleted, and enriched samples. While the precise IR capabilities of the analyzer and the microplasma cannot be dissected at this point, it is clear that the combination of the LS-APGD and the Orbitrap MS itself generate figures of merit that are quite respectable, with precisions of ~1.08% relative standard deviation (% RSD).
Experimental
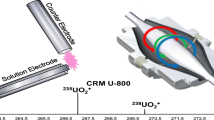
The basic components of the LS-APGD ion source have been described previously and are shown in Figure 1 [16–18]. The ion source consists of a anode that is made of a metal electrode (SS, weldable feedthrough, MDC Vacuum Products,LLC, Hayward California) that has a potential applied to it from a Glassman (High Bridge, NJ) Model EH power supply (0-100 mA, 0-1 kV) and a cathode. The cathode consists of an inner silica capillary (280 μm i.d., 580 μm o.d., Restek Corporation, Bellefonte, PA, USA) that delivers the sample solution to the plasma and an outer capillary (316 SS, 0.8 mm i.d., 1.6 mm o.d., IDEX Health and Science, Oak Harbor, WA, USA) that delivers the helium gas which acts as a cooling/sheath gas. The cathode is grounded as depicted in Figure 1. All experiments were run under continuous sample flow using an NE-1000 (New Era Pump Systems, Inc., Farmingdale, NY, USA) syringe pump. As the LS-APGD requires that a feed solution be electrolytic in nature, all solutions were prepared in aqueous 2% nitric acid. A 5 μg mL-1 natural-abundance U solution was prepared to complete a parametric optimization of both the Exactive and the LS-APGD. The natural U had an assumed 235U/238U value of 0.00725. Three additional solutions were prepared to evaluate and correct for isotopic mass bias; a 5 μg mL–1 sample of enriched uranium standard (CRM U-030a; New Brunswick Laboratory, Chicago, IL, USA) with a certified 235U/238U value of 0.03093, a natural-abundance uranium standard at 5 μg mL–1 (CRM-129a; New Brunswick Laboratory) with a certified 235U/238U value of 0.007258, and an in-housed determined 5 μg mL–1 of depleted uranium (235U/238U = 0.00192).
Additional elements having different chemical reactivity and diverse isotopic abundances were employed to gain insights into chemical and IR measurement issues. The mass spectrometric characteristics and IR performance for Ag, Rb, Tl, Ce, Ba, Pb, and U were evaluated using 5 μg mL–1 solutions. Isotopic analysis was performed using the atomic ions for each of the elements except for U. As is well known from early work in ICP-MS on Paul-type traps [25,26], atomic metal ions are quite reactive towards residual atmospheric gases (particularly O2) in the trapping cell. The extent of oxide formation is a function of the metal ion’s native oxygen affinity. In the case of measuring U, the oxide form UO2 + was utilized because little U+ or UO+ were detected, even after the system was optimized. In the case of Ba, the Ba+/BaO+ was approximately unity. In that case, a cursory comparison of the IR performance of the atomic and oxide ions surprisingly showed better performance for the oxides than the atomic ions. This point is addressed subsequently with reference to Table 2.
The Exactive Orbitrap located at the Environmental and Molecular Sciences Laboratory (EMSL) of the Pacific Northwest National Laboratory (PNNL) was used without any modification other than removing the standard electrospray/APCI source. The Exactive was operated in the positive ion mode and was controlled using the Thermo Xcalibur operating and data acquisition software. The in-source collision induced dissociation and the high-energy collisional dissociation (HCD) in the integrated HCD cell/trap were used to obtain clean spectra and to reduce the number of interfering molecular ions (principally H2O-related clusters). For this work, the Thermo software package, Tune, defines the data collection as a series of micro scans and scans. A microscan is the injection of ions into the Orbitrap for detection and the recording of that transient signal. A scan is the average of a selectable number of microscans. Scans themselves can be viewed independently or averaged again. Owing to the inherent variability from one scan to the next, a minimum of 10 scans was averaged together, defined as one acquisition. Data for the respective isotopes were processed as the integrated signals as this provided better precision than peak heights. To complete the parametric optimization, three acquisitions of 10 scans each made up a single data point.
