Abstract
Introduction
It is unclear whether adding basal insulin or enhancing incretin signaling with a glucagon-like peptide-1 receptor agonist (GLP-1RA) is more effective as an up-titration strategy after dipeptidyl peptidase-4 inhibitor (DPP-4i)-based oral antidiabetic drug (OAD) therapy. GLP-1RAs can be injected without dose adjustment, unlike basal insulin. Our objective was to examine the efficacy of changing patients inadequately controlled with oral DPP-4i-based OAD therapy to injectable GLP-1RA and discontinuing the DPP4i versus adding basal insulin glargine (IGlar) with the continuation of the oral DPP4i.
Methods
Sixty patients with type 2 diabetes (T2DM) and glycated hemoglobin (HbA1c) between 7.0% and 10.0% on DPP-4i-based OAD therapy were randomized to either adding IGlar and remaining on the DPP-4i or liraglutide and discontinuing the DPP-4i for 24 weeks. Patients in the IGlar group started with 0.1 unit/kg and were titrated according to the algorithm. In the liraglutide group, the DPP-4i was replaced with liraglutide 0.9 mg/day, the maximum dose in Japan. We evaluated HbA1c, glycated albumin (GA), and anthropometrics.
Results
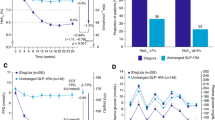
HbA1c was significantly lower at week 24 (− 1.0 ± 0.9% in the IGlar group and − 0.6 ± 0.8% in the liraglutide group), but the difference between groups was not significant. Changes in GA were similar (− 2.9 ± 3.2% vs. − 2.6 ± 3.2%) in both groups. Body weight (BW) was significantly lower only in the liraglutide group (+ 0.5 ± 2.6 kg vs. − 2.2 ± 2.0 kg). The rate of minor hypoglycemic episodes was similar for both groups.
Conclusion
For poorly controlled T2DM on DPP-4i-based OAD therapy, switching to single-dose liraglutide to enhance incretin signaling is as effective as dose-titrated basal IGlar, but significant BW reduction was only seen in the liraglutide group. These results suggest that enhancing incretin signaling with a single-dose injectable GLP-1 RA might be an alternative to dose-titrated basal insulin therapy in patients with T2DM poorly controlled with DPP-4i-based OAD therapy. These findings should be confirmed in a longer and larger trial.
Trial Registration
Trial Registry (UMIN-CTR) as UMIN000012224.
Similar content being viewed by others
Introduction
To reduce the risk of long-term diabetic complications, guidelines in Europe, the USA, and Japan have recommended early partitioning of injectable therapy as part of stepwise intensification of treatment to reduce glycated hemoglobin (HbA1c) below the target of 7.0% (53 mmol/mol) [1,2,3,4]. As part of an up-titration algorithm, insulin is the next choice of medication to achieve a HbA1c target below 7.0% after oral antidiabetic drugs (OADs). However, use of insulin is not widespread because of fears of hypoglycemia and challenges in dose titration.
Incretin is a hormone that controls glucose metabolism via an intestinal signal that senses various nutritional stimuli [5]. Glucagon-like peptide-1 (GLP-1) analogues are alternatives to insulin as additions to OAD therapy. Gliptins, a relatively new class of OADs, act by inhibiting dipeptidyl peptidase-4 (DPP-4), which inactivates incretins, namely glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 [5]. Incretin-based therapies are attracting attention in terms of treatment efficacy, cost-effectiveness, and improved cardiovascular outcomes for patients with type 2 diabetes mellitus (T2DM) [6,7,8]. Two types of incretin-based therapy are now available to treat T2DM: injectable GLP-1 receptor agonists (GLP-1RAs, incretin mimetics) and oral DPP-4 inhibitors [9]. DPP-4 inhibitors are the most commonly prescribed OADs in Japan, taken by more than 70% of patients with T2DM [10]. Incretin-based therapy is thought to be more effective for T2DM in Asian populations than non-Asian counterparts [11,12,13]. However, there are many patients who are inadequately controlled with DPP-4 inhibitors.
Basal insulin or GLP-1RA injections have generally been accepted as second- or third-line therapy in American Diabetes Association/European Association for the Study of Diabetes consensus statements [2]. Earlier introduction of injection therapy is desired in patients with diabetes poorly controlled with OADs. Two studies showed that switching from sitagliptin to liraglutide in T2DM has a similar effect on the HbA1c, although the active comparator was a DPP-4 inhibitor [14, 15]. Unlike insulin, GLP-1RAs can be injected without dose adjustment. However, when using GLP-1RAs, we must pay attention to tachyphylaxis [16]. It is also unclear why changes in mean HbA1c and fasting plasma glucose (FPG) levels occur after the third month of intervention and the effect may occur later.
