Abstract
Objectives
Metformin is the first-line therapy for most patients with type 2 diabetes, but the majority require treatment intensification at some stage due to the progressive nature of the disease. The 1860-LIRA-DPP-4 trial showed that liraglutide exhibited greater improvements compared with sitagliptin in glycated hemoglobin and body mass index in patients with type 2 diabetes inadequately controlled on metformin monotherapy. As a follow-up to a previously published cost-effectiveness analysis of 1.2 mg liraglutide versus sitagliptin in Spain, the aim of this analysis was to compare long-term projections of the clinical and cost implications associated with 1.8 mg liraglutide and sitagliptin.
Methods
For the modeling analysis, 52-week treatment effect data (as opposed to 26-week data in the previous analysis) were taken from the 1860-LIRA-DPP-4 trial, for adults with type 2 diabetes receiving 1.8 mg liraglutide or 100 mg sitagliptin daily in addition to metformin. Long-term (patient lifetime) projections of clinical outcomes and direct costs (2012 EUR) were made using a published and validated model of type 2 diabetes, with modeling assumptions as per the 1.2 mg liraglutide analysis.
Results
Liraglutide was associated with increased life expectancy (14.24 versus 13.87 years) and quality-adjusted life expectancy [9.24 versus 8.84 quality-adjusted life years (QALYs)] over sitagliptin. Improved clinical outcomes were attributable to the improvement in glycemic control, leading to a reduced incidence of diabetes-related complications, including renal disease, cardiovascular disease, ophthalmic and diabetic foot complications. Liraglutide was associated with increased direct costs (EUR 56,628 versus EUR 52,450), driven by increased pharmacy costs. Based on these estimates, liraglutide was associated with an incremental cost-effectiveness ratio of EUR 10,436 per QALY gained versus sitagliptin.
Conclusions
A previous analysis has suggested that 1.2 mg liraglutide is cost-effective from a healthcare payer perspective in Spain, and the present analysis suggests that the 1.8 mg dose is also likely to be cost-effective.
Similar content being viewed by others
Introduction
Hyperglycemia in type 2 diabetes results from insulin resistance in the peripheral tissues, insulin deficiency due to insufficient pancreatic output, and excessive hepatic glucose output and is associated with serious microvascular and macrovascular complications [1]. Early initiation of treatment can delay disease progression, and achieving evidence-based clinical goals by implementing effective management strategies substantially reduces the risk of morbidity and mortality and ultimately improves patient outcomes [2–4].
Metformin remains the first-line therapy for most patients with type 2 diabetes mellitus. However, due to the progressive nature of the disease, with beta cell function declining over time, the majority of patients require additional therapy to maintain glycemic control. Long-standing second-line interventions include sulfonylureas and thiazolidinediones, and whilst these therapies are effective in achieving glycemic control, they are associated with weight gain, increased risk of hypoglycemic events, and/or cardiovascular concerns [5].
In an attempt to improve treatment of type 2 diabetes, a number of new therapies targeting the incretin axis have been developed [6]. These therapies exert effects in a number of different target tissues to address the complex pathophysiology of the disease. Incretin-based therapy has been shown to stimulate glucose-dependent insulin secretion, reduce glucagon secretion, improve beta cell function, slow gastric emptying, increase satiety, reduce appetite, and general benefits beyond the pancreas. Two classes of incretin therapy have been developed: degradation-resistant glucagon-like peptide-1 (GLP-1) receptor agonists (such as liraglutide and exenatide) which mimic the actions of endogenous GLP-1, and dipeptidyl peptidase-4 (DPP-4) inhibitors (such as sitagliptin and saxagliptin) which inhibit the inactivation of incretin hormones by the enzyme DPP-4. Both GLP-1 receptor agonists and DPP-4 inhibitors are associated with reductions in glycated hemoglobin (HbA1c), but reductions may be more substantial with GLP-1 receptor agonists (0.5–1.6% reduction versus 0.5–1% reduction) [7]. Furthermore, GLP-1 receptor agonists are associated with weight loss [8–13] whereas DPP-4 inhibitors have been associated only with the prevention of weight gain [14–17].
Whilst the interventions that target the incretin axis provide a more rounded approach to treatment of type 2 diabetes than traditional second-line interventions, they also come at an increased cost in the short term, although this can be partially offset by avoidance of treatment of diabetes-related complications over a patient’s lifetime as a result of better control. In a publically funded healthcare system, such as Spain, the aim is to maximize health outcomes across the population with the finite resources available. Healthcare payers must make decisions on how best to allocate these scarce resources, and economic evaluation of new and existing healthcare interventions is playing an increasingly important role in informing these decisions [18, 19].
A previous study investigating the cost-effectiveness analysis of liraglutide 1.2 mg versus sitagliptin in the Spanish setting demonstrated that liraglutide was associated with improved life expectancy and quality-adjusted life expectancy but was associated with increased costs [20]. The analysis concluded that liraglutide 1.2 mg was cost-effective compared to sitagliptin over patient lifetimes. However, liraglutide is available in two doses, either 1.2 or 1.8 mg per day. The liraglutide trial program has shown that the increased dose is associated with improved clinical outcomes over the lower dose, but this increased efficacy comes at an increased pharmacy cost.
As a follow-up analysis to the previously published cost-effectiveness analysis of liraglutide 1.2 mg versus sitagliptin, the present analysis aimed to assess the cost-effectiveness of liraglutide 1.8 mg versus sitagliptin in patients failing to achieve adequate glycemic control on metformin monotherapy in the Spanish setting. A secondary analysis was also conducted, in which the cost-effectiveness of delaying GLP-1 receptor agonist therapy, with a year of DPP-4 inhibitor therapy first, was investigated.
Methods
Modeling Analysis
The present analysis is a further extension of the cost-effectiveness analysis of liraglutide 1.2 mg versus sitagliptin in Spain, with two major differences. The first is the evaluation of the 1.8 mg dose of liraglutide (rather than the 1.2 mg dose), and the second is the use of trial data from the 52-week endpoint (rather than the 26-week endpoint). The methods used in this analysis are consistent with the previously published cost-effectiveness analysis of liraglutide 1.2 mg versus sitagliptin, and therefore are only outlined briefly here [20]. The analysis was performed using the CORE Diabetes Model (IMS Health, Basel, Switzerland), a non-product specific diabetes policy analysis tool. The model functionality has been previously described, and the long-term outcomes projected by the model have been validated against real-life data at first publication in 2004 and following a series of updates in 2013 [21–23].
The model was used to project life expectancy, quality-adjusted life expectancy, cumulative incidence of diabetes-related complications, time to onset of diabetes-related complications and direct medical costs for patients receiving liraglutide 1.8 mg daily or sitagliptin 100 mg daily in the Spanish setting.
In line with published health economic guidance for Spain, future costs and clinical benefits were discounted symmetrically by 3% per annum [24]. The time horizon was set to patient lifetimes in the base case to capture all relevant long-term complications, associated costs, and to assess their impact on life expectancy and quality-adjusted life expectancy.
Simulated Cohort
The baseline cohort characteristics were taken from the 1860-LIRA-DPP-4 (NCT00700817) trial [25, