Abstract
Introduction
The appearance of anti-insulin antibodies or an allergy to insulin occasionally causes clinical problems with glycemic control in insulin users.
Methods
In the present report, we describe a therapeutic approach that was employed for a man with type 2 diabetes who had insulin allergy, anti-insulin antibodies, and anti-insulin receptor antibodies that developed during his insulin treatment.
Results
We started the patient on liraglutide, a glucagon-like peptide-1 receptor agonist, and attained glycemic control without incurring any side effects. Two years after liraglutide induction, his blood glucose was being maintained at a healthy level by liraglutide monotherapy.
Conclusion
Liraglutide may be a therapeutic option for patients with insulin allergy, anti-insulin antibodies, and type B insulin resistance syndrome, as it represents an alternative strategy to insulin.
Similar content being viewed by others
Introduction
In patients on insulin, the synthesis of anti-insulin antibodies or the expression of insulin allergy occasionally induces a problematic and uncontrolled glycemic profile. As has recently been reported by Chakraborty et al., avoiding a faulty injection technique is important for preventing injection-site allergies in patients with insulin-treated diabetes [1]. Insulin allergy and anti-insulin antibodies often cause difficulties with glycemic control during insulin therapy. Here, we describe a therapeutic approach that was employed for a patient with type 2 diabetes who had insulin allergy and was positive for serum anti-insulin antibodies and anti-insulin receptor antibodies.
Case Report
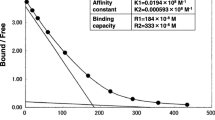
A 62-year-old man (body mass index: 18.5 kg/m2) with type 2 diabetes who was undergoing treatment with vildagliptin (100 mg/day) was referred to our hospital for the treatment of dilated cardiomyopathy with a left ventricular assist device (LVAD). On admission, his glycemic control was good, with a glycated hemoglobin level of 7.4% (NGSP). After approximately 1 year, he was transferred to our hospital because of cerebral hemorrhage, and craniotomy was performed to remove the hematoma; however, left-side hemiparesis remained. On the first day of admission he was administered glargine (6 units), and insulin aspart (6–6–4 units) was subsequently added. Despite this multi-insulin injection therapy, glycemic control was poor (fasting plasma glucose level was about 120 mg/dL, postprandial plasma glucose was 180–230 mg/dL), and he developed skin reactions (redness, itching, and swelling) at the subcutaneous insulin injection site. Thus, insulin aspart (30 mix, 20–0–10 units) and insulin degludec (12–0–4 units) were initiated, but both triggered local skin reactions. He had no family history of diabetes and no past medical history including collagenous diseases. Even with insulin therapy, glycemic control was poor, and insulin allergy was suspected due to the appearance of the allergic skin reaction. Anti-insulin antibodies (>50 U/mL), anti-insulin-specific immunoglobulin E (27 UA/mL), and anti-insulin receptor antibodies (25.2%) were detected in his serum. Furthermore, hyperinsulinemia (fasting plasma glucose level of 160 mg/dL; C-peptide level of 7.22 ng/mL; immunoreactive insulin level of 1150 μU/mL) was detected. Therefore, he was diagnosed with type B insulin resistance syndrome and insulin allergy. Acanthosis nigricans was not found, only allergic skin reactions. In addition, he had no history of Helicobacter pylori infection. There was no renal impairment, and glutamic acid decarboxylase antibody was negative, but low complement levels (serum C4, 7 mg/dL; CH50 27 U/mL) were found. Scatchard analysis revealed that anti-insulin antibodies with high-affinity sites had a low affinity constant (K 1, 0.0109 × 108/M) and a high binding capacity (R 1, 47.4 × 10−8 M) [2]. Both the type B insulin resistance syndrome and the high titer of anti-insulin antibodies caused severe insulin resistance, leading to hyperinsulinemia without hypoglycemia. Considering his urinary tract infection, we believed that a lower glycemic control target was needed. The fact that insulin treatment was inefficient led us to switch the patient from insulin administration to liraglutide treatment. Liraglutide was initiated at 0.3 mg/day, and the dose was increased by 0.3 mg/day each week until a dose of 0.9 mg/day was being administered. He received no other drugs aside from liraglutide. After initiating liraglutide, his glycemic control gradually improved (fasting plasma glucose level: 90–110 mg/dL, postprandial plasma glucose level: <160 mg/dL) without incurring allergic skin reactions. He had no liraglutide-related side effects (i.e., liver or kidney dysfunction and pancreatitis). There was no hypoglycemic event during liraglutide treatment. One month after the initiation of liraglutide, the patient’s laboratory parameters had improved (fasting plasma glucose level: 101 mg/dL; C-peptide level: 4.1 ng/mL; immunoreactive insulin level: 732 μU/mL). At the same time, we rechecked the levels of anti-insulin antibodies and anti-insulin receptor antibodies. The anti-insulin antibody level was unchanged (>50 U/mL) but the anti-insulin receptor antibody level was reduced (17.9%). We have now been following this patient for about 2 years; his glycemic control has been good since he was started on liraglutide therapy only (fasting plasma glucose level: 100 mg/dL; HbA1c: 6.4%). Informed consent was obtained from the patient before they were included in the study.
