Abstract
Introduction
Randomized controlled trials (RCTs) have demonstrated the efficacy of dulaglutide in adults with type 2 diabetes mellitus (T2DM), but results may not be generalizable in routine practice. This pragmatic literature review aimed to summarize real-world evidence (RWE) for dulaglutide.
Methods
The MEDLINE, EMBASE, NHS Economic Evaluation Database, and Health Technology Assessment databases were searched from January 2014 to July 2019 for studies providing RWE for dulaglutide in adults with T2DM regarding at least one outcome of interest (change in glycated hemoglobin [HbA1c]; weight; adherence; persistence; discontinuation; costs; healthcare resource utilization; health-related quality of life; patient satisfaction; and preference). Relevant congress abstracts were identified from EMBASE.
Results
A total of 29 studies (11 articles; 18 abstracts) were included. RWE for dulaglutide was not identified for all outcomes of interest. Dulaglutide reduced HbA1c from baseline to 3–24 months by 0.5–2.2% across studies (n = 20), and 23.4–55.7% of patients achieved HbA1c < 7.0%. Weight was reduced by 2.1–6.4 kg across studies of 3–12 months (n = 15). Based on outcomes from ten studies, 27.2–61.0% of dulaglutide patients were adherent. Mean persistence was 146–152 days and > 250 days in 6- and 12-month studies, respectively. Most studies reported discontinuation rates of 26.2–37.0%. Adherence and persistence were consistently reported to be greater in dulaglutide-treated patients in RW settings compared with other glucagon-like peptide-1 receptor agonists. Dulaglutide was associated with lower costs per 1% reduction in HbA1c compared with exenatide, liraglutide, or basal insulin (n = 3 studies).
Conclusion
Evidence from RWE studies suggests that dulaglutide may be associated with clinically relevant reductions in HbA1c, with a favorable adherence, persistence, and discontinuation profile in patients with T2DM in routine clinical practice. These findings provide additional insights regarding the potential value of dulaglutide in real-world settings that may assist healthcare decision makers in the delivery of patient-centered care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Randomized controlled trials (RCT) provide robust evidence for the efficacy of dulaglutide in the management of type 2 diabetes mellitus (T2DM), but because they are performed in targeted populations in controlled environments, their results may lack external validity and may not fully reflect the situation in the general disease population who exhibit more diverse characteristics. |
Given the significant global economic and humanistic burden of T2DM, it is important that healthcare decision makers better understand the clinical effectiveness of interventions for T2DM (including dulaglutide) in a broader patient population in everyday clinical practice. |
Based on a pragmatic review of the literature, we investigated evidence from real-world studies to support the clinical effectiveness of dulaglutide in more representative samples of patients with T2DM. |
What was learned from the study? |
It appeared that the efficacy of dulaglutide previously observed in RCTs likely translated into therapeutic benefits with respect to outcomes such as glycemic control, weight loss, adherence and persistence, and costs in routine clinical practice among patients that are more generalizable to the T2DM disease population at large. |
The results of the real-world studies identified and summarized in this review may provide healthcare decision makers with additional insights regarding the potential value of dulaglutide in the delivery of patient-centered care. |
Introduction
Randomized controlled trials (RCTs) are considered among the highest levels of evidence for the efficacy and safety of new medicines [1]. With good internal validity due to narrowly defined study populations, randomization, blinding, and inclusion of control groups, RCTs offer robust evidence that a clinical outcome is due to the intervention under study and not because of confounding factors or bias [2, 3]. However, because RCTs are performed in targeted populations typically observed in controlled environments, their results may not reflect the efficacy of an intervention in the general disease population who exhibit more diverse characteristics and medication behaviors [3, 4]. The importance of real-world evidence (RWE) for a treatment’s effectiveness and outcomes in routine practice settings outside the controlled environment of an RCT is becoming increasingly recognized [3]. RWE can complement RCTs to provide, for example, longer-term data on treatment trends and adverse events, patient adherence, durability of clinical outcomes, and economic data that are fundamental for healthcare decision makers [1, 5, 6]. Such data are particularly important in chronic and disabling diseases such as diabetes mellitus (DM), which exerts a high global economic and humanistic burden [7].
The main aim of treatment for type 2 diabetes mellitus (T2DM) is to achieve target blood glucose levels and so prevent or delay complications and maintain quality of life [8]. Several guidelines for the treatment of T2DM are available, which generally recommend initial management with lifestyle and dietary changes [8,9,10]. If individualized glycated hemoglobin (HbA1c) targets are not achieved with diet and exercise, it is recommended that pharmacologic therapy is commenced. Guidelines recommend specific approaches to the choice of glucose-lowering medications in patients with T2DM according to the presence of comorbidities, safety concerns, or healthcare environment [8]. Given the chronic and progressive nature of T2DM, many patients will require injectable glucose-lowering medications within 5–10 years [8]. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are an important advancement in the treatment of T2DM as they significantly improve glycemic control while reducing weight and with a low risk of hypoglycemia [11]. Guidelines recommend the use of GLP-1 RAs as one of the options in patients with T2DM who have failed to achieve target HbA1c levels after treatment with metformin [8]. Real-world data from prescription databases suggest that dulaglutide is initiated in patients who previously used a median of 2–3 antihyperglycemic therapy classes in the previous 6 months, most commonly biguanides or sulfonylureas [12]. Recent guideline updates have raised the priority of GLP-1 RAs, further recommending their use in patients with T2DM and atherosclerotic cardiovascular (CV) disease, at high/very high CV risk, or to reduce CV events [8, 13, 14].