Results and Discussion
Previous LS-APGD efforts on this Orbitrap instrument and on the Thermo Scientific LCQ Advantage Max at Clemson University resulted in similar dependencies relative to the plasma operation conditions of solution feed rate, discharge current, and ion sampling distance [18]. Those works dealt specifically with the responses of atomic ions. As noted above, the nature of the trapping process, first in the C-trap and then the Orbitrap cell yields a much higher percentage of the di-oxide (UO2 +) cation for uranium. Therefore, a cursory reevaluation of the plasma parameters relative to the yield of those species was performed. As suggested above, under the supposition that the oxides are formed in the mass analyzer, the initial plasma parameters were the same as those found for atomic ion analysis; more atomic ions lead to greater amounts of oxides. Ultimately, the LS-APGD was run at the following conditions throughout this effort: electrolyte (sample) flow = 30 μL min–1, He sheath gas flow = 0.5 L min–1, and a discharge current = 30 mA, equivalent to the previous works [17,18]. The parameters that were varied and optimized on the Orbitrap were the Fourier transform (FT) digitization window, the ion accumulation time prior to injection into the Orbitrap (termed injection time in the system software), the number of averaged micro scans that are averaged, and the number of scans making up the spectral data.
Use of Collisional Dissociation to Simplify Spectral Complexity
As will be described in the following section, one of the basic tenants of performing high quality mass spectrometry wherein Fourier transform processing is applied (i.e., Fourier transform-ion cyclotron resonance (FT-MS) and Orbitrap), is the minimization of spectral components, the noise of which is distributed across the entire spectrum [27]. Likewise, in all forms of trapping MS, sensitivity is limited when non-analyte ions make up a large fraction of the ion population; limiting the number of analyte ions that can be effectively trapped and analyzed (i.e., space charge effects) [28–33]. Finally, regardless of the resolving power of the mass analyzer, it is always beneficial to remove species that simply add spectral complexity. In the case of the LS-APGD microplasma, the unwanted matrix species in question are principally water-related ions of the general form of (H2O)nH+.
The Exactive Orbitrap provides two avenues for removing water-related background ions from the ion population that would eventually be injected from the C-trap to the Orbitrap detection cell; in-source collision-induced dissociation (CID) at the exit of the ion transfer capillary and transfer of the ions to the HCD cell for CID with helium gas atoms. These two routes were investigated as means of reducing the prevalence of undesirable background ions. Figure 2a and b illustrate the efficacy of in-source CID for the standard case of no potential difference between the capillary end and the ion lens stack, and the use of 50 eV accelerating potential for a 5 μg mL–1 U solution. As seen in Figure 2a (no CID), the resultant mass spectrum is dominated by water-related ions, though with a strong signal representing the UO2 + ion. Use of 50 eV in-source activation dramatically reduces the prevalence of the background species (Figure 2b), but as would be expected, has no appreciable effect on the signal intensity of the analyte species.
Activation in the HCD cell should affect the reduction of background molecular ion ((H2O)nH+) interferents as well as more robust species. The utility of HCD is illustrated in Figure 2c and d, for an example case of a pair of ions in the mass region of 267 Da. As seen in Figure 2, the most prominent uranium-related ion is 238UO2 + at nominal mass 270 Da. Of course, that species should be paired with the same chemical form for 235U, nominal mass 267 Da. The mass spectrum obtained for the 40 eV HCD energy presented in Figure 2c shows two distinct peaks. While the mass accuracy of the Exactive would allow ready identification of the target ion, CID at an energy of 70 eV (Figure 2d) completely removes the higher intensity, lower mass peak with only the 238UO2 + species remaining in that spectral region. Important to note here as well, the intensity of that robust oxide species is not diminished in the least in the HCD process. Based on a cursory evaluation of the two CID approaches, all subsequent work was undertaken at an in-source CID energy of 50 eV and an HCD set at 70 eV.
