Abstract
Aim
The primary objective of this document is to develop practice-based expert group opinion on certain important but less discussed endocrine and metabolic effects of modern sulfonylureas (SUs) and their usage in the management of diabetes mellitus (DM).
Background
Modern SUs may be considered a panacea in DM care with their beneficial extra-pancreatic, pleiotropic, and cardiovascular effects. Safe glycemic control with SUs could be achieved with appropriate patient selection, drug and dosage selection, and patient empowerment. Additionally, sulfonylureas also exhibit certain endocrine and metabolic effects, which could be considered beneficial in the management of DM. In this regard, a group of international clinical experts discussed the less known beneficial aspects of SUs and safe and smart prescription of modern SUs in DM care.
Results
The concept of glucocrinology or the relationship of glycemia with the endocrine system was emphasized during the meetings. Clinical experts arrived at a consensus for the usage of modern SUs in the presence of other endocrine dysfunction and the impact of these drugs on endocrine health. The beneficial pleiotropic and cardiovascular effects of modern SUs were also discussed. The key discussion points were considered to develop clinical expert opinions for the use of modern SUs in persons with DM. Clinical expert opinions were developed for indications, pleiotropic benefits, cardiovascular outcomes, adherence, and safe use of modern SUs.
Conclusions
Appropriate clinical judgement coupled with a patient-centered approach is crucial to achieve the best outcome in persons with DM. Owing to their safety, efficacy, extra-pancreatic benefits including effects on endocrine and metabolic aspects, and low cost of therapy, modern SUs could be considered as drugs/agents of choice for the treatment of diabetes.
Funding
Sanofi India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral antidiabetics (OADs) are oral medications prescribed to patients with type 2 diabetes mellitus (T2DM). Various factors that are considered in the management of diabetes mellitus with oral medications include efficacy, safety, tolerability, and cost. In addition to these factors, when prescribing oral medications, clinicians should also consider other endocrine and metabolic factors that could impact the clinical outcome.
Among the several OADs available, sulfonylureas (SUs) constitute one of the key pharmacotherapeutic agents in the management of T2DM. The latest guidelines published by the World Health Organization (WHO) recommend the use of metformin and SUs as preferred agents for the control of blood glucose levels in patients with DM. But, despite their well-established efficacy profile, the clinical utility of SUs and their place in therapy have become debatable. This may be because SUs are clubbed under one group, although in reality all SUs are different.
Sulfonylureas are classified on the basis of their hierarchy of development as conventional and modern and on the basis of duration of action as short-acting, intermediate-acting, and long-acting [1, 2].
Modern SUs offer several benefits when compared to conventional SUs. Their efficacy profile is better compared to that of conventional SUs. Moreover, they possess extra-pancreatic effects and are available at considerably lower cost, which makes them one of the drugs/agents of choice for the treatment of diabetes. Modern SUs also possess certain additional benefits in terms of endocrine effects, metabolic effects, and anti-inflammatory or immunomodulatory effects, which are less discussed.
In the management of DM, a holistic approach encompassing vasculo-metabolic aspects and endocrine facets of diabetes is very important. The concept of glucocrinology focuses on the association of various endocrine glands and diabetes, the role of endocrinopathic drugs in unmasking latent diabetes, and the role of antidiabetic drugs in modulating endocrine disease. The concept of glucocrinology emphasizes the consideration of endocrine aspects in the management of diabetes. It promotes a comprehensive assessment of and aids in the quest for novel targets in the management of DM.
In this context, an initiative by a multinational team of experts aimed to encourage safe and smart prescription of modern SUs while keeping the glucocrinologic aspects of these drugs in mind [1]. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Methods
During a 2-day international meeting, experts reviewed available literature evidence, provided their individual insights based on their experience in the management of DM with primary focus on pleiotropic effects and cardiovascular (CV) benefits of modern SUs, and charted out key opinions. Important topics were discussed by panel members to arrive at expert opinion on standardization of various OADs that could be considered safe and unsafe in the management of patients with DM and CV risk or comorbidity. Drugs that could be considered safe in the use of DM patients with associated endocrinopathies were charted out. The panel members’ key discussion points, which were based on scientific evidence and collective clinical judgment from practice, considered as “clinical expert opinions” for each of these topics, were developed and have been summarized in this document.
Results
Glucocrinology: Concept of Interplay and Interlink of Glucose Homeostasis and Endocrine Glands
Diabetes mellitus is a complex multifaceted syndrome characterized by a state of decreased insulin secretion and/or insulin resistance with hyperglycemia as the primary abnormality. Several endocrine glands, including adipose tissue, the gastrointestinal endocrine system, pituitary, thyroid, parathyroid, adrenals, and gonads, play an important role in the development of DM. Glucocrinology has been defined as the study of medicine that describes the correlation between glycemia and the endocrine system [3]. The important aspects of glucocrinology are listed below:
-
1.
Endocrinopathies may cause secondary diabetes:
-
Acromegaly
-
Cushing syndrome
-
Pheochromocytoma
-
Hyperthyroidism
-
Hyperaldosteronism
-
Hyperparathyroidism
-
-
2.
Endocrinopathies may be associated with metabolic syndrome:
-
Polycystic ovary syndrome
-
Hypothyroidism
-
Subclinical Cushing syndrome
-
-
3.
Endocrine dysfunction and diabetes may coexist:
-
Autoimmune polyglandular syndromes
-
Multiple endocrine neoplasia
-
Mitochondrial disorders
-
-
4.
Endocrine dysfunction may be the etiology of refractory hyperglycemia in diabetes:
-
Hyperthyroidism
-
Cushing syndrome
-
Acromegaly
-
-
5.
Endocrinopathies associated with an increased risk of hypoglycemia with diabetes treatment:
-
Adrenal insufficiency
-
Hypothyroidism
-
Growth hormone deficiency
-
-
6.