Discussion
We have described a case of insulin allergy and type B insulin resistance that was successfully controlled with liraglutide. An allergic reaction to insulin products or insulin itself is an important clinical problem in patients using insulin, as it influences glycemic control [3]. In a case with anti-insulin antibodies or insulin allergy, modifying the insulin therapy is a common strategy, although there is no established method for doing so. Type B insulin resistance syndrome is an autoimmune disease caused by the production of antibodies to the insulin receptor, resulting in severe insulin resistance. It may be complicated by the presence of collagenous disease. The following treatment options have been suggested: insulin-like growth factor 1, rituximab, plasmapheresis, immunosuppressive agents (e.g., cyclosporine, cyclophosphamide, azathioprine, and steroids), and an intensive combination therapy incorporating some of these options [4]. For the treatment of diabetic patients with heart failure, empagliflozin (a sodium-glucose co-transporter 2 inhibitor, SGLT2I) is a promising therapeutic option according to EMPA-REG OUTCOME [5]. However, the present patient had severe heart failure treated with an implanted LVAD and a urinary tract infection. We did not choose SGLT2I because of his susceptibility to urinary infectious complications. In addition, altering the insulin therapy was not an effective approach in this patient due to a high titer of anti-insulin antibodies. Moreover, he had type B insulin resistance syndrome. Because his endogenous insulin secretion was preserved, we attempted to use a glucagon-like peptide-1 receptor (GLP-1R) agonist. Liraglutide therapy eventually resulted in good glycemic control with an insulin-free state via monotherapy. Although a few cases of the successful use of liraglutide therapy to control hyperglycemia in patients with insulin allergy and type B insulin resistance have been reported [6, 7], the biological mechanism associated with the use of liraglutide to treat type B insulin resistance syndrome, anti-insulin antibodies, and insulin allergy is unclear. GLP-1 acts at multiple sites with both insulinotropic pancreatic and extrapancreatic effects, including the intestinal– and cephalic–pancreas axes [8]. It may exert a glucagon-suppressive effect or increase the residence time of food in the stomach, thus contributing to the improvement in glycemic control. Hirai et al. reported a patient with anti-insulin antibodies to exogenously injected insulin and noted that liraglutide may decrease the amount of anti-insulin antibodies and suppress further production [7]. However, after 1 year of follow-up, liraglutide did not affect the serum concentration of anti-insulin antibodies; spontaneous remission of type B insulin resistance syndrome reversed the insulin receptor signaling and partially improved the glucose profile. Indeed, this patient did not show any typical clinical features of type B insulin resistance syndrome: acanthosis nigricans, collagenous disease. The titer of anti-insulin receptor antibody was also not so high. It has been reported that there are some patients without these typical clinical features of type B insulin resistance syndrome [4]. Further study is required to elucidate the mechanistic details associated with the potential benefit of liraglutide in the treatment of type B insulin resistance syndrome, anti-insulin antibodies, and insulin allergy.
Conclusion
These findings suggest that liraglutide is a useful therapeutic option for treating type B insulin resistance syndrome, anti-insulin antibodies, and insulin allergy.
References
Chakraborty PP, Biswas SN, Patra S. Faulty injection technique: a preventable but often overlooked factor in insulin allergy. Diabetes Ther. 2016;7:163–7.
Suzuki K, Hirayama S, Ito S. A case of a non-insulin dependent diabetic patient with regular spontaneous hypoglycemic attacks, which were due to insulin-binding antibodies induced by human insulin therapy. Tohoku J Exp Med. 1997;2:163–73.
Ghazavi MK, Johnston GA. Insulin allergy. Clin Dermatol. 2011;29:300–5.
Zhang S, Wang G, Wang J. Type B insulin resistance syndrome induced by systemic lupus erythematosus and successfully treated with intravenous immunoglobulin: case report and systematic review. Clin Rheumatol. 2013;32:181–8.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Kawanami D, Ito T, Watanabe Y, et al. Successful control of a case of severe insulin allergy with liraglutide. J Diabetes Investig. 2013;4:94–6.
Hirai H, Ogata E, Kikuchi N, et al. The effects of liraglutide on both hypereosinophilic insulin allergy and the characteristics of anti-insulin antibodies in type 2 diabetes mellitus: a case report. J Med Case Rep. 2016;10:202.
Drucker DJ. Deciphering metabolic messages from the gut drives therapeutic innovation: the 2014 Banting Lecture. Diabetes. 2015;64:317–26.
Acknowledgements
No funding or sponsorship was received for this study or the publication of this article. Article processing charges were funded by the authors. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Yuko Matsumoto, Hodaka Yamada, Shunsuke Funazaki, Daisuke Suzuki, Masafumi Kakei, and Kazuo Hara have nothing to disclose.
Compliance with Ethics Guidelines
Informed consent was obtained from the patient before they were included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/90FBF0604B855849.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Matsumoto, Y., Yamada, H., Funazaki, S. et al. Effect of Liraglutide on Type B Insulin Resistance Syndrome and Insulin Allergy in Type 2 Diabetes: A Case Report. Diabetes Ther 8, 1191–1194 (2017). https://doi.org/10.1007/s13300-017-0291-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-017-0291-2