Role of Fourier Transform Digitization Window Width
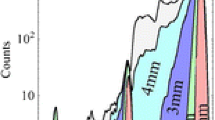
One of the common attributes of Fourier transform processing is the distribution of spectral noise across the entire spectrum [27]. In simple terms, in the flicker-noise limit, it is counterproductive to digitize signals/frequencies that are not of analytical relevance. For example, the propagation of spectral noise is the limiting aspect in the use interferometry, where Fourier transforms are used to extract spectral information, in the UV-VIS portions of the electromagnetic spectrum. As a result, interferometry in ICP-OES can yield incredible wavelength precision, but noise distributed across the spectrum results in poor signal-to-noise characteristics [34,35]. The design of the Exactive Orbitrap instrument allows all ions above m/z ≈ 50 to enter the storage C-trap, eventually to be injected in to the Orbitrap cell. Figure 3 illustrates the role of mass/frequency digitization window on the mass spectral composition and ultimately the IR ratio values obtained for 235U/238U (measured as the di-oxide ions). As a demonstration of the ionic species that contribute to the digitized mass spectra, Figure 3a presents the mass spectrum derived from processing the frequencies corresponding to the mass range of 70–470 Da. Likewise, Figure 3b shows the equivalent spectrum with the frequency range equating to 265–275 Da. In this case, the dynamic range in the digitization yields a much higher analyte signal response. This dynamic range extends to aspects relating to the 235U/238U IR, as the minor isotope is seen on the latter presented scale. In fact, the dynamic range plays a role in the resulting IRs, which changes dramatically as a function of the mass/frequency window as plotted in Figure 3c. In the case of the broadest mass range, the resultant 235U/238U ratio for this natural-abundance uranium was 0.00582. However, when the mass range was narrowed to 10 Da, the measured 235U/238U ratio increased to 0.00665, much closer to the accepted value (0.00725).
Potential explanations for the differences in the measured ratios in the Orbitrap might fall into two categories—space charge effects and the transient signal digitization/processing. In the Exactive Orbitrap instrument used here, there is no way to limit or mass-select the ions that enter the trap, and so space charge effects would be consistent across these experiments. The second factor that could affect the measured 235U/238U ratios revolves around the signal processing. As suggested in the previous paragraph, there is a limited dynamic range within the digitization process inclusive of all of the frequencies being sampled. Beyond this contribution, though, is a background subtraction process that occurs automatically in the Tune operation module used to extract the IRs. The criteria for this background correction are not available (nor controllable) to the user. In terms of performing IRs for ions that differ intensity by more than 100× (as is the case for 235U and 238U), any background subtraction will reduce the peak height/areas of the lower intensity isotope to a greater extent (proportionally) by this automatic process. With reference to the changes in the digitization window, wide mass ranges would be expected to propagate more noise across the spectrum and, as such, results in greater amounts of background subtraction. This greater influence is seen as the determined ratio increases with decreasing mass/frequency window widths. As will be discussed further, the influence of the automatic background correction is believed to play a key role in the accuracy and precision of extreme IRs. These points made, the fact that replicate ratios under the same conditions vary by ~1.08% RSD in all cases cannot be lost. Additionally, the accuracy of the measured values is also quiet good considering no internal standards are employed. As a result of these observations, all subsequent uranium IR data were processed using a collection window of 10 Da (m/z 265–275).
Roles of Ion Accumulation Time and Discharge Conditions
The potential deleterious effects of high ion densities in trapping-type mass analyzers are well documented [28–33,36]. Space-charge effects due to overfilling of traps can result in suppression of anticipated signal levels and corresponding intra-species ratios, broadening in the mass/frequency domains, and shifts in apparent ion masses. Thus, the injection time into the C-trap, the number of microscans equating to one scan, and the role of discharge conditions (controlling the ion introduction rate) were evaluated. The Exactive Orbitrap employs a pre-scan function which, based on ion source output, sets the maximum width of the ion injection time, referred to as automatic gain control. As such, the operator actually only has control of the maximum injection time per microscan. In initial testing, the injection time into the C-trap was limited by the system to 30–40 ms, even when the maximum injection time was set to 1000 ms. The 235U/238U ratio for UO2 was recorded as 0.0068 when operating in this mode. It was found that reducing the sheath gas flow to the LS-APGD from 1 L min–1 to 0.5 L min–1 resulted in increases in actual injection times and increased 235U/238U ratios, moving in a positive fashion to 0.00700. Decreasing the gas flow any further resulted in melting the inner capillary. The primary effect of reducing the gas flow is a relative lowering of the amount of water-related ions making up the mass spectrum. Thus, the ions eventually injected in to the Orbitrap cell are predominately analyte-related, and since the total number of ions is less, longer injection times can be employed with relatively lower amounts of spectral noise to eventually be subtracted. As discussed above, the large quantity of interfering ions limits the dynamic range of the system and thus reduces the measured 235U/238U values. The ability to use longer injection times should also result in better precision via time-averaging. To be clear, though, there is no evidence to this point that any of the typical space charge effects listed above (e.g., frequency shifts) are occurring in this LS-APGD/Orbitrap coupling.