Endocrinopathic drugs may worsen diabetes:
-
Glucocorticoids
-
Thyroid hormones
-
Inotropes
-
Growth hormone
-
Estrogen
-
Somatostatin analogs
-
The concept of glucocrinology emphasizes the importance of endocrinology and role of endocrinologists in the management of diabetes mellitus. It also aids in delivering a comprehensive approach to treatment of persons with diabetes mellitus.
Sulfonylureas: An Established Treatment of DM
Classification of SUs
Sulfonylureas are classified into various categories on the basis of their hierarchy of development and duration of action. In terms of development, SUs are classified into conventional (e.g., glibenclamide) and modern SUs (glimepiride, gliclazide modified release [MR], glipizide MR, and gliclazide). In terms of the duration of action, they are classified into short-acting (tolbutamide), intermediate-acting (glipizide and gliclazide), and long-acting SUs (glibenclamide, glimepiride, gliclazide MR, and glipizide MR) [1].
Mechanism of Action
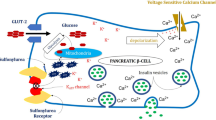
Sulfonylureas act by stimulating endogenous insulin secretion via blockade of adenosine triphosphate-sensitive potassium channels (KATPs) on pancreatic β-cells. Sulfonylureas bind to a common SU receptor (SUR) subunit present on the β-cell plasma membrane causing closure of the KATP channels and inhibition of K+ efflux, consequently depolarizing the membrane and facilitating influx of Ca2+ ions. This, in turn, stimulates the exocytosis of insulin-secretory vesicle [1].
Modern SUs (such as glimepiride) stimulate secretion of insulin by binding to a specific site on the KATP channel of pancreatic β-cells. Modern SUs deploy allosteric inhibition of the SUR complex. The distinct feature of modern SUs leads to a lower inhibition of KATP channel and, hence, there is a reduced risk of hypoglycemia in comparison to conventional SUs [1].
Indications of SUs
Sulfonylureas are an effective second-line OADs used in the management of T2DM. Modern SUs may be considered as a treatment option in persons who do not respond to metformin. They are superior to conventional SUs in reducing mortality, bringing better outcomes, and preserving renal function [1].
Reduced Risk of Hypoglycemia with Modern SUs
Hypoglycemia is one of the most common adverse reactions associated with sulfonylureas. However, modern sulfonylureas such as glimepiride differ from conventional sulfonylureas and are associated with fewer hypoglycemic episodes. This could be attributed to equivalent metabolic control and lower stimulation of insulin levels with glimepiride as compared to glibenclamide [4]. In an international prospective study, diabetic patients treated with glimepiride had fewer hypoglycemic episodes compared to those treated with glibenclamide (105 vs. 150) [2].
Glipizide and modern SUs are preferred in renal failure patients and T2DM patients who are at increased risk of developing hypoglycemia.
American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) Recommendations for SUs in T2DM Management
Modern SUs confer a lower risk of hypoglycemia and have favorable cost, efficacy, and safety profiles. Sulfonylureas constitute a reasonable choice among glucose-lowering medications, especially when cost is the key consideration. Patient education and use of variable dosing of modern sulfonylureas should be considered to mitigate the risk of hypoglycemia. Glipizide, glimepiride, and gliclazide have lower risk of hypoglycemia compared to conventional sulfonylureas. Sulfonylureas should be used with great caution in patients who are at increased risk of hypoglycemia, such as those with chronic kidney disease and older patients.
Hidden Facets of SUs
Selection of a specific SU should be done on the basis of efficacy, safety, and tissue specificity with respect to the β-cell [5]. Modern SUs exhibit additional benefits over conventional SUs, which guide the choice of treatment in the management of DM. A few of these benefits have been listed below:
-
1.
Effects on the pancreas
-
2.
Extra-pancreatic effects
Hidden Pleiotropic Effects of Modern SUs
Modern SUs have multiple pleiotropic benefits. Some of them are listed below:
-
1.
Immunomodulatory/anti-inflammatory effects
-
Modern SUs exert antioxidative effects (by decreasing toxic advanced glycation end-products [AGEs] and receptors of AGEs) [7–9].
-
Modern SUs exert anti-inflammatory effects (by reducing high-sensitivity C-reactive protein, interleukin-6, and tumor necrosis factor-α levels) [7–9].
-
Modern SUs exert anti-angiogenic effects (by reducing plasma vascular endothelial growth factor and fibroblast growth factor-2 levels) [7–9].
-
-
2.
Endocrine effects
-
Human chorionic gonadotropin-induced testosterone secretion by Leydig cells is inversely related to insulin sensitivity among men with varying degrees of glucose tolerance. Thus, the lesions resulting in hypogonadism in obesity and T2DM may occur at several levels of the hypothalamic–pituitary–gonadal axis. However, the absence of an increase in gonadotropin concentrations indicates that the primary defect in T2DM and obesity is at the hypothalamo-hypophyseal level.
-
A study evaluated the impact of sulfonylurea as an initial treatment for hypogonadism in T2DM patients. In the study, the initial dose of oral glimepiride was 1 mg/day and the dose was titrated according to blood glucose levels for 16 weeks. Results indicated that as compared with the healthy control group, the middle-aged men with type 2 diabetes had significantly decreased total testosterone levels and a lower testosterone secretion index.
-
3.
Other effects
-
Glimepiride, a modern SU, is cardiovascular neutral as compared to other SUs. The degree of inhibition of KATP channels in T2DM patients is less severe during treatment with glimepiride. Therefore, this drug can be safely used in T2DM patients with concurrent coronary artery disease (CAD) [10, 11].
-
Another modern SU, gliclazide, has also been associated with reduced risk of hypoglycemic episodes and long-term cardiovascular safety when compared with other OADs in the treatment of DM [12].