Dulaglutide is a once-weekly GLP-1 RA approved in 2014 for glycemic control in adults with T2DM. The safety and efficacy of dulaglutide is supported by a large evidence base from a comprehensive phase 3 trial program [15]. Dulaglutide 0.75 mg and 1.5 mg were superior to placebo, metformin, sitagliptin, exenatide twice daily (BID), and insulin glargine, and dulaglutide 1.5 mg demonstrated non-inferiority versus liraglutide 1.8 mg with respect to reduction in HbA1c from baseline [15]. The composite end point of HbA1c < 7.0% with no hypoglycemia, no severe hypoglycemia, and no weight gain was achieved in significantly more patients randomized to dulaglutide compared with metformin, sitagliptin, exenatide BID, and insulin glargine [15]. Furthermore, the beneficial effects of dulaglutide on glycemic control were observed early in treatment and lasted up to 104 weeks, and treatment was well tolerated [15].
Less is known about the RWE relating to dulaglutide and whether the treatment benefits in patients with T2DM included in pivotal clinical trials translate into individuals receiving the drug in routine clinical practice. As such, this literature review was undertaken to identify and summarize studies that provide RWE for dulaglutide with respect to clinical effectiveness, adherence, persistence, costs, healthcare resource utilization (HCRU), health-related quality of life (HRQOL), and patient preference and satisfaction.
Methods
This literature review aimed to identify and describe studies that detail RWE for dulaglutide in patients with T2DM. Several outcomes of interest were prespecified, including those related to clinical effectiveness, adherence, persistence, economic outcomes, and HRQOL. A robust and reproducible protocol was developed that specified the focus of the review with respect to patient population, outcomes, and study types and outlined the methods for the search, study selection, and data extraction. The protocol minimized any author biases, ensured transparency and accountability, and maximized the chances of accurate data extraction.
Search Strategy
A search for relevant publications was done on 16 July 2019: MEDLINE (Ovid), EMBASE (Ovid), the NHS Economic Evaluation Database, and the Health Technology Assessment database were searched. In addition, a hand search of the bibliographies of eligible publications was undertaken to identify any relevant studies that, for whatever reason, were not found by the original search.
Records that were marked as congress abstracts in EMBASE were also reviewed and any that were relevant included. Congress abstracts that were identified as being a resubmission of another abstract presented at an earlier meeting and already included in the review were treated as exclusions, if they contained identical data. Also excluded were any congress abstracts that included data subsequently published as a full journal article that was already included in the review.
The main search strategy consisted of two concepts: T2DM AND dulaglutide. These were captured using subject headings and text word searches in title, abstract, and keyword heading word fields (and Registry Number/Name of Substance/Name of Substance Word fields for drug terms). Search terms for the T2DM concept included terms for non-specific DM and terms for explicit T2DM. Terms relating to RWE study types or to the outcomes were not specified since these are not always used consistently or well defined in the literature. In particular, there appear to be no validated search filters or agreed search approach for the identification of RWE studies, and the term itself is broad. In addition, due to the specification for “dulaglutide” publications, the search was limited in size and was, therefore, more robust to search for all dulaglutide records.
The search terms for the dulaglutide concept included the terms LY2189265 (Eli Lilly and Company identification number) and Trulicity (the brand name). Full details of the MEDLINE search strategy, which was adapted for the other databases, are included in the supplementary materials (Table S1).
Study Eligibility Criteria
Eligible studies were those published in English between 2014 (when dulaglutide was first licensed) and July 2019 that provided RWE for dulaglutide in adults (≥ 18 years) with T2DM. Studies including pediatric patients (< 18 years) with T2DM were excluded.
Studies could include placebo as a comparator, usual care as a comparator (only pharmacologic comparators were considered), or no comparator (in which case outcomes post-dulaglutide initiation were compared with pre-initiation values). In addition, studies had to include data on one or more of the outcomes of interest. Effectiveness outcomes were effects on HbA1c (change in absolute values and proportion of patient reaching targets) and body weight (in lb or kg). Other outcomes included adherence, persistence, discontinuation, economic factors (costs and HCRU), HRQOL (using validated instruments only), and patient satisfaction or preference. Studies were excluded from the review if they provided an analysis of predictors of response only.
Data from the following types of studies were eligible for inclusion: cross-sectional, non-interventional case-control, non-interventional cohort study with primary data, non-interventional cohort study with secondary data, administrative or claims database, electronic health record (EHR), or registry. Ineligible studies were case studies, RCTs, pragmatic clinical trials, utility studies, preference or satisfaction studies based on hypothetical drug profiles, and economic evaluations such as cost-effectiveness analyses.
Study Selection and Data Extraction
Search results were assessed by two reviewers using a two-phase approach consisting of (1) a broad review (title and/or abstract) of results and (2) a subsequent full text review of potentially eligible studies. Any studies that failed to meet the study criteria at stage 2 were excluded and the reason for exclusion recorded. Disagreements between reviewers regarding study inclusion were resolved by discussion until consensus was met.
Data extraction was performed on a standardized data extraction form by one reviewer, with the second undertaking a quality review of all records. Variables extracted included study population (sample size, demographic and baseline disease characteristics), interventions, study type and methods (including data source), study duration, and specific outcome data.
Compliance with Ethics Guidelines
This article is a review and analysis of previously published studies and does not include any new studies on human or animal subjects performed by any of the authors.