The cumulative effects of the setting of the maximum injection time, the sheath gas flow rate, and the number of microscans making up a scan are presented in Figure 4. A complex set of relationships results, for which ambiguities about how the Exactive processes the spectral data are important. When the maximum injection time is set to 10 ms and the number or micro scans is set to 1, the 235U/238U is depressed, with no appreciable effect seen in terms of the gas flow. Significant scatter in the values is observed. Increasing the number of microscans to 10 does improve the ratio with regards to the accepted value of 0.00725, but still shows little gas flow rate dependence. In principle, increasing the number of microscans averaged to equate to a single scan should not appreciably affect the determined ratio, but perhaps improve the precision. Looking at the raw data for the extracted peak areas shows that these values increase somewhat as a function of the number of microscans, though not proportionally. It is not at all clear how this is the case; a true averaging should yield the same nominal peak intensities, with a reduction in the standard deviation of the background. Such an improvement may lower the count level of background that is automatically subtracted; this would yield higher observed ratios as discussed above. Furthermore, there is significant scatter in the data and no clear trend can be seen.
When the maximum injection time is increased to 1000 ms, a very different set of relationships is seen. In this data, the role of gas flow rate and the percentages of the spectra that are made up of unwanted background ions become quite clear. As the relative amount of background species is reduced with lower gas flow rates, the observed ratios increase. This is in line with the role of digitization bandwidth; fewer background ions result in lower amounts of background subtraction and subsequently higher IR values. The longer maximum injection times in this case do as would be expected in terms of producing lower variability. As in the case of the of the 10 ms injections, there is a slight increase in IR values using 1000 microscans. These are attributed to lower amounts of background that are automatically subtracted due to better averaging of background variations. Under these conditions, the recorded 235U/238U trends towards the expected value of 0.00725.
With a better appreciation of the potential influences of the water-related background species on the IR characteristics, a closer look at the roles of discharge conditions on producing those species was in order. The optimization of the liquid sample flow rate and current was done while keeping the maximum injection time (1000 ms), number of micro scans (10), and the sheath gas flow rate (0.5 L min–1) constant (though they were varied initially to be sure that interdependences did not exist). As can be seen in Figure 5, as the discharge current is increased from 20 to 40 mA, the IR increased; however, the liquid flow rate had no appreciable impact on the 235U/238U value. Across all of the liquid flow rates and discharge currents, the LS-APGD operates in a total consumption mode; that is, all of the electrolytic solution/sample are vaporized. What changes appreciably as the current increases, though, is the ability to vaporize the eluate and desolvate (decluster) hydrated analyte ions. This set of trends is entirely consistent with the more detailed parametric evaluation of the microplasma on the Paul-trap instrument [18]. To this end, as seen in the previous sections, the 235U/238U ratio increases as the amount of spectral background is decreased. While the data presented in Figure 6 suggests operation at conditions of 30 mA and 40 μL min–1, a current of 30 mA provided greater long-term stability of the plasma, which was felt to be more important in general than a higher accuracy.
235U/ 238U Isotope Ratio Statistics
With the parametric optimization complete, the IR figures of merit of the LS-APGD for natural, depleted, and enriched uranium were determined. The standard operating conditions were gas flow = 0.5 L min–1, discharge current = 30 mA, solution flow = 30 μL min–1, maximum injection time = 1000 ms, number of microscans = 10, mass range = 265–275 Da. Much different from results using a Paul-trap, it was observed that the reported 235U signal could vary significantly from run-to-run as the discharge was extinguished to change solution feedstocks or from experimental session to session. For example, when the plasma was turned off and then restarted, the signal intensity could vary significantly, ranging from intensities of 102 to 104 for the 235U isotope even though there was no change in the concentration of the solution. This observation would have been of concern if the determined ratio had been dependent on the signal intensity. However, when the 235U/238U ratio collected over a number of days to yield eight random data points was plotted against the corresponding 235U intensities (Figure 6) there was no appreciable change in the IR. In fact, the percentage relative standard deviation (% RSD) for these eight data acquisitions was a surprisingly good 1.33%.