-
Key recommendations of the international task force
Key recommendations | Evidence and/or rationale |
|---|---|
Modern SUs (such as glimepiride and gliclazide MR) should be preferred to conventional SUs especially in | Low rate of hypoglycemia and weight gain conferred by modern SUs as compared to conventional SUs could be attributed to its lower binding affinity (2–3 fold) and quick association and dissociation with sulfonylurea receptor (SUR proteins). Conventional SUs inhibit the mitochondrial KATP channels in cardiac myocytes, which contributes to impairment of ischemic preconditioning; however, modern SUs do not exert this effect and preserve myocardial ischemic preconditioning [13] |
Overweight/obese T2DM patients | |
Patients at a high risk of hypoglycemia | |
Patients at a high risk of CV diseases | |
Modern SUs (such as glimeperide and gliclazide MR) should be preferred to conventional SUs with the aim to reduce mortality, bring better outcomes, and preserve renal function | A meta-analysis of randomized clinical trials conducted by Varvaki Rados et al. [14] evaluated the association between SU use and all-cause and cardiovascular mortality in patients with T2DM. Sulfonylureas were not associated with all-cause (OR 1.12 [95% CI 0.96–1.30]) or cardiovascular mortality (OR 1.12 [95% CI 0.87–1.42]) Modern SUs exhibit several extra-pancreatic effects, apart from glycemic control, and thereby contribute to better clinical outcomes [1] Modern SUs are mainly excreted as unchanged drug or inactive metabolite. Therefore, they may produce less hypoglycemia in patients with renal impairment. Glimepiride has been reported to be safe and effective in diabetic patients with renal impairment [1] |
The panel suggests that the patients/family members should be educated on the appropriate use (dose, time, route, and adherence) of modern SUs | Self-management plan on a day-to-day basis is very important in management of diabetes mellitus. Diabetic education enables the patients to effectively manage the disease without any complications. Self-monitoring of blood glucose (SMBG) at home and self-down-titration of doses in case of hypoglycemia by patients are recommended. The patient should be trained in the safe use of fixed-dose combination (FDC) containing SUs and should be able to detect the hypoglycemic complications. Therefore, patients along with their family members should be educated about the usage of SMBG systems [1] |
The expert group addressed the safety issues of conventional SUs. Conventional SUs may cause hypoglycemia and weight gain in most patients with T2DM. Additionally, older SUs are believed to increase β-cell apoptosis, risk of ischemic complications, and thereby result in non-fatal CV outcomes and all-cause mortality | A prospective study conducted by Lee and Chou [15] determined the impact of administration of different SUs on cardioprotective effects in T2DM patients undergoing coronary angioplasty. Nondiabetic patients treated with glimepiride had a significantly lowered ischemic burden (assessed by an ST-segment shift, chest pain score [3.4 ± 0.9 vs. 5.5 ± 1.5; p = 0.02], and myocardial lactate extraction ratios [− 59 ± 21% vs. − 26 ± 16% in non-diabetics; p = 0.007]) compared with glibenclamide-treated patients, demonstrating that acute administration of glimepiride does not abolish cardioprotection |
The pleiotropic effects of modern SUs, including beneficial effects on the pancreas, extra-pancreatic effects, immunomodulatory effects, and other effects on endocrine functions, were reinforced in the meeting | Sulfonylureas (SUs) exhibit several extra-pancreatic effects, apart from glycemic control, including inhibition of metabolic clearance rate of insulin, inhibition of glucagon secretion from pancreatic α-cells, insulin sensitization, increases adiponectin levels, exerts antioxidative and anti-angiogenetic effects, and preserves ischemic preconditioning [1] |
Cardiovascular Effects of SUs
Cardiovascular Phenotype
Definition of Cardiovascular Phenotype
Cardiovascular phenotype is the term used by diabetes experts to describe congenital cardiac anomalies as well as vascular and cardiac dysfunction associated with DM. It is a checklist of various clinical parameters to be assessed before therapeutic intervention for the management of DM. The clinical parameters include pulse rate, blood pressure, weight, lipid status, systolic function, diastolic function, orthostatic hypotension, coronary health, cerebrovascular health, and peripheral arterial health [16].
The concept of cardiovascular phenotype is a useful clinical decision-making tool to help determine appropriate OAD therapy in diabetic patients with high cardiovascular risk, and allows easier assessment of the impact of such therapy on cardiovascular health [16].
Cardiovascular Phenotype in Diabetes
Modern SUs, such as glimepiride, are found to maintain myocardial ischemic preconditioning with fewer CV side effects as compared to conventional SUs. In addition, modern SUs were not associated with all-cause or CV mortality. They are also not associated with an increased risk of myocardial infarction or stroke. In light of this, modern SUs can be considered cardiac-friendly [1, 14].
The Clinical Expert Group Endorsed Newer SUs Because of CV Safety
Since modern SUs (such as gliclazide MR and glimepiride) are associated with a lower risk of all-cause and CV-related mortality compared to conventional SUs in T2DM patients, the clinical expert group opinion suggests that the modern SUs can be safely used in T2DM patients with CV risk, myocardial infarction, or stroke. A nationwide registry comprising 1310 DM patients with acute myocardial infarction revealed that the mortality was lower in patients previously treated with modern SUs when compared to those treated with other oral medications or insulin [1, 17]. The risk–benefit analysis of the IDF 2017 Clinical Practice Recommendations also showed that SUs are associated with neutral effects on the major CV events and congestive heart failure (HF) [18].
Influence of Cardiovascular Phenotype on OAD Choice
OAD Choice and (Risk of) Heart Failure
Metformin is the drug of choice in this scenario.
Effect of Metformin Therapy on Prognosis of Patients with HF and New-Onset DM
A study conducted by Romero et al. suggested that metformin therapy was associated with a decreased mortality and hospitalization rate. However, it was not associated with an improved prognosis of HF patients [19].