It remains unclear which injections are better as an additional treatment option for T2DM when OADs, including DPP-4 inhibitors, do not provide adequate control in terms of achieving target HbA1c: basal-supported oral therapy (BOT) with insulin or switching to a GLP-1RA to strengthen incretin-based therapy. It is also unclear whether GLP-1 incretin enhancement remains an option after oral incretin or DPP-4 inhibitor therapy. Therefore, we performed this 24-week randomized controlled study to find out whether adding basal insulin glargine U100 (IGlar) or switching to liraglutide is more likely to be an option for achieving HbA1c below 7.0% and better in terms of patient safety.
Methods
Study Design
This 24-week, multicenter, open-label, randomized, parallel-group trial took place at six sites (university hospitals and diabetes clinics). Participants were enrolled between June 2013 and December 2015. Inclusion criteria included T2DM with inadequate glycemic control despite diet and exercise therapy and OADs consisting of a DPP-4 inhibitor with metformin, sulfonylureas (SUs), glinides, alpha-glucosidase inhibitors, or thiazolidinediones.
Sixty T2DM patients treated with OADs, including a DPP-4 inhibitor in addition to lifestyle modification for 12 or more weeks, were randomized to either IGlar or liraglutide once daily in a 1:1 ratio for a 24-week intervention period (Fig. 1). The randomization scheme, incorporating a random number generator, was produced by an independent researcher who was not directly involved in data collection or delivery of the study drugs. Randomization was performed using the minimization method with HbA1c (%) and age. Four or more weeks before the start of this trial, after written informed consent was obtained, all types of DPP-4 inhibitors were replaced with 50 mg of sitagliptin daily and SUs were changed to 1 mg of glimepiride, the highest prescribed dose in Japan for each class, to minimize the effect of individual drugs. To evaluate the impact of the two investigational treatments, adding basal insulin (IGlar) with the oral incretin sitagliptin versus the injectable incretin liraglutide, we aimed to structure OAD therapy to the same extent prior to the beginning of the intervention. Most of the subjects in this study were treated with glimepiride 1 mg, but one subject in each group was prescribed 2 mg. Neither subject had been taking other SUs such as glibenclamide or gliclazide before the study period. The change in HbA1c from more than 4 weeks before the intervention to the end of the run-in period was not statistically significantly different (IGlar group, 8.19 ± 0.84% to 8.27 ± 1.01%; liraglutide group, 8.06 ± 0.46% to 8.16 ± 0.46%; P = 0.22). The change in glycated albumin over the same period was similar between the two groups (IGlar group, 21.0 ± 2.68% to 20.8 ± 3.58%; liraglutide group, 22.4 ± 4.14% to 21.0 ± 3.73%; P = 0.40). Patients visited the hospital or clinic once every 2 weeks up to week 8 for dose adjustments; thereafter, visits were every 4 weeks. The IGlar group was started at 0.1 unit/kg and titrated up according to an algorithm to a target FPG of less than 110 mg/dL (6.1 mmol/L) without discontinuing sitagliptin (Table 1). In the liraglutide group, sitagliptin was replaced by liraglutide at a starting dose of 0.3 mg, which was increased by 0.3 mg at least once weekly so the final dose was 0.9 mg, the maximum dose in Japan, by week 12, which was continued until the end of the trial. Blood samples were collected after an overnight fast at visits every 4 weeks. As a general rule, we did not change OADs during the trial period unless hypoglycemia occurred.
Participants
We included Japanese men and women with T2DM for at least 6 months, who were at least 20 years of age, currently treated with OADs for at least 12 weeks, and had HbA1c levels ranging from 7.0% to less than 10%. Subjects were excluded if they had been treated with insulin or GLP-1RA injection therapy, or had impaired renal or hepatic function, pancreatic disease, malignancy (either known or previous malignancy and strongly suggestive of recurrence), significant cardiovascular disease (heart failure or coronary artery disease) within 3 months, or unstable proliferative retinopathy.
All study protocols and procedures were in accordance with the ethical standards of the Ethics Committee of the Toho University Omori Medical Center Hospital and the 1964 Helsinki Declaration and its amendments. The study objectives and intended measures were explained to all the participants individually. Informed consent was obtained from all study participants.
Endpoints and Assessments
The primary endpoints were the change in HbA1c from baseline to week 24 and the proportion of patients achieving HbA1c below 7% at week 24. Secondary efficacy endpoints included change in HbA1c regarding taking SUs or not, and change in body weight (BW). Secondary safety endpoints assessed over 24 weeks included the number of hypoglycemic episodes. The sample size calculation assumed a mean difference in the change in HbA1c between the two groups of 0.18% and a standard deviation (SD) of 0.22%, based on the results of two prior studies of basal insulin compared to GLP-1 analogue therapy [17, 18]. At a significance level of 5% and power of 80%, 18 patients were required (24 per group). Therefore, we planned to enroll 30 patients per group to account for potential loss to follow-up.
Statistical Analysis
The results are expressed as means ± SD. Differences between groups were first estimated using one-way analysis of variance (ANOVA), the chi-squared test, or the t test for comparison. When a significant effect was found with ANOVA, the results were further compared using the Bonferroni multiple range test. Cochran–Mantel–Haenszel (CMH) modeling was used to analyze the proportion of patients who achieved the HbA1c target at week 24, with or without SUs. Differences were considered to be significant with a two-sided P < 0.05.
This trial was registered with the University Hospital Medical Information Network-Clinical Trials Registry (UMIN-CTR) as UMIN000012224.
Results
In the IGlar group, three subjects withdrew consent. The trial was stopped in one subject at the discretion of the physician because of high glucose levels. In the liraglutide group, one subject withdrew consent. These five subjects were excluded from the analysis (Fig. 2).
Baseline characteristics of the study patients are summarized in Table 2. The mean dose of IGlar was 6.5 ± 2.8 units at the start of the trial, which was titrated to 14.4 ± 9.1 units at the end of the trial (range, 6–40 units/day). The dose of liraglutide was increased to 0.9 mg in all subjects by week 8, but two subjects had dose reductions to 0.6 mg because of adverse effects (gastrointestinal symptoms). Although we did not plan to introduce self-monitoring of blood glucose for subjects, we approved it for the five subjects in the IGlar group and two subjects in the liraglutide group who wished to do so (P = 0.17).
The mean change in HbA1c from baseline to week 24 was significant in both groups (− 1.0 ± 0.9% in the IGlar group and − 0.6 ± 0.8% in the liraglutide group) but there was no statistically significant difference between the groups (P = 0.16; Fig. 3a). Results were similar for glycated albumin (GA) (mean change − 2.9 ± 3.2% in the IGlar group and − 2.6 ± 3.2% in the liraglutide group; P = 0.53; Fig. 3b). The proportion of patients who achieved HbA1c below 7% at week 24 was not statistically significant different (42.3% in the IGlar group and 24.1% in the liraglutide group; chi-squared P = 0.15, CMH P = 0.25; Supplementary Fig. S1). We examined changes in HbA1c with or without SUs separately, but there was no statistically significant difference (Supplementary Fig. S2).
Mean BW was significantly increased from baseline in the IGlar group (+ 0.5 ± 2.6 kg), whereas in the liraglutide group it decreased significantly (− 2.2 ± 2.0 kg) (Table 3), but the difference between groups was not significant (p = 0.37).
A few minor hypoglycemic episodes occurred in both groups (four episodes in the IGlar group and three in the liraglutide group; P = 0.58). The minimum FPG level was 77 mg/dL, which occurred in the IGlar group. Lipid parameters, as well as human immunoreactive insulin and proinsulin levels, were similar to baseline levels and between groups (Table 3). Blood pressure in the two groups was similar during the study period.
Discussion
Injectable After Oral Incretin Therapy
We wish to find room to enhance incretin signaling to improve glucose control in patients with primary OAD failure, including DPP-4 inhibitors, compared to BOT. In this study, we showed the efficacy of an up-titration strategy for T2DM that was inadequately controlled with DPP-4 inhibitors and other OADs. Adding a single-dose GLP-1 incretin enhancement had a therapeutic effect that was similar to the effect of adding dose-titrated basal insulin. Parallel analysis of a series of predefined parameters related to glucose control showed that adding a single-dose GLP-1 analogue (i.e., changing an oral incretin to an injectable incretin) and adding dose-titrated basal insulin treatment had similar effects. However, BW reduction was only observed with single-dose GLP-1 analogue therapy. The clinical safety and efficacy profile of GLP-1 analogues compared to insulin has been assessed in several meta-analyses [19, 20]. However, at this point, GLP-1 analogue therapy after insufficient OAD therapy might be considered as an alternative to basal insulin treatment used as an adjunct to OADs. Therefore, there is an increasing desire to understand the possibilities and limitations of GLP-1 analogues and basal insulins.
Efficacy Analysis
Adding titrated basal insulin to oral therapy is typically indicated when HbA1c is 1.5–2.0% above target HbA1c in patients with T2DM [21]. Our trial investigated the effectiveness of a simple fixed-dose incretin enhancement to multiple OAD therapy, which was designed to support the initiation of injection therapy in patients with T2DM in the primary care setting. Furthermore, we aimed to set simple clinical protocols compared to the dose-titration algorithms used when initiating basal insulin. Regarding reducing hyperglycemia, mean changes in HbA1c from baseline to week 24 were not significantly different between the two groups. The rates of achieving HbA1c below 7.0% after 24 weeks of treatment, as recommended in international guidelines, were 24% in the simple fixed-dose GLP-1 analogue group and 42% in the titrated basal insulin group (P = 0.15), suggesting that intensifying incretin signaling and oversupplying basal insulin were not sufficient to achieve optimized glycemic control. IGlar was used as an adjunct to DPP4 inhibitor-based OAD therapy. In this study, physicians specializing in diabetes treatment tried to titrate the dose of IGlar on the basis of an algorithm to achieve a target FPG level of less than 110 mg/dL (6.1 mmol/L), in contrast to patients adjusting the basal insulin IGlar dose frequently. Study visits occurred at intervals of 2–6 weeks, which might not be effective at lowering fasting plasma glucose. The reason for poor achievement, including a sedentary lifestyle, might be related to patient factors. Inclusion criteria for this trial included resistance to multiple drugs for controlling blood glucose. In diabetes management, inability to improve glycemia with the addition of a GLP-1 analogue and basal insulin suggests that multiple daily injections are imperative in some patients inadequately controlled with OADs.
Limitations
The present study has some limitations. First, this was an open-label trial, as a result of different titration algorithms for basal insulin and the GLP-1 analogue, which would have led to more investigator or participant bias. Second, at the end of the 24-week trial, the proportion of subjects who achieved HbA1c below 7.0% was relatively low, 24–42%, suggesting that most subjects might eventually need multiple injections daily. However, this rate was similar to the rate in a previous trial comparing the effect of IGlar to liraglutide in patients with OAD failure, who were not taking DPP-4 inhibitors [22]. The small sample size probably explains why reduced BW was seen in the IGlar group and there were very limited safety and efficacy concerns. Finally, we could not figure out the mechanism of action that explains the differences between adding basal insulin and GLP-1 analogue injection in patients with OAD failure. Hypoglycemia was seen in both groups to the same extent. The structure and dynamics of hypoglycemia should be monitored using a continuous glucose monitoring system in future studies, which we did not use. To address these limitations, a larger sample size and continuous glucose monitoring should be used to investigate the rate of hypoglycemia.
Conclusions
For patients with T2DM inadequately treated with OADs including DPP-4 inhibitors, either adding IGlar or switching to liraglutide is safe and effective, but significant BW reduction was only observed in the liraglutide group. Our results suggest that liraglutide can be a good treatment option for such patients because, unlike IGlar, it is effective without dose adjustment. Given the short study period and small sample size, future studies with more participants are needed to confirm the results of this study.
References
Hocher B, Tsuprykov O. Diabetic nephropathy: renoprotective effects of GLP1R agonists and SGLT2 inhibitors. Nat Rev Nephrol. 2017;13(12):728–30.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429–42.
American Diabetes Association. Standards of Medical Care in Diabetes 2017. Diabetes Care. 2017;40(Suppl. 1):S1–135.
Suzuki S, Oura T, Takeuchi M. Treatment guide for diabetes 2014–2015. In: Japan Diabetes Society (ed.). Bunkodo, Tokyo; 2016. http://www.fa.kyorin.co.jp/jds/uploads/Treatment_Guide_for_Diabetes_2014-2015.pdf.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705.
Qin L, Chen S, Flood E, et al. Glucagon-like peptide-1 receptor agonist treatment attributes important to injection-naïve patients with type 2 diabetes mellitus: a multinational preference study. Diabetes Ther. 2017;8(2):321–34.
Evans M, McEwan P, O’Shea R, George L. A retrospective, case-note survey of type 2 diabetes patients prescribed incretin-based therapies in clinical practice. Diabetes Ther. 2013;4(1):27–40.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18(3):203–16.
Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(Suppl 1):102–9.
Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and Non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708.
Kim YG, Hahn S, Oh TJ, et al. Differences in the HbA1c lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:900–9.
Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig. 2015;6:495–507.
Bailey TS, Takács R, Tinahones FJ, et al. Efficacy and safety of switching from sitagliptin to liraglutide in subjects with type 2 diabetes (LIRA-SWITCH): a randomized, double-blind, double-dummy, active-controlled 26-week trial. Diabetes Obes Metab. 2016;18(12):1191–8.
Takeshita Y, Takamura T, Kita Y, et al. Vildagliptin vs liraglutide as a second-line therapy switched from sitagliptin-based regimens in patients with type 2 diabetes: a randomized, parallel-group study. J Diabetes Investig. 2015;6(2):192–200.
Nauck MA, Kemmeries G, Holst JJ, et al. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60(5):1561–5.
Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther. 2007;29(11):2333–48.
Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32(5):762–8.
Abd El Aziz MS, Kahle M, Meier JJ, et al. A meta-analysis comparing clinical effects of short- or longacting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes Metab. 2017;19(2):216–27.
Singh S, Wright EE Jr, Kwan AY, et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19(2):228–38.
Blak BT, Smith HT, Hards M, et al. A retrospective database study of insulin initiation in patients with type 2 diabetes in UK primary care. Diabet Med. 2012;29(8):e191–8.
D’Alessio D, Häring HU, Charbonnel B, et al. Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab. 2015;17(2):170–8.
Acknowledgements
The authors are especially grateful to Hiroshi Yoshino MD, Kayoko Ikehara MD, and Shuki Usui MD of Toho University Graduate School of Medicine; Takamasa Ichijo MD of Saiseikai Yokohama-city Tobu Hospital; and the BOOST2 investigators.
Funding
No funding or sponsorship was received for this study. The article processing charges were funded by the authors.
Editorial Assistance
The manuscript has received editorial assistance in the form of proofreading by a native English editor certified by ZENIS Co., Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors made significant contributions to study design, analysis, and interpretation of data, as well as preparing and reviewing the manuscript.
Disclosures
The authors have no other relevant affiliations or financial involvement with any organizations or entities with a financial interest in or financial conflict with the subject or materials discussed in the manuscript apart from those disclosed.
Munehide Matsuhisa has received research support from Astellas, Biomedicalnet, Boehringer Ingelheim, Daiichi-Sankyo, Merck (MSD), Nihon Unysis, Novartis, Tanabe-Mitsubishi, and Welby, and speaker honoraria from Astellas, Eli Lilly, Novo Nordisk, Sanofi, Tanabe-Mitsubishi, and Takeda.
Takahisa Hirose is on an advisory panel for Eli Lilly, Novo Nordisk, and Sanofi; research support from Astellas, AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Dainippon-Sumitomo, Eli Lilly, Kissei, Merck (MSD), Novo Nordisk, Ono, Sanofi, Takeda, and Tanabe-Mitsubishi; and received speaker honoraria from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Kissei, Merck (MSD), Novo Nordisk, Ono, Sanofi, Takeda, and Tanabe-Mitsubishi.
The remaining authors Masahiko Miyagi, Hiroshi Uchino, Naoki Kumashiro, Mariko Higa, Koki Shin, Makiko Sasamoto, Hiroji Kitazato and Motoyuki Tamaki have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the Toho University Omori Medical Center Hospital and with the 1964 Helsinki Declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Prior Presentation
Some of the findings from this study were presented at the 60th Annual Meeting of The Japan Diabetes Society, Nagoya, Japan, May, 2017.
Data Availability
The datasets generated during and/or analyzed during the current study are available in the University Hospital Medical Information Network-Clinical Trials Registry (UMIN-CTR), number UMIN000012224.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6894653.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Miyagi, M., Uchino, H., Kumashiro, N. et al. Up-Titration Strategy After DPP-4 Inhibitor-Based Oral Therapy for Type 2 Diabetes: A Randomized Controlled Trial Shifting to a Single-Dose GLP-1 Enhancer Versus Adding a Variable Basal Insulin Algorithm. Diabetes Ther 9, 1959–1968 (2018). https://doi.org/10.1007/s13300-018-0486-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-018-0486-1