Looking at the cumulative results of the depleted, natural (CRM-129a) and enriched (U-030a) uranium samples points to the situation where the influence of background subtraction on low-abundance isotopes is seen. The results presented in Table 1 reflect 10 data sets, each composed of 10 acquisitions of 10 scans, with the sample solutions changed in between each data set. In general, the intra-data sets showed lower standard deviations than across the entire set; as would be expected. As might be expected, the imprecision of the obtained values (% RSD) is the highest for the depleted sample (3.05% RSD), improving to more or less equivalent values of 1.37% and 1.27% RSD for the samples having higher 235U fractions. It is clear from the tabulated data that the imprecision is controlled by the measurement precision of the minor isotope samples. Indeed, the precision values for the ratio are virtually identical to those of the 235U.
Beyond the resultant IR precision statistics, the accuracy of the obtained values must be analyzed relative to potential error/biases. Deviations from expected IR values can be the result of non-uniform recoveries in the sample processing steps, preferential vaporization/ionization yields in the ion source, or differences in ion separation/detection efficiencies in the mass spectrometry step [37]. In the vast majority of elemental IR measurements bias is typically the result of the non-uniformity mass spectrometer throughput characteristics, with differences also attributable to the actual ionization source, detection modality, and the specific type of mass analyzer [38,39]. Correction (bias) factors were determined using the certified CRM-129a and U-030a solutions, dividing the certified ratio values by the experimentally determined values presented in Table 1. When this was done, the correction factor for the CRM-129a sample is 1.0572, with the correction factor for the enriched U-030a being 1.0499. In practice, the Orbitrap detection process (sensing of image currents) for the two analytes (235UO2 and 238UO2), should be virtually identical as the masses differ by only 3 Da. The bias from a value of unity seen here points in both cases to an underestimation of the lower abundance isotope, likely due to contributions in the background correction process.
IR Measurement Characteristics of Diverse Elements
The primary impetus for this effort was an assessment of the combined capabilities of the LS-APGD microplasma, which is a candidate for transportable instrumentation, and the Orbitrap, a mass analyzer that may hold great potential in elemental MS, for the measurement uranium IRs. There are natural questions regarding what aspects of the results are unique to the specific element. In order to evaluate the utility of the LS-APGD as an ion source for IRMS, Ag, Rb, Tl, Pb, Ba, and Ce were also studied using the same plasma and MS data acquisition conditions used above. These elements represent a range of potential IRs and tendencies to form oxides. Among this group of elements, Ag, Rb, and Tl have virtually no tendency to form oxides and so the IR performance was based on measurement of atomic ions. Pb, Ba, and Ce tend to yield higher oxide fractions. Based on the relative M+/MO+ fractions, Pb was analyzed as the atomic ion, Ba was evaluated in both atomic and oxide forms, and Ce (the strongest oxide former) was evaluated as the monoxide ion. The IR characteristics were evaluated using the same MS protocol as in the U studies with solution concentrations of 5 μg mL–1. Different from the uranium IR data reported in Table 1, each element was evaluated using a single set of 10 acquisitions versus 10 sets of 10 acquisitions (i.e., 10× few data points).
As presented in Table 2, the IR performance for these elements correlates well with the results reported for U. For those IRs having values of >0.5, the IR precision is generally better than 0.5% RSD, with the precision for Tl being <0.1% RSD. These values are considered to be quite good given the state of development of this instrumentation coupling, and are certainly of sufficient quality to be of use in many trace element applications. In each instance, the IR precision is severely degraded in cases were the minor isotope is a very small relative composition to the major isotope. Using Pb as an example, the IR precision for 206Pb/208Pb and 207Pb/208Pb have precision values of <0.25% RSD, whereas 204Pb/208Pb, which has a ratio value of 0.0236, has a precision of 3.31% RSD. This type of relationship is seen for all of the elements, with the eventual IR values underestimated for each isotopic pair relative to the natural abundances. Thus, the correction factors have values of >1.0. Across the entirety of the isotopic suite, it can be generally said that the correction values are much smaller in relative terms than are common across ICP-MS [38,39] (i.e., there is greater detection uniformity in the case of the Orbitrap analyzer).
As a final point of comparison, the respective IR performance for the atomic and monoxide ion forms of Ba were evaluated. In this specific case, the ion signals for the atomic and monoxide forms of the element are fairly equivalent (BaO+/Ba+ ≈ 1). As seen in Table 2, there is minimal difference in the derived IRs for specific isotope pairs in using atomic or monoxide ion forms. Additionally, there is no significant difference in the precisions observed in the given measurements. Although this is a reassuring phenomenon in terms of performance, it is somewhat surprising as the formation of monoxide ions (likely within the trapping steps) would be expected to show greater variability that the single step of forming the atomic ions.
Conclusions
The coupling of the liquid sampling-atmospheric pressure glow discharge (LS-APGD) microplasma and the Exactive Orbitrap has been previously presented with respect to multi-element analysis of aqueous solutions and laser ablation-produced particulates [16,17,21]. The ultimate goal in this task’s work on the LS-APGD technique is to provide an ionization source that facilitates portable or field-deployable mass spectrometry. To this end, the IR must be initially determined and ultimately shown to be relevant to the challenges at the application at hand. Ideally, those characteristics could be evaluated on mass analyzer systems of known IR performance. In the absence of such instruments, we describe here the initial characterization on the Orbitrap platform. To date, no detailed analysis of IR performance has been reported. As such, the efforts here look to assess the combined performance in hopes of gaining insight into how both components could be employed in IR mass spectrometry.
At this point, the IR performance observed to date for this combination of ion source and mass analyzer is very encouraging. The key aspects of LS-APGD operation to yield sensitive mass spectroscopic elemental analysis were confirmed. Of greater relevance was the derivation of Orbitrap operation parameters that yield greater spectral clarity and lower amounts of spectral noise that can be propagated across the product mass spectrum. The quantitative aspects presented in Tables 1 and 2 bode well for the target application of the microplasma for field-deployable isotopic analysis. Moving forward, a far greater level of understanding of the background subtraction methods utilized in the Exactive operating system is needed. In addition, methods of precluding entry of unwanted ions from the Orbitrap cell are a logical step towards improving performance. As these points are addressed, focus will turn to any limitations imposed by the microplasma that can be assessed.
References
Cristallini, O., Esteban, A., Gautier, E., Machado da Silva, L., Fernández Moreno, S., Renha, G. Jr., Bonino, A., Almeida, S., Pereira de Oliveira O. Jr., Llacer, C., Pardo, L., Dias, F., Galdoz, E., Amaraggi, D., Mason, P.: Qualification for Safeguards of the Sampling of UF6 using Alumina – “ABACC‐Cristallini Method” – Progress Achieved. Proceedings of the 56th Meeting of the Institute of Nuclear Materials Management, Indian Wells, CA, 12–16 July 2015
Anheier, N.C.: A Laser-Based Method for Onsite Analysis of UF6 and Environmental Samples at Enrichment Plants. Proceedings of the Symposium on International Safeguards: Linking Strategy, Implementation, and People – IAEA CN-220, Vienna, 20–24 October 2014
Regulations for the Safe Transport of Radioactive Material, 2012 Edition, Specific Safety Requirements No. SSR-6, International Atomic Energy Agency, Vienna, Austria
Rios, A., Escarpa, A., Simonet, B.: Miniaturization of Analtyical Systems: Principles, Designs, and Applications. John Wiley and Sons, Chichester (2009)
Le Gac, S., van den Berg, A.: Miniaturization and Mass Spectrometry. The Royal Society of Chemistry, Cambridge (2009)