Results
Overview of Search Results
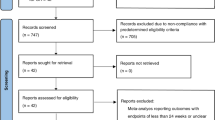
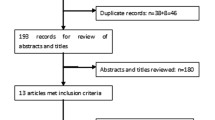
An overview of study selection is provided in Fig. 1. After de-duplication, the database searches yielded 807 records (including 630 records from the main search and 177 marked as congress abstracts in EMBASE). Review of titles/abstracts resulted in exclusion of 612 full publications from the main search and 145 congress abstracts. The full texts of 18 publications were further reviewed for relevance, and seven excluded since they did not include data on dulaglutide or were not RWE. No additional publications of relevance were identified from a hand search of the bibliographies of included full papers.
Of the abstracts identified for inclusion, six were encore/resubmission abstracts (three of which had subsequently been fully published), and eight more had also been published in full. Consequently, these 14 abstracts were treated as exclusions as outlined in the Methods. The review, therefore, included 29 studies with unique data (18 abstracts and 11 full journal publications).
Study Characteristics
Characteristics of included studies are summarized in Table 1. Most of the included publications described retrospective observational studies. Only two studies were of a prospective observational design: data were derived from a patient-completed questionnaire assessing medication satisfaction and adherence in one study [16] and from pharmacy records and a DM clinic portal in the other [17]. A variety of different data sources were employed in retrospective observational studies including medical records from DM/endocrinology clinics or outpatient departments in 12 records [18,19,20,21,22,23,24,25,26,27,28,29], claims databases in 7 [12, 30,31,32,33,34,35], EHR databases in 5 [36,37,38,39,40], and longitudinal prescriptions databases in 2 [41, 42]. In addition, one study used a patient registry [43].
Dulaglutide was compared with another glucose-lowering agent(s) in several studies (Table 1). It was most commonly compared with other GLP-1 RAs including liraglutide (n = 12 studies), exenatide once weekly (QW) (n = 9), and exenatide BID (n = 4). Lixisenatide was an active comparator in three studies and albiglutide in one. Other comparators included insulin (glargine and basal) and sodium-glucose co-transporter-2 (SGLT-2) inhibitors. Dulaglutide outcomes were compared pre- and post-initiation (i.e., no active comparator) in 13 studies (Table 1). Five studies evaluated the real-world effectiveness of dulaglutide in combination with other glucose-lowering agents including an SGLT-2 inhibitor plus metformin with or without insulin (n = 2 studies), an SGLT-2 inhibitor (not specified, n = 1), canagliflozin specifically (n = 2), or insulin (n = 1) (Table 1).
Study durations ranged from 1 month in a small Malaysian study [23] up to 2 years in a Japanese study [22], with the majority being between 3 months and 1 year in duration.
Regarding the geographic reach of the included studies, most were conducted in the USA (n = 10), India (n = 7), and the UK (n = 3) (Table 1). Two records described studies conducted in Italy, while one record each described studies conducted in Canada, Germany, Japan, Malaysia, the Republic of Korea, and Sweden. Another record described a multinational study performed in populations from Belgium, Canada, France, Germany, Italy, and The Netherlands [12].
Approximately half of the studies reported no sponsorship (n = 15) and half reported pharmaceutical company sponsorship (n = 14) (Table 1).
Patient Characteristics
The mean age of patients with T2DM treated with dulaglutide ranged from 48.2 to 62.0 years (Table 1). Across studies, 37.0–76.2% of the population was male. Duration of T2DM was reported infrequently across studies and ranged from 5.6 years in a subpopulation of patients aged ≤ 70 years in one study [22] to 10.3 years in a registry study including dulaglutide-treated patients with a mean age of 61.2 years [43]. Baseline weight was reported in nine studies, with body weight ranging from 74.4 to 115.0 kg. Mean baseline HbA1c was recorded in most studies and was in the range of 7.9–10.1% (Table 1).
Outcomes
Various outcomes were included in the search strategy that underpins this review. Dulaglutide data were not identified for all outcomes of interest. For example, no studies were found that described the effect of dulaglutide on HCRU, HRQOL, or patient preference. HbA1c was the most studied outcome in 20 publications (Fig. 2 and Table 2), followed by weight change in 15 (Fig. 3). Compliance outcomes, including adherence, persistence, and discontinuation, were reported in ten studies (Table 3) and cost data in three (Table 4). A single study reported limited data on patient satisfaction with treatment [16].
Mean change in HbA1c from baseline in patients with T2DM treated with dulaglutide in real-world practice. *p ≤ 0.05; ** p < 0.01; † p ≤ 0.001 versus baseline. Note, not all studies provided statistical analyses or provided information on dulaglutide dose. aIndex dulaglutide dose: 0.75 mg, 49% and 1.5 mg, 51% [37]; 0.75 mg, 56.5% and 1.5 mg, 43.5% [32]; 0.75 mg, 22.6% and 1.5 mg, 77.4% [28]. bDulaglutide data are from the cohort propensity score matched with once-weekly exenatide (similar findings reported for liraglutide matched cohort). [A] congress abstract; CAN canagliflozin, DU dulaglutide, HbA1c glycated hemoglobin, INS insulin, Met metformin, NR not reported, SGLT-2i sodium-glucose co-transporter-2 inhibitor, T2DM type 2 diabetes mellitus
Mean weight change (kg) from baseline in patients with T2DM treated with dulaglutide in real-world practice. *p ≤ 0.01; **p ≤ 0.001; †p ≤ 0.0001 versus baseline. Note, not all studies provided statistical analyses or provided information on dulaglutide dose. aIndex dulaglutide dose: 22.6% 0.75 mg and 77.4% 1.5 mg. bStudy duration of 12 months, but weight reduction presented was at 16 weeks. [A] congress abstract, CAN canagliflozin, DU dulaglutide, Met metformin, NR not reported, SGLT-2i sodium-glucose co-transporter-2 inhibitor, T2DM type 2 diabetes mellitus
Glycemic Control
In total, 20 studies reported the effect of dulaglutide on HbA1c levels; most presented values for change in HbA1c (Fig. 2) and eight evaluated the proportion of patients reaching prespecified targets for glycemic control (Table 2).
Change in HbA1c
HbA1c levels were consistently reduced with use of dulaglutide from pre- to post-initiation. Reductions in the range of 0.5–2.2% were observed across studies with a duration of 3–24 months (Fig. 2). Outcomes by dose of dulaglutide were rarely reported across studies, but it is assumed that study participants are treated according to approved dosing (0.75–1.5 mg QW). One Indian study presented in a congress abstract reported HbA1c reductions associated with dulaglutide 1.5 mg QW according to percentage of patients: a decrease of 1.4% was observed in 71% of patients at week 16 (p < 0.0001) and of 1.7% in 18% of patients at week 32 (p = 0.0002) [26]. This study is not shown in Fig. 2. Dulaglutide was associated with improved glycemic control when used alone or in combination with other glucose-lowering agents such as SGLT-2 inhibitors [19, 20, 25, 26, 39] or insulin [38]. The extent of HbA1c reduction was generally similar in patients treated with dulaglutide compared with other GLP-1 RAs [16, 18, 20, 33] and was significantly greater with dulaglutide in studies that compared it with insulin glargine or basal insulin (p ≤ 0.05) [34, 36]. One study evaluated differences in glycemic control according to age, showing that patients with T2DM aged > 71 years experienced a greater reduction in HbA1c compared with those aged ≤ 70 years [22] (Fig. 2).
Proportion of Patients Achieving HbA1c Targets
Eight publications reported data on the proportion of patients with T2DM achieving various HbA1c targets after receiving dulaglutide in real-world settings. HbA1c targets of < 7.0% are advocated in several T2DM management guidelines depending on individual patient characteristics [8, 10, 13, 44]. Seven of the included studies demonstrated that 23.4–55.7% of patients achieved HbA1c levels of < 7.0% following treatment with dulaglutide (Table 2), with the proportion meeting targets increasing from baseline to follow-up in those studies making the comparison. The proportion of patients attaining the less stringent targets of 7.0 to < 8.0% ranged from 30.0% up to 64.5% in a study evaluating a combination of dulaglutide and an SGLT-2 inhibitor (Table 2). In studies that compared dulaglutide with other GLP-1 RAs, no significant differences between treatments with respect to HbA1c targets were reported [33, 40].
Weight Change
Weight change was reported in 15 studies; 12 reported mean weight reduction in kg from baseline as shown in Fig. 3. Dulaglutide was associated with weight loss from baseline ranging from 2.1 to 6.4 kg in 3–12-month studies. Of the three remaining studies, two reported weight loss as a percentage, with patients experiencing a reduction of 0.6% or 3.3% in body weight following treatment for 6 months with dulaglutide [18, 24]. The third study reported 71% of dulaglutide patients having a loss of 0.4–4.8 kg at 1-month post-initiation [23].
When weight change was compared across different GLP-1 RAs, dulaglutide was associated with no difference in weight loss versus liraglutide in two studies [16, 20] and with a numerically smaller weight reduction after treatment for 6 months in a further two investigations [18, 24]. Similarly, weight reduction with dulaglutide at 6 months was reported to be inferior to twice-daily exenatide and lixisenatide in one study [18], but superior to once-weekly exenatide and albiglutide in another (− 2.7 vs. − 1.4 and − 1.6 kg, respectively; p = 0.0002 vs. exenatide) [40].
Adherence, Persistence, and Discontinuation
RWE for the adherence, persistence, and discontinuation of patients with T2DM receiving dulaglutide was described in ten studies (Table 3). Adherence, as measured by the proportion of days covered (PDC), was reported in four studies. Patients with a PDC of ≥ 0.80 were considered adherent to medication. Across studies, the mean PDC for dulaglutide ranged from 0.50 as reported in a congress abstract describing compliance in an elderly population of patients with T2DM (mean age, 71 years) to 0.76 in a T2DM population with mean age of 53 years (Table 3) [32, 35]. Overall, 27.2–61.0% of dulaglutide-treated patients were adherent. Patients who were more adherent with dulaglutide therapy experienced improved glycemic outcomes compared with less adherent individuals [32, 33]. Furthermore, over treatment for 12 months, a significantly greater reduction in HbA1c was reported by dulaglutide-treated patients compared with liraglutide-treated patients (− 0.98% vs. − 0.77%; p = 0.03) and a numerically greater reduction was reported compared with exenatide QW (− 1.00% vs. − 0.77%; p = 0.056) [33].
Across studies, persistence with medication was generally measured as the number of days of continuous therapy until discontinuation or the end of follow-up. Mean persistence with dulaglutide was 146–152 days in studies with a duration of 6 months [30, 32] and was > 250 days in a study with a duration of 12 months depending on matched cohort [33] (Table 3); median persistence was not calculable in two studies since a high proportion of dulaglutide patients were still on treatment at the end of the specified follow-up period [41, 43]. The proportion of patients on dulaglutide that remained persistent over 6–12 months ranged from 36.8% to 85.0% across the studies identified (Table 3). In six out of seven studies, rates of discontinuation of dulaglutide across studies with a duration of 6–12 months ranged from 26.2% to 37.0% (Table 3). One study, a UK audit, did, however, report discontinuation rates with dulaglutide as high as 62.1% at 6 months [18].
Most studies provided a comparison of compliance outcomes for dulaglutide with other GLP-1 RAs. Both adherence and persistence were consistently reported to be greater in dulaglutide-treated patients in real-world settings compared with patients receiving other GLP-1 RAs including twice-daily or once-weekly exenatide, liraglutide, or lixisenatide (Table 3). Furthermore, dulaglutide-treated patients were less likely to discontinue medication compared with other GLP-1 RAs (Table 3) across several countries.
Patient Satisfaction
A single study identified in this search and presented as a congress abstract provided some RWE regarding patient satisfaction with dulaglutide therapy [16]. This prospective observational study evaluated patient-reported outcomes among individuals initiating GLP-1 RAs in a Canadian DM specialist practice. There was a numerical trend toward better medication and device satisfaction in dulaglutide-treated patients compared with liraglutide-patients as measured by slightly greater improvements in the Diabetes Medication Satisfaction score and the Treatment-Related Impact Measure-Diabetes Device score [16].
Costs
Three studies identified by this search explored the costs associated with dulaglutide treatment in patients with T2DM in real-world practice in the USA [31, 33, 34]. Two studies compared costs between dulaglutide and exenatide QW or liraglutide at 6 months and 12 months post-initiation [31, 33]. Dulaglutide-treated patients had similar DM-related costs compared with liraglutide in patients with T2DM, but higher pharmacy and total costs compared with exenatide QW after 6 months (Table 4). This may be due, in part, to higher medication use in patients receiving dulaglutide versus exenatide (mean post-index prescriptions were 4.0 vs. 2.9; p < 0.0001), which is consistent with previously demonstrated higher adherence for dulaglutide [31]. In the 12-month study, dulaglutide was associated with higher DM-related pharmacy costs compared with liraglutide, but these were offset by lower DM-related medical costs such that DM-related total costs were similar between treatment groups [33] (Table 4). DM-related medical costs, pharmacy costs, and total costs were higher in dulaglutide-treated patients at 12 months compared with exenatide-treated patients. Dulaglutide was, however, associated with lower costs per 1% reduction in HbA1c compared with exenatide or liraglutide [33] (Table 4). Similar findings were also reported in a 12-month comparison of dulaglutide with basal insulin, in which costs per 1% reduction in HbA1c were significantly lower in dulaglutide users [34] (Table 4).
Discussion
This review identified numerous studies that support the effectiveness of dulaglutide in patients with T2DM as treated in routine real-world clinical practice. Studies consistently demonstrated that dulaglutide improved glycemic control by reducing HbA1c levels from pre-treatment. Furthermore, treatment with dulaglutide was associated with weight loss.
The RWE described in the included studies is supportive of data from the dulaglutide clinical trial program. The Assessment of Weekly AdministRation of dulaglutide in Diabetes (AWARD) trials included an evaluation of the efficacy of dulaglutide across the various stages of the T2DM treatment continuum, with study durations ranging from 24 to 104 weeks [15]. Across the AWARD trials, dulaglutide was associated with HbA1c reductions of 0.78–1.64% [15]. In the real-world studies described herein, dulaglutide was also associated with a reduction in HbA1c ranging from 0.5 to 2.2% across studies with durations of 3–24 months, values that are largely consistent with the observations from RCTs. Furthermore, the RW studies included in this review indicated that a considerable number of patients treated with dulaglutide (23.4–55.7%) met guideline-recommended HbA1c targets of < 7.0%. These values are slightly lower than those observed across the AWARD program (dulaglutide 1.5 mg, 53–78%; 0.75 mg, 37–69%) [15], which may reflect the influence of real-world factors on treatment effectiveness including lower follow-up intensity and lower medication adherence. Indeed, the influence of adherence on glycemic control has been demonstrated in real-world practice, with greater reductions in HbA1c being reported in patients with T2DM who were adherent with GLP-1 RA therapy (including dulaglutide) compared with those who were non-adherent with treatment [33].
Baseline weight and BMI were often poorly recorded across the studies included in this review, many of which were congress abstracts, perhaps reflecting their poor capture in databases in general. However, among the RW studies described herein that did adequately record weight change, dulaglutide was consistently associated with significant weight loss among patients with T2DM ranging from a loss of 2.1 to 6.4 kg. These values are slightly higher than those reported across the AWARD trials (− 0.9 to − 3.0 kg for dulaglutide 1.5 mg) [15].
Real-world studies also provide the ability to evaluate medication compliance in routine practice, which is not possible in stringently controlled clinical trials. It is well known that medication adherence is a key component in achieving good glycemic control, and it has been suggested that lack of adherence in clinical practice accounts for a considerable proportion of the efficacy gap between RCTs and RWE [4]. Poor medication adherence has been linked to an increased risk of DM-related complications, mortality, hospitalization, and healthcare costs [8], so it is pertinent to better understand compliance behaviors in real-world settings. Unsurprisingly, T2DM management guidelines recognize the importance of adherence and indicate that, when selecting a glucose-lowering agent, facilitation of medication adherence (including persistence) should be specifically considered [8]. Among the studies evaluating dulaglutide adherence, persistence, and discontinuation in this review, the mean PDC for dulaglutide was between 0.50 and 0.76, and overall 27.2–61.0% of dulaglutide-treated patients were considered adherent to treatment. The lower range of adherence was reported in a study of elderly patients [35], perhaps reflecting the unique challenges associated with disease management in this patient population [45]. Mean persistence with dulaglutide across studies with a duration of 6 months was 146–152 days and was > 250 days in studies with a duration of 12 months. Most studies compared compliance outcomes between dulaglutide and other GLP-1 RAs and consistently demonstrated that dulaglutide was associated with better adherence, longer persistence, and lower rates of discontinuation [12, 16, 18, 30, 33, 35, 41,42,43]. Furthermore, greater improvements in glycemic control were observed in patients with T2DM who were considered adherent to dulaglutide treatment compared with non-adherent individuals [32, 33]. The improved adherence with dulaglutide is likely related to dulaglutide’s convenient dosing schedule and method of delivery by ready-to-use, single-dose pen [12, 33]. Indeed, studies have indicated that these medication attributes are key components of patient preference for GLP-1 RAs [46], the importance of which is considered fundamental to the individualization of T2DM treatment goals and in the delivery of patient-centered care as advocated by management guidelines [8, 9].
Another aspect of T2DM management to which RWE can contribute a better understanding is the economic burden. Globally, some 463 million people are estimated to be living with DM, of which 90% have T2DM [7]. Total global health expenditure on DM has been estimated to be as high as US$ 760 billion and rising [7]. Given this high economic burden, choosing an intervention that accounts for both cost and effectiveness is important. Real-world studies can provide insight into such aspects of treatment, and some have used the metric of cost per 1% HbA1c reduction to articulate the value of a medication in terms that may be relevant to healthcare decision makers who need to balance budgets with patient needs. For example, it has been demonstrated that even though mean total DM-related costs for dulaglutide were similar compared with liraglutide and significantly higher compared with exenatide QW, when glycemic control was accounted for, the all-cause and DM-related 12 month costs per 1% reduction in HbA1c were lowest for dulaglutide-treated patients [33]. Similarly, in a comparison of dulaglutide and basal insulin, the total cost per 1% HbA1c reduction was lower for dulaglutide despite there being no between-group difference in total DM-related costs at 12 months [34].
The current review is subject to limitations that relate to the search itself, the evidence base, and constraints inherent in the methods employed in RWE studies. The search was restricted to English-language papers, but it is likely that other relevant studies could have been published in foreign-language journals. Also, further relevant studies could inevitably have been published since our searches were undertaken. While we searched for congresses indexed in EMBASE, it is possible abstracts have been presented at congresses not indexed in the database but that would be of relevance.
While the search included terms for several clinical outcomes, only five of the outcomes of interest were evaluated in the literature with respect to RWE for dulaglutide. Data are lacking on important outcomes such as HCRU, HRQOL, and patient satisfaction and preference. In addition, few studies had a duration > 12 months, so limited data are available for dulaglutide with respect to long-term treatment trends, patient adherence over more lengthy treatment periods, durability of clinical outcomes, and development of safety signals. Most of the studies included in the review employed a retrospective observational approach, with only two being of a prospective observational design. Prospective observational studies, however, offer some important advantages over retrospective designs since they provide more control over data collection, allow for assessment of temporality of outcomes, and eliminate the issue of recall bias. For example, The Real-world Observational Prospective study of Health outcomes with dulaglutIde and liraglutide in typE 2 diabeteS patients (TROPHIES) study is an ongoing prospective observational study being conducted in France, Germany, and Italy [47]. The aim of the study is to evaluate treatment patterns, clinical outcomes, HRQOL and other patient-reported outcomes, and HCRU over 24 months in patients with T2DM initiating first injectable treatment with dulaglutide or liraglutide [48, 49].
Finally, although this review has attempted to draw comparisons between studies, given the range of different methods employed, use of different databases with varying structures, and poorly described and variable adjustment for confounding factors within individual studies, it is difficult to draw firm conclusions across evaluations. Furthermore, a lack of clarity in the description of methods for data collection was frequently encountered, and details of many of the studies included in the review were from congress abstracts wherein methodology description is very limited.
Conclusions
Findings from this pragmatic review of real-world studies suggest that the efficacy of dulaglutide observed in RCTs may translate into therapeutic benefits with respect to outcomes such as glycemic control, weight loss, adherence and persistence, and costs in routine clinical practice among patients that are more generalizable to the T2DM disease population at large. The results of these real-world studies may provide healthcare decision makers with additional insights regarding the potential value of dulaglutide in the delivery of patient-centered care.
References
Chatterjee S, Davies MJ, Khunti K. What we have learnt from “real world” data, observational studies and meta-analyses. Diabetes Obes Metab. 2018;20(Suppl 1):47–58.
Katkade VB, Sanders KN, Zou KH. Real world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J Multidiscip Healthc. 2018;11:295–304.
Blonde L, Bailey T, Strong J, Levin P. Real-world evidence in diabetes: relevant to clinical practice. J Fam Pract. 2019;68(3 Suppl):jfp_6803l.
Carls GS, Tuttle E, Tan T-D, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40:1469–78.
Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35:1763–74.
Guerci B, Charbonnel B, Gourdy P, et al. Efficacy and adherence of glucagon-like peptide-1 receptor agonist treatment in patients with type 2 diabetes mellitus in real-life settings. Diabetes Metab. 2019;45:528–35.
International Diabetes Federation (IDF) (2019) Diabetes atlas. 9th ed. https://diabetesatlas.org/en/resources/. Accessed 7 Jan 2020
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;2018(61):2461–98.
American Diabetes Association. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes 2019. Diabetes Care. 2020;43(Suppl 1):S98–110.
National Institute of Health and Care Excellence (NICE) (2015) Type 2 diabetes in adults: management. NICE guideline NG28, December 2015. https://www.nice.org.uk/guidance/NG28. Accessed 6 Jan 2020.
Levin PA, Nguyen H, Wittbrodt ET, Kim SC. Glucagon-like peptide-1 receptor agonists: a systematic review of comparative effectiveness research. Diabetes Metab Syndr Obes. 2017;10:123–39.
Divino V, Boye KS, Lebrec J, DeKoven M, Norrbacka K. GLP-1 RA treatment and dosing patterns among type 2 diabetes patients in six countries: a retrospective analysis of pharmacy claims data. Diabetes Ther. 2019;10:1067–88.
Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–32323.
Buse JB, Wexler DJ, Tsapas A, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:487–93.
Anderson JE, Thieu VT, Boye KS, Hietpas RT, Garcia-Perez L-E. Dulaglutide in the treatment of adult type 2 diabetes: a perspective for primary care providers. Postgrad Med. 2016;128:810–21.
Brown RE, Abitbol A, Bajaj HS, et al. Patient-reported outcomes following initiation of glucagon-like peptide-1 receptor agonists (GLP-1RA) in patients with type 2 diabetes-progress-diabetes study. Diabetes. 2018;67(Suppl 1):A292.
Pacitti S, Smith C, Deosaran J. Observational prospective analysis of real-world experience of glucagon-like peptide-1 receptor agonist dulaglutide in patients with type 2 diabetes in Clyde. Diabetic Med. 2018;35(Suppl 1):147–8.
Atkinson R, Bharaj J, Basu A, Krishnan S, Banerjee M. Efficacy or convenience: which should we consider for glucagon-like peptide (GLP)-1 analogue therapy? Diabetic Med. 2018;35(Suppl 1):70.
Bhattacharyya S. Clinical effectiveness of combination therapy with dulaglutide, SGLT2 inhibitor and metformin with or without insulin in Indian adults with type 2 diabetes: a real-world retrospective study. Indian J Endocrinol Metab. 2018;22(7 Suppl 1):S28–S29.
Ghosal S, Sinha B. Liraglutide and dulaglutide therapy in addition to SGLT-2 inhibitor and metformin treatment in Indian type 2 diabetics: a real world retrospective observational study. Clin Diabetes Endocrinol. 2018;4:11.
Jha S, Waghdhare S, Chhabra N, Panda M, Yadav A, Batul N. Clinical effectiveness of dulaglutide in real-world setting: evidence of improved glycaemic and weight control in overweight/obese Indian patients with type 2 diabetes. Int J Diabetes Develop Countries. 2018;38(Suppl 2):S138–S139.
Kaneko S, Ueda Y, Tahara Y. Dulaglutide has favorable outcomes in elderly or renal impairment patients with type 2 diabetes. Diabetes. 2018;67(Suppl 1):A294.
Kang WH, Rohana J, Norasyikin AW, Norlaila M, Norlela S, Azmi KN. Weight loss and A1c reduction: our experience with dulaglutide. J Diabetes Investig. 2018;9(Suppl 1):132.
Maccora C, Formichi C, Crisci I, et al. Efficacy of liraglutide, dulaglutide and SGLT2 inhibitors in obese/overweight patients with type 2 diabetes mellitus. Obes Facts. 2019;12(Suppl 1):71.
Nigam A. Improved clinical outcomes with the dual therapies of a SGLT-2I and a GLP-1RA in overweight/obese people with T2 diabetes, real world data from the Indian subcontinent. Endocrine Pract. 2016;22(Suppl 6):26–7.
Nigam A. Once-weekly dulaglutide (DU) and canagliflozin (CAN) combination therapy in obese T2 diabetes (T2D) patients—one year real-world evidence from India. Diabetes. 2018;67(Suppl 1):LB29.
Wasir JS, Mithal A, Agarwal P, Mittal A. Once weekly dulaglutide therapy in type 2 diabetic subjects, real-world evidence from a tertiary care diabetes center in India. Indian J Endocrinol Metab. 2018;22:728–34.
Yoo JH, Cho YK, Lee J, et al. Clinical efficacy and parameters affecting the response to dulaglutide treatment in patients with type 2 diabetes: a retrospective, real-world data study. Diabetes Ther Res. 2019;10:1453–63.
Zouras S, Stephens JW, Price DE, Syed W. Experience with dulaglutide in a real-world setting. Diabetic Med. 2018;35(Suppl 1):186.
Alatorre C, Fernandez L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19:953–61.
Mody R, Alatorre C, Yu M, Fernandez Lando L, Davis B, Juneau P. Health care resource utilization and costs of patients with type 2 diabetes treated with dulaglutide vs exenatide QW or liraglutide in the US. Value Health. 2017;20(Suppl 5):A178.
Mody R, Grabner M, Yu M, et al. Real-world effectiveness, adherence and persistence among patients with type 2 diabetes mellitus initiating dulaglutide treatment. Curr Med Res Opin. 2018;34:995–1003.
Mody R, Huang Q, Yu M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12-month follow-up in a real-world setting in the United States. Diabetes Obes Metab. 2019;21:920–9.
Mody R, Huang Q, Yu M, et al. Real-world economic outcomes among patients with type 2 diabetes (T2DM) treated with dulaglutide (DU) vs basal insulin (BI) in the US: the DISPELTM study. Value Health. 2019;22(Suppl 2):S149–S150.
Yu M, Xu Y, Kwan A, et al. Real-world adherence and persistence among elderly patients with T2DM initiating dulaglutide vs liraglutide or exenatide once-weekly in the US. Value Health. 2019;22(Suppl 2):S159.
Boye KS, Mody R, Wu J, Lage MJ, Botros FT, Woodward B. Effects of dulaglutide and insulin glargine on estimated glomerular filtration rate in a real-world setting. Clin Ther. 2018;40:1396–407.
Mody R, Yu M, Fernandez L, et al. Real-world effectiveness of dulaglutide in patients with type 2 diabetes. Value Health. 2017;20(Suppl 5):A164.
Mody R, Kallenbach L, Yu M, et al. Characteristics and glycemic response of patients with uncontrolled type 2 diabetes initiating dulaglutide alone or in combination with insulin in us real world practice. Value Health. 2018;21(Suppl 1):S71.
Srivastava B, Deepa M, Anjana RM, Siddharth M, Priyasandhya P, Mohan V. Combination therapy with once weekly GLP 1 receptor agonist dulaglutide and SGLT2 inhibitors in Asian Indians with type 2 diabetes. Endocrine Pract. 2018;24(Suppl 1):38–9.
Unni S, Wittbrodt E, Ma J, et al. Comparative effectiveness of once-weekly glucagon-like peptide-1 receptor agonists with regard to 6-month glycaemic control and weight outcomes in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:468–73.
Federici MO, McQuillan J, Biricolti G, et al. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Italy: a retrospective cohort study. Diabetes Ther Res. 2018;9:789–801.
Otto T, Myland M, Jung H, Lebrec J, Richter H, Norrbacka K. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Germany: a retrospective cohort study. Curr Med Res Opin. 2019;35:893–901.
Toll A, Eliasson B, Lebrec J, et al. Treatment persistence in patients with type 2 diabetes treated with GLP-1 receptor agonists in clinical practice in Sweden: nationwide retrospective cohort study. Value Health. 2018;21(Suppl 3):S137.
American Diabetes Association. Glycemic targets: standards of medical care in diabetes, 2019. Diabetes Care. 2020;43(Suppl 1):S66–S76.
Bradley D, Hsueh W. Type 2 diabetes in the elderly: challenges in a unique patient population. J Geriatr Med Gerontol. 2016;2(2):14. https://doi.org/10.23937/2469-5858/1510014.
Thieu VT, Robinson S, Kennedy-Martin T, Boye KS, Garcia-Perez L-E. Patient preferences for glucagon-like peptide 1 receptor–agonist treatment attributes. Patient Prefer Adherence. 2019;13:561–76.
García-Pérez LE, Sapin H, Norrbacka K, et al. The real-world observational prospective study of health outcomes with dulaglutIde and liraglutide in typE 2 diabetes patients (TROPHIES)—design and baseline characteristics. Poster presented at The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) European Conference, 2019 (PDB116). https://www.ispor.org/heor-resources/presentations-database/presentation/euro2019-3120/94861. Accessed 16 Dec 2019.
Sapin H, García-Pérez LE, Norrbacka K, et al. The real-world observational prospective study of health outcomes with dulaglutide and liraglutide in type 2 diabetes patients (TROPHIES)—country-specific baseline characteristics. Poster presented at The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) European Conference, 2019 (PDB82). https://www.ispor.org/heor-resources/presentations-database/presentation/euro2019-3120/94620. Accessed 16 Dec 2019.
Boye K, García-Pérez LE, Sapin H, et al. The real-world observational prospective study of health outcomes with dulaglutIde and liraglutide in type 2 diabetes patients (TROPHIES)—patient-reported outcomes at baseline. Poster presented at The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) European Conference, 2019 (PDB113). https://www.ispor.org/heor-resources/presentations-database/presentation/euro2019-3120/93919. A ccessed 16 Dec 2019.
Acknowledgements
The authors thank Mick Arber (York Health Economic Consortium [YHEC]) for assistance with the literature search.
Funding
This study and the Rapid Service Fee were funded by Eli Lilly and Company (Indianapolis, IN, USA).
Medical Writing and Editorial Assistance
The authors thank Rebecca Mant and Alison Terry of KMHO Ltd. for assistance with writing and editing, respectively, which was funded by Eli Lilly and Company (Indianapolis, IN, USA).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Disclosures
Alena Strizek is a former employee of Eli Lilly and Company and is now an employee of Amgen Australia Pty Ltd. Kristina S Boye, Manige Konig, Reema Mody, and Raleigh E. Malik are employees and minor shareholders of Eli Lilly and Company. Susan Robinson and Tessa Kennedy-Martin are employees of KMHO, who received funding from Eli Lilly for time spent conducting this research.
Compliance with Ethics Guidelines
This article is a review and analysis of previously published studies and does not include any new studies on human or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as, since this is a review, no datasets were generated or analyzed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12279920.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Robinson, S., Boye, K.S., Mody, R. et al. Real-World Effectiveness of Dulaglutide in Patients with Type 2 Diabetes Mellitus: A Literature Review. Diabetes Ther 11, 1437–1466 (2020). https://doi.org/10.1007/s13300-020-00839-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00839-5