Increase in Risk of Hospitalizations for HF in Patients Treated with Saxagliptin: Reports from the SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes) Trial
A study conducted by Scirica et al. demonstrated that the use of saxagliptin was associated with an increased rate of hospitalization for HF [20].
Addition of Sitagliptin to Usual Care is Not Associated with an Increased Risk of Hospitalization for HF: Data from the (Trial Evaluating Cardiovascular Outcome with Sitagliptin) TECOS Study
The Trial Evaluating Cardiovascular Outcome with Sitagliptin (TECOS) study conducted by Green et al. demonstrated that the addition of sitagliptin to an existing therapy did not influence the rate of hospitalizations for HF in patients with T2DM [21].
Reduction in Hospitalization for HF with Empagliflozin: Reports from the (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial) EMPA-REG OUTCOME Trial
The EMPA-REG OUTCOME trial conducted by Zinman et al. demonstrated that the administration of once-daily empagliflozin was associated with 35% of relative risk reduction in hospitalization for HF, when compared to the placebo group [22].
Increased Incidence of HF in DM Patients Treated with Pioglitazone: PROactive Study
The pioglitAzone Clinical Trial In macroVascular Events (PROactive Study) conducted by Erdmann et al. proved that a larger number of patients treated with pioglitazone were reported to have serious HF compared to those treated with placebo. However, subsequent mortality or morbidity was not increased in patients with serious HF treated with pioglitazone [23].
Reduction in Risk of Cardiovascular Death or Hospitalized HF in DM Patients Treated with Canagliflozin: Results from the CANVAS (Canagliflozin Cardiovascular Assessment Study) Program
The CANVAS Program (Canagliflozin Cardiovascular Assessment Study) conducted by Rådholm et al. on 10,142 patients with T2DM and high cardiovascular risk proved that treatment with canagliflozin resulted in reduced risk of cardiovascular death or hospitalized HF across a broad range of different patient subgroups. Benefits were found to be greater in those patients with a history of HF at baseline [24].
Efficacy and Safety of Dapagliflozin in Patients with T2DM and Concomitant HF
A study conducted by Kosiborod et al. investigated the efficacy and safety of dapagliflozin in patients with T2DM and HF. Hospitalizations due to HF were found to be rare with dapagliflozin (0.6%) when compare to placebo (4.7%). Point estimates for hazard ratios of composite cardiovascular outcomes also favored dapagliflozin vs. placebo [25].
OAD Choice and Risk of Myocardial Infarction
Non-statistical Trend in Reduction of Non-fatal Myocardial Infarction: Results from CV Safety Outcome Trials (CVSOTs)
A non-statistical trend was observed in the reduction of non-fatal MI in most of the CVSOTs, including SAVOR-TIMI, TECOS, LEADER, ELIXA, and EMPA-REG [21, 26].
Glimepiride Was Associated with Reduced Mortality Rates in DM Patients with CAD
A retrospective cohort study conducted by Pantalone et al. suggested that the use of glimepiride was associated with reduced mortality rates in diabetic patients with CAD when compared with the use of glyburide [27].
OAD Choice and Risk of Stroke
OADs with Neutral Outcome on Non-fatal Stroke: Evidence from CVSOTs
Evidence from CVSOTs suggests that neutral outcome on non-fatal stroke was reported from the following trials: EXAMINE, TECOS, and LEADER. From these trial reports, it is evident that rates of non-fatal stroke in patients treated with OADs, including alogliptin, sitagliptin, and liraglutide, were non-significantly lower than in those treated with placebo [21, 22, 26].
Pioglitazone Reduces Stroke in DM Patients
A meta-analysis conducted by Lee et al. on three randomized controlled studies reported that use of pioglitazone in stroke patients with insulin resistance, prediabetes, and DM was associated with a lower risk of recurrent stroke and future major vascular events [28].
Cardiovascular Safety of Modern SU Gliclazide: ADVANCE Study
In the ADVANCE trial, 11,140 patients with type 2 diabetes were randomized to either standard glucose control or intensive glucose control with gliclazide (modified release) plus other drugs as required to achieve HbA1c ≤ 6.5%. The median duration of follow-up was 5 years.
Intensive glucose control was associated with a 10% relative reduction in the combined outcome of major macrovascular and microvascular events. Intensive glucose control was also associated with a 6%, 2%, 8%, and 2% reduction in major macrovascular events, non-fatal MI, major coronary events, and all coronary events [29].
Ongoing CVOTs for SUs: The CAROLINA Trial
The Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes (CAROLINA) trial has investigated the long-term impact on CV morbidity and mortality, relevant efficacy parameters (e.g., glycemic parameters), and safety (e.g., weight and hypoglycemia) of linagliptin in patients with type 2 diabetes at elevated CV risk receiving usual care and compared the outcome against glimepiride.
The primary outcome is time to the first occurrence of any of the following adjudicated components of the primary composite endpoint: CV death (including fatal stroke and fatal MI), non-fatal MI (excluding silent MI), or non-fatal stroke.
Linagliptin was non-inferior to glimepiride for time to first major adverse CV event in adults with type 2 diabetes at high CV risk. To date, detailed results are awaited [30].
Key recommendations of the international task force
Key recommendations | Evidence and/or rationale |
|---|---|
Modern SUs (such as glimepiride) are found to maintain myocardial ischemic preconditioning with fewer CV side effects as compared to conventional SUs | A preclinical trial conducted by Mocanu et al. [10] compared the effect of glimepiride vs. glibenclamide on ischemic preconditioning (IP) protection and the protection afforded by diazoxide, an opener of mitochondrial KATP channels. The protective actions of IP or diazoxide were not eliminated by glimepiride; however, glibenclamide eliminated the infarct-limiting effects of IP and diazoxide |
Use of OADs in HF patients should be considered on the basis of the stages of HF | Patients with diabetes mellitus are at increased risk of developing heart failure because of the abnormal cardiac handling of glucose and free fatty acids (FFAs), and also due to the effect of the metabolic derangements of diabetes on the cardiovascular system. The metabolic risk of diabetes in heart failure is increased by the effect of most OADs, as the use of certain antidiabetic agents increases the risk of mortality and hospitalization for heart failure both in patients with and without heart failure. Therefore it is important to use OADs on the basis of the stage of HF [31] |
Strong suggestion for avoidance of metformin use in patients with acute stroke was proposed. However, it was decided that metformin could be considered for use in patients with stable HF | Experimental studies suggest that neuronal AMP-activated protein kinase (AMPK) activation induced by metformin during the acute phase of stroke has adverse clinical implications, while glial AMPK activation plays a beneficial role. The experimental evidence also suggests that cerebral AMPK activation by metformin is detrimental to stroke outcomes, while peripheral AMPK activation by metformin reduces stoke-enhanced serum glucose levels [32] A study conducted by Romero et al. [19] suggested that metformin therapy was associated with a decreased mortality and hospitalization rate in patients with HF and new-onset DM |
Modern SUs (such as gliclazide MR and glimepiride) are associated with a lower risk of all-cause and CV-related mortality compared to conventional SUs in T2DM patients. The clinical expert group suggests that the modern SUs can be safely used in T2DM patients with CV risk, myocardial infarction, or stroke | Simpson et al. [33] conducted a network meta-analysis to compare the relative risk of mortality and adverse CV events among SUs. Network meta-analysis using both direct and indirect evidence showed that gliclazide and glimepiride were associated with a lower risk of all-cause and CV-related mortality compared with glibenclamide, whereas glipizide use had a similar risk. No significant differences were observed among SUs, neither on traditional nor on network meta-analysis on the incidence of MI |
The expert group listed out all the conditions and the OADs that could be considered safe or unsafe in each of the following conditions | |
Recommendations on use of various OADs in patients with stable CAD | |
Preferred choice: Metformin, modern SU | Preferred choice Metformin reduces CV events significantly and reduces blood pressure and low-density lipoprotein levels (LDL). The United Kingdom Prospective Diabetes Study (UKPDS), a subpopulation study that included overweight patients with diabetes, found that metformin, when initiated early in the disease, is associated with significant risk reductions of 32% for any diabetes-related endpoint (sudden death, fatal or non-fatal myocardial infarction [MI], angina, heart failure, stroke, and amputation), 42% for diabetes-related death (death from MI, stroke, peripheral vascular disease), and 36% for all-cause mortality [34] Modern SUs: A nationwide registry comprising 1310 DM patients with acute myocardial infarction revealed that the mortality was lower in patients previously treated with modern SUs when compared to those treated with other oral medications or insulin [1, 17] Use with caution Conventional SU: According to South Asia Consensus Statements, modern SUs should be preferred over conventional SUs in patients with CAD [1] |
May use: Dipeptidyl peptidase-4 inhibitor (DPP4i), alpha-glucosidase inhibitors (AGI) | |
Use with caution, only if necessary: Conventional SUs | |
Recommendations on use of various OADs in patients with unstable CAD | |
Preferred choice: Pioglitazone | Preferred choice Pioglitazone is associated with reduced CV risk, all-cause mortality, non-fatal MI, and stroke and therefore is preferred OAD in patients with unstable CAD. A meta-analysis conducted by Lee et al. [28] on three randomized controlled studies reported that use of pioglitazone in stroke patients with insulin resistance, prediabetes, and DM was associated with a lower risk of recurrent stroke and future major vascular events Use with caution Metformin should be avoided in patients with unstable CAD [35] EMPA-REG OUTCOME Study: In the study, although beneficial effect of empagliflozin was reported on mortality and hospitalization for heart failure, it failed to reduce hospitalization from unstable angina [22] |
May use: Modern SU, DPP4i, AGI | |
Use with caution, only if necessary: Metformin, SGLT2i | |
Recommendations on use of various OADs in patients with HF EF preserved | |
Preferred choice: Metformin, SGLT2i | Preferred choice Metformin: In failing hearts, metformin improves myocardial energy metabolic status through the activation of AMP (adenosine monophosphate)-activated protein kinase (AMPK) and the regulation of lipid and glucose metabolism. By increasing nitric oxide (NO) bioavailability, limiting interstitial fibrosis, reducing the deposition of advanced glycation end-products (AGEs), and inhibiting myocardial cell apoptosis, metformin reduces cardiac remodeling and hypertrophy, and thereby preserves left ventricular systolic and diastolic functions [36]. A study conducted by Romero et al. [19] suggested that metformin therapy was associated with a decreased mortality and hospitalization rate in patients with HF and new-onset DM SGLT2 inhibition promotes natriuresis and osmotic diuresis, leading to plasma volume contraction and reduced preload, as well as decreases in blood pressure, arterial stiffness, and afterload, thereby improving subendocardial blood flow in patients with HF. SGLT2 inhibition is also associated with preservation of renal function [37] Absolute contraindication Pioglitazone: The pioglitAzone Clinical Trial In macroVascular Events (PROactive Study) conducted by Erdmann et al. [23] proved that a larger number of patients treated with pioglitazone were reported to have serious HF compared to those treated with placebo |
May use: Modern SU, DPP4i, AGI | |
Use with caution, only if necessary: Conventional SU | |
Absolute contraindication: Pioglitazone | |
Recommendations on use of various OADs in patients with HF low EF | |
Preferred choice: SGLT2i | Preferred choice SGLT2i: The CANVAS Program (Canagliflozin Cardiovascular Assessment Study) conducted by Rådholm et al. [24] on 10,142 patients with T2DM and high cardiovascular risk proved that the treatment with canagliflozin resulted in reduced risk of cardiovascular death or hospitalized HF across a broad range of different patient subgroups. Benefits were found to be greater in those patients with a history of HF at baseline Absolute contraindication Pioglitazone: The pioglitAzone Clinical Trial In macroVascular Events (PROactive Study) conducted by Erdmann et al. [23] proved that a larger number of patients treated with pioglitazone were reported to have serious HF compared to those treated with placebo Conventional SUs: Conventional SUs do not preserve ischemic preconditioning and therefore should be used with caution in patients with HF only if necessary [1] |
May use: Modern SU, metformin, AGI | |
Use with caution, only if necessary: DPP4i | |
Absolute contraindication: Pioglitazone, conventional SU | |
Recommendations on use of various OADs in patients with stroke | |
Preferred choice: Modern SU, metformin, pioglitazone | Preferred choice Modern SU: Meta-analysis of 47 RCTs by Varvaki Rados et al. [14] reported that SUs are not associated with increased risk for all-cause mortality, CV mortality, myocardial infarction, or stroke Metformin: The United Kingdom Prospective Diabetes Study (UKPDS), a subpopulation study that included overweight patients with diabetes, found that metformin, when initiated early in the disease, is associated with significant risk reductions of 32% for any diabetes-related endpoint (sudden death, fatal or non-fatal myocardial infarction [MI], angina, heart failure, stroke, and amputation), 42% for diabetes-related death (death from MI, stroke, peripheral vascular disease), and 36% for all-cause mortality [34] Pioglitazone: A meta-analysis conducted by Lee et al. [28] on three randomized controlled studies reported that use of pioglitazone in stroke patients with insulin resistance, prediabetes, and DM was associated with a lower risk of recurrent stroke and future major vascular events |
May use: DPP4i, AGI | |
Use with caution, only if necessary: SGLT2i, conventional SU | |
Recommendations on use of various OADs in patients with peripheral artery disease | |
Preferred choice: Modern SU, metformin, DPP4i | Preferred choice Modern SUs: Evidence from a randomized controlled study suggests that glimepiride exerts an inhibitory effect on the initiation and development of atherosclerosis [38] Metformin and DPP4i delay the progression of atherosclerosis by improving endothelial dysfunction and are thereby preferred in patients with peripheral arterial disease [39] Use with caution SGLT2i and conventional SU increase the atherosclerotic plaque and therefore should be used with caution [39] |
May use: Pioglitazone, AGI | |
Use with caution, only if necessary: SGLT2i, conventional SU | |
Recommendations on use of various OADs in patients with dyslipidemia uncontrolled | |
Preferred choice: Modern SU, metformin, DPP4i, SGLT2i, pioglitazone | Preferred choice Modern SU: Glimepiride increases high-density lipoprotein cholesterol by increasing adiponectin levels [40] Metformin: Metformin reduces LDL cholesterol and triglycerides and increases HDL cholesterol [41] DPP4i, SGLT2i, and pioglitazone: Increase HDL cholesterol [41] Use with caution Conventional SU: Increases LDL [41] |
May use: AGI | |
Use with caution, only if necessary: conventional SU | |
Recommendations on use of various OADs in patients with arrhythmias | |
Preferred choice: Metformin | Preferred choice Metformin: In a population-based cohort study, metformin use was associated with a decreased risk of atrial fibrillation in patients with T2DM who were not using other antidiabetic medication. Reduced atrial fibrillation risk could be attributed to attenuation of atrial cell tachycardia-induced myolysis and oxidative stress [42] Use with caution Conventional SU: glibenclamide interferes with the beneficial action of KATP channel opening during acute ischemia–reperfusion events and therefore should be cautiously used in patients with arrhythmias [1] |
May use: Modern SU, DPP4i, SGLT2i, AGI | |
Use with caution, only if necessary: Conventional SU, pioglitazone | |
Modern SUs Enhance Adherence and Are the Preferred Management Option
Importance of Medication Adherence in Achieving Glycemic Control
Despite the growing understanding of diabetes and the availability of new medications and technologies for the management of DM, a substantial number of individuals are not able to achieve their glycemic goals [28, 29, 43, 44]. According to real-world data, the proportion of patients with poor glycemic control (HbA1c > 7%) was 85.8% between 1999 and 2002; 91% between 2003 and 2006; and 91.7% between 2007 and 2010. This proportion has not improved through 2014, despite the development of many new medications [28, 29, 43, 44].
The key contributing factor for the inadequate glycemic control in a real-world setting is poor medication adherence. Medication non-adherence accounted for approximately three-quarters of the gap between real-world data and expected randomized controlled trial results (gap = 0.51%) [30, 45].
Patient Adherence
Adherence to and persistence with antidiabetic medications are crucial in patients with DM to achieve optimal clinical benefits. Increased adherence to medications is associated with a decrease in HbA1c, decreased mortality rates, fewer all-cause hospitalizations, and lower healthcare expenditure. Approximately 699,000 emergency room visits and 341,000 hospitalizations per year can be averted with long-term adherence to and persistence with antidiabetic medications, resulting in yearly savings of nearly US$5 billion [31, 46].
Adherence to medications is important to achieve an effective therapeutic outcome. A retrospective cohort study conducted by Ho et al. evaluated the association between medication non-adherence and all-cause hospitalization and all-cause mortality. It was found that the non-adherent patients had higher all-cause hospitalization and all-cause mortality when compared to adherent patients [32, 47]. Compliance to and non-persistence with prescribed medication regimens also resulted in increased morbidity and mortality as well as increased healthcare costs [33, 48].
Physicians’ Communication
Physicians’ communication has a major impact on adherence. Communication contributes to a better understanding among patients about the illness and the risks and benefits of treatment. Support, empathy, understanding, collaborative partnerships, and patient-centered interviewing are essential for improving effective communication and enhancing adherence [34]. In a meta-analysis conducted by Haskard Zolnierek et al. of across 106 studies, a strong relationship was identified between patient adherence and physicians’ communication. Non-adherence was found to be more than 1.47 times greater among individuals whose physician was a poor communicator. The odds ratio of a patient adhering is 2.16 times better if the physician is a good communicator [34, 49].
A study conducted by Kurlander et al. suggested that non-adherence is influenced primarily by financial reasons, whereas patients who selectively reduce their diabetes treatments are influenced by their mood and medication beliefs [35, 50].
Affordability and Improved Adherence with Modern SUs
Modern SUs offer superior glycemic efficacy and are also available at a reasonable cost. Treatment with modern SUs is associated with a lower economic burden, and hence they are an effective alternative to other newer antidiabetic drugs. Sulfonylureas have an oral route of administration (vs. injectable insulins and GLP-1 analogs) and a once-daily dosing schedule (vs. once to twice daily for metformin and three times daily for alpha-glucosidase inhibitors and glinides). The once-daily dosing ensures better patient adherence to the medication, unlike its comparator drugs [1].
Key recommendations of the international task force
Key recommendations | Evidence and/or rationale |
|---|---|
Treatment with modern SUs is associated with a lower economic burden and better patient adherence, and hence can be considered as an effective alternative to other newer antidiabetic drugs | South Asia consensus statement recommends modern SUs as an effective alternative to other antidiabetic medications; SU-containing dual or triple fixed dose combinations, if available, (with drugs that have complementary modes of action) reduce cost, offer convenience, and improve patient adherence [1] |
The panel also highlighted the role of physicians in proper communication about the illness and the risks and benefits of treatment. Support, empathy, understanding, collaborative partnerships, and patient-centered interviewing are essential for improving effective communication and enhancing adherence [34] | In a meta-analysis conducted by Haskard Zolnierek et al. of across 106 studies, a strong relationship was identified between patient adherence and physicians’ communication [49] |
Conclusion
Sulfonylureas are an asset in diabetes care. Owing to their safety, efficacy, extra-pancreatic benefits, and low cost of therapy, modern SUs could be considered as drugs of choice for the treatment of diabetes. The concept of glucocrinology should be implemented in the management of diabetes to achieve holistic care and best clinical outcomes.
References
Kalra S, Aamir AH, Raza A, et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: a consensus statement. Indian J Endocrinol Metab. 2015;19(5):577–96.
WHO. Guidelines on second-and third-line medicines and type of insulin for the control of blood glucose levels in non-pregnant adults with diabetes mellitus. http://apps.who.int/iris/bitstream/handle/10665/272433/9789241550284-eng.pdf?ua=1. Accessed 3 Oct 2018.
Kalra S, Priya G, Gupta Y. Glucocrinology. J Pak Med Assoc. 2018;68(6):963–5.
Basit A, Riaz M, Fawwad A. Glimepiride: evidence-based facts, trends, and observations (GIFTS) [corrected]. Vasc Health Risk Manag. 2012;8:463–72.
Del Prato S, Pulizzi N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metabolism. 2006;55(5 Suppl 1):S20–7.
Barzilai N, Groop PH, Groop L, et al. A novel mechanism of glipizide sulfonylurea action: decreased metabolic clearance rate of insulin. Acta Diabetol. 1995;32(4):273–8.
ter Braak EW, Appelman AM, van der Tweel I, et al. The sulfonylurea glyburide induces impairment of glucagon and growth hormone responses during mild insulin-induced hypoglycemia. Diabetes Care. 2002;25(1):107–12.
Nagasaka S, Taniguchi A, Aiso Y, et al. Effect of glimepiride on serum adiponectin level in subjects with type 2 diabetes. Diabetes Care. 2003;26(7):2215–6.
Inukai K, Watanabe M, Nakashima Y, et al. Efficacy of glimepiride in Japanese type 2 diabetic subjects. Diabetes Res Clin Pract. 2005;68(3):250–7.
Mocanu MM, Maddock HL, Baxter GF, et al. Glimepiride, a novel sulfonylurea, does not abolish myocardial protection afforded by either ischemic preconditioning or diazoxide. Circulation. 2001;103(25):3111–6.
Lee TM, Chou TF. Impairment of myocardial protection in type 2 diabetic patients. J Clin Endocrinol Metab. 2003;88(2):531–7.
Landman GWD, de Bock GH, van Hateren KJJ, et al. Safety and efficacy of gliclazide as treatment for type 2 diabetes: a systematic review and meta-analysis of randomized trials. PLoS One. 2014;9(2):e82880. https://doi.org/10.1371/journal.pone.0082880.
Kalra S, Bahendeka S, Sahay R, et al. Consensus recommendations on sulfonylurea and sulfonylurea combinations in the management of type 2 diabetes mellitus—international task force. Indian J Endocrinol Metab. 2018;22(1):132–57.
Varvaki Rados D, Catani Pinto L, Reck Remonti L, et al. The association between sulfonylurea use and all-cause and cardiovascular mortality: a meta-analysis with trial sequential analysis of randomized clinical trials. PLoS Med. 2016;13(4):e1001992.
Lee TM, Chou TF. Impairment of myocardial protection in type 2 diabetic patients. J Clin Endocrinol Metab. 2003;88(2):531–7.
Kalra S, Gupta Y, Kishor K. The cardiovascular phenotype: impact on choice of glucose-lowering therapy. J Pak Med Assoc. 2016;66(4):480–2.
Zeller M, Danchin N, Simon D, et al. Impact of type of preadmission sulfonylureas on mortality and cardiovascular outcomes in diabetic patients with acute myocardial infarction. J Clin Endocrinol Metab. 2010;95(11):4993–5002.
Aschner P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res Clin Pract. 2017;132:169–70.
Romero SP, Andrey JL, Garcia-Egido A, et al. Metformin therapy and prognosis of patients with heart failure and new-onset diabetes mellitus. A propensity-matched study in the community. Int J Cardiol. 2013;166(2):404–12.
Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26.
Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42.
Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME™). Cardiovasc Diabetol. 2014;19(13):102.
Erdmann E, Charbonnel B, Wilcox RG, et al. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08). Diabetes Care. 2007;30(11):2773–8.
Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2019;138(5):458–68.
Kosiborod M, Gause-Nilsson I, Xu J, Sonesson C, Johnsson E. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and concomitant heart failure. J Diabetes Complicat. 2017;31(7):1215–21.
White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascularoutcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162(4):620–6.e1.
Pantalone KM, Kattan MW, Yu C, et al. The risk of overall mortality in patients with type 2 diabetes receiving glipizide, glyburide, or glimepiride monotherapy: a retrospective analysis. Diabetes Care. 2010;33(6):1224–9.
Lee M, Saver JL, Liao HW, et al. Pioglitazone for secondary stroke prevention: a systematic review and meta-analysis. Stroke. 2017;48(2):388–93.
Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72.
Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69–79.
Rosano GMC, Vitale C, Seferovic P. Heart failure in patients with diabetes mellitus. Card Fail Rev. 2017;3(1):52–5.
Jia J, Cheng J, Ni J, et al. Neuropharmacological actions of metformin in stroke. Curr Neuropharmacol. 2015;13(3):389–94.
Simpson SH, Lee J, Choi S, et al. Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol. 2015;3(1):43–51.
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;35(9131):854–65.
American Diabetes Association. Cardiovascular disease and risk management. Standard of medical care in diabetes 2019. Diabetes Care. 2019;42(Suppl. 1):S103–23.
Dziubak A, Wojcicka G, Wojtak A, et al. Metabolic effects of metformin in the failing heart. Int J Mol Sci. 2018;19(10):2869.
Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136(17):1643–58.
Koshiba K, Nomura M, Nakaya Y, et al. Efficacy of glimepiride on insulin resistance, adipocytokines, and atherosclerosis. J Med Invest. 2006;53(1–2):87–94.
Papanas N, Maltezos E. Oral antidiabetic agents: anti-atherosclerotic properties beyond glucose lowering? Curr Pharm Des. 2009;15(27):3179–92.
Araki T, Emoto M, Konishi T, et al. Glimepiride increases high-density lipoprotein cholesterol via increasing adiponectin levels in type 2 diabetes mellitus. Metabolism. 2009;58(2):143–8.
Azimova K, Juan ZS, Mukherjee D. Cardiovascular safety profile of currently available diabetic drugs. Ochsner J. 2014;14(4):616–32.
Chang SH, Wu LS, Chiou MJ, et al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13:123.
Ali MK, Bullard KM, Saaddine JB, et al. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med. 2013;368(17):1613–24.
Carls G, Huynh J, Tuttle E, et al. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther. 2017;8(4):863–73.
Carls GS, Tuttle E, Tan RD, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40(11):1469–78.
Farr AM, Sheehan JJ, Curkendall SM, et al. Retrospective analysis of long-term adherence to and persistence with DPP-4 inhibitors in US adults with type 2 diabetes mellitus. Adv Ther. 2014;31(12):1287–305.
Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–41.
Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–7.
Haskard Zolnierek KB, Di Matteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47(8):826–34.
Kurlander JE, Kerr EA, Krein S, Heisler M, Piette JD. Cost-related nonadherence to medications among patients with diabetes and chronic pain: factors beyond finances. Diabetes Care. 2009;32(12):2143–8.
Acknowledgements
We acknowledge Shalini Menon, Senthilnathan Mohanasundaram, Romik Ghosh, and S. Amarnath from Sanofi India for their logistics assistance, guidance and expertise in convening an expert forum meeting. The content published herein represents the views and opinions of the various contributing authors and does not necessarily represent the views or opinion of Sanofi and/or its affiliates. The details published herein are intended for informational, educational, academic, and/or research purposes and are not intended to substitute for professional medical advice, diagnosis, or treatment.
Funding
This expert opinion initiative has been funded by Sanofi India. The Rapid Service Fee was received by the journal and was paid for by Sanofi India. All authors had full access to the articles reviewed in this manuscript and take complete responsibility for the integrity and accuracy of this manuscript.
Medical Writing and Editorial Assistance
Medical writing and editorial support was provided by Dr Rajshri Mallabadi and Dr Kavitha Ganesha from BioQuest Solutions Pvt. Ltd. which was paid for by Sanofi, India.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published
Disclosures
Sanjay Kalra is a member of the journal’s Editorial Board. A. K. Das, M. P. Baruah, A. G. Unnikrishnan, Arundhati Dasgupta, Parag Shah, Rakesh Sahay, Rishi Shukla, Sambit Das, Mangesh Tiwaskar, G. Vijayakumar, Manoj Chawla, Fatimah Eliana, Ketut Suastika, Abbas Orabi, Aly Ahmed Abdul Rahim, Andrew Uloko, Silver Bahendeka, Abdurezak Ahmed Abdela, Fariduddin Mohammed, Faruque Pathan, Muhammed Hafizur Rahman, Faria Afsana, Shajada Selim, Muaz Moosa, Moosa Murad, Pradeep Krishna Shreshtha, Dina Shreshtha, Mimi Giri, Wiam Hussain, Ahmed Al-Ani, Kaushik Ramaiya, Surender Singh, Syed Abbas Raza, Than Than Aye, Chaminda Garusinghe, Dimuthu Muthukuda, Muditha Weerakkody, Shyaminda Kahandawa, Charlotte Bavuma, Sundeep Ruder, Koy Vanny, Manish Khanolkar, and Leszek Czupryniak have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8263688.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kalra, S., Das, A.K., Baruah, M.P. et al. Glucocrinology of Modern Sulfonylureas: Clinical Evidence and Practice-Based Opinion from an International Expert Group. Diabetes Ther 10, 1577–1593 (2019). https://doi.org/10.1007/s13300-019-0651-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-0651-1