McMahon, G.: Analytical Instrumentation: A Guide to Laboratory, Portable and Miniaturized Instruments. Wiley, Chichester (2007)
Karanassios, V.: Microplasmas for chemical analysis: analytical tools or research toys? Spectrochim. Acta B Atomic Spectrosc. 59, 909–928 (2004)
Gianchandani, Y.B., Wright, S.A., Eun, C.K., Wilson, C.G., Mitra, B.: Exploring microdischarges for portable sensing applications. Anal. Bioanal. Chem. 395, 559–575 (2009)
Mezei, P., Cserfalvi, T., Janossy, M., Szocs, K., Kim, H.J.: Similarity laws for glow discharges with cathodes of metal and an electrolyte. J. Phys. D Appl. Phys. 31, 2818–2825 (1998)
Miclea, M., Franzke, J.: Analytical detectors based on microplasma spectrometry. Plasma Chem. Plasma Proc. 27, 205–224 (2007)
Venzie, J.L., Marcus, R.K.: Micro-scale analytical plasmas for liquid chromatography detection. Anal. Bioanal. Chem. 381, 96–98 (2005)
Webb, M.R., Hieftje, G.M.: Spectrochemical analysis by using discharge devices with solution electrodes. Anal. Chem. 81, 862–867 (2009)
Cserfalvi, T., Mezei, P.: Direct solution analysis by glow discharge-electrolyte cathode discharge spectrometry. J. Anal. At. Spectrom. 9, 345–349 (1994)
Kim, H.J., Lee, J.H., Kim, M.Y., Cserfalvi, T., Mezei, P.: Development of open-air type electrolyle-as-cathode glow discharge-atomic emission spectrometry for determination of trace metals in water. Spectrochim. Acta Part B Atomic Spectrosc. 55, 823–831 (2000)
Marcus, R.K., Davis, W.C.: An atmospheric pressure glow discharge optical emission source for the direct sampling of liquid media. Anal. Chem. 73, 2903–2910 (2001)
Marcus, R.K., Quarles, C.D., Barinaga, C.J., Carado, A.J., Koppenaal, D.W.: Liquid sampling-atmospheric pressure glow discharge ionization source for elemental mass spectrometry. Anal. Chem. 83, 2425–2429 (2011)
Quarles, C.D., Carado, A.J., Barinaga, C.J., Koppenaal, D.W., Marcus, R.K.: Liquid sampling-atmospheric pressure glow discharge (LS-APGD) ionization source for elemental mass spectrometry: preliminary parametric evaluation and figures of merit. Anal. Bioanal. Chem. 402, 261–268 (2012)
Zhang, L.X., Manard, B.T., Kappel, S.K., Marcus, R.K.: Evaluation of the operating parameters of the liquid sampling-atmospheric pressure glow discharge (LS-APGD) ionization source for elemental mass spectrometry. Anal. Bioanal. Chem. 406, 7497–7509 (2014)
Zhang, L.X., Marcus, R.K.: Mass spectra of diverse organic species utilizing the liquid sampling-atmospheric pressure glow discharge (LS-APGD) microplasma ionization source. J. Anal. Atom. Spectrom. 31, 145–151 (2016)
Bruins, A.P.: Atmospheric pressure ionization mass spectrometry. 1. Instrumentation and ionization techniques. TrAC Trend Anal. Chem. 13, 37–43 (1994)
Carado, A.J., Quarles, C.D., Duffin, A.M., Barinaga, C.J., Russo, R.E., Marcus, R.K., Eiden, G.C., Koppenaal, D.W.: Femtosecond laser ablation particle introduction to a liquid sampling-atmospheric pressure glow discharge ionization source. J. Anal. At. Spectrom. 27, 385–389 (2012)
Marcus, R.K., Burdette, C.Q., Manard, B.T., Zhang, L.X.: Ambient desorption/ionization mass spectrometry using a liquid sampling-atmospheric glow discharge (LS-APGD) ionization source. Anal. Bioanal. Chem. 405, 8171–8184 (2013)
Scigelova, M., Makarov, A.: Orbitrap mass analyzer—overview and applications in proteomics. Proteomics 6, 16–21 (2006)
Eliuk, S., Makarov, A.: Evolution of Orbitrap mass spectrometry instrumentation. Annu. Rev. Anal. Chem. 8, 61–80 (2015)
Koppenaal, D.W., Barinaga, C.J., Smith, M.R.: Performance of an inductively-coupled plasma source ion-trap mass-spectrometer. J. Anal. At. Spectrom. 9, 1053–1058 (1994)
Barinaga, C.J., Koppenaal, D.W.: Ion-trap mass-spectrometry with an inductively-coupled plasma source. Rapid Commun. Mass Spectrom. 8, 71–76 (1994)
Marshall, A.G., Verdun, F.R.: Fourier Transforms in NMR, Optical, and Mass Spectrometry: A User’s Handbook. Elsevier Science Publishers B.V., Amsterdam (1990)
March, R.E., Hughes, R.J., Todd, J.F.J.: Quadrupole Storage Mass Spectrometry. Wiley-Interscience, New York (1989)
Cox, K.A., Cleven, C.D., Cooks, R.G.: Mass shifts and local space-charge effects observed in the quadrupole ion-trap at higher mass resolution. Int. J. Mass Spectrom. Ion Proc. 144, 47–65 (1995)
Han, S.J., Shin, S.K.: Space-charge effects on Fourier transform ion cyclotron resonance signals: experimental observations and three-dimensional trajectory simulations. J. Am. Soc. Mass Spectrom. 8, 319–326 (1997)
Wong, R.L., Amster, I.J.: Experimental evidence for space-charge effects between ions of the same mass-to-charge in Fourier-transform ion cyclotron resonance mass spectrometry. Int. J. Mass Spectrom. 265, 99–105 (2007)
Uechi, G.T., Dunbar, R.C.: Space-charge effects on relative peak heights in Fourier transform-ion cyclotron resonance spectra. J. Am. Soc. Mass Spectrom. 3, 734–741 (1992)
Kharchenko, A., Vladimirov, G., Heeren, R.M.A., Nicolaev, E.N.: Performance of Orbitrap mass analyzer at various space charge and non-ideal field conditions: simulation approach. J. Am. Soc. Mass Spectrom. 23, 977–987 (2012)
van Veen, E.H., de Loos-Vollebregt, M.T.C.: Application of mathematical procedures to background correction and multivariate analysis in inductively coupled plasma-optical emission spectrometry. Spectrochim. Acta B Atomic Spectrosc. 53, 639–669 (1998)
Travis, J.C., Winchester, M.R., Salit, M.L., Wythoff, B.J., Scheeline, A.: UV/visible Fuurier-transform spectroscopy using an inductively-coupled plasma-dual channel noise cancellation. Spectrochim. Acta B Atomic Spectrosc. 48, 691–709 (1993)
Zubarev, R.A., Makarov, A.: Orbitrap mass spectrometry. Anal. Chem. 85, 5288–5296 (2013)
Adams, F., Gijbels, R., Van Grieken, R.: Inorganic Mass Spectrometry. John Wiley and Sons, New York (1988)
Fielder, R.: Total evaporation measurements: experience with multi-collector instruments and a thermal ionization quadrupole mass spectrometer. Int. J. Mass Spectrom. Ion Proc. 146/147, 91–97 (1995)
Cheng, H., Edwards, R.L., Shen, C.-C., Polyak, V.J., Asmerom, Y., Woodhead, J., Hellstrom, J., Wang, Y., Kong, X., Spotl, C., Wang, X., Alexander, E.C., Jr.: Improvements in 230Th dating, 230Th, and 234U half-life values, and U–Th isotopic measurements by multi-collector inductively coupled plasma mass spectrometry. Earth Planet. Sci. Lett. 371/372, 82–91 (2013)
Acknowledgments
This research was supported in part by the U.S. National Nuclear Security Administration Office of Nonproliferation and Arms Control (NA-24) within the U.S. Department of Energy under Contract DE-AC05-76RL01830. PNNL is a multi-program national laboratory operated by Battelle for the U.S. Department of Energy. The Exactive MS capability was provided by the W. R. Wiley Environmental Molecular Science Laboratory, a national scientific user facility sponsored by the U.S. Department of Energy’s Office of Biological and Environmental Research (BER) program. Funding for this work (to E.D.H.) was provided by the U.S. National Nuclear Security Administration’s (NNSA) Office of International Nuclear Safeguards and the Next Generation Safeguards Initiative. Support for Clemson University activities from the Defense Threat Reduction Agency, Basic Research Award # HDTRA1-14-1-0010 is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoegg, E.D., Barinaga, C.J., Hager, G.J. et al. Preliminary Figures of Merit for Isotope Ratio Measurements: The Liquid Sampling-Atmospheric Pressure Glow Discharge Microplasma Ionization Source Coupled to an Orbitrap Mass Analyzer. J. Am. Soc. Mass Spectrom. 27, 1393–1403 (2016). https://doi.org/10.1007/s13361-016-1402-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-016-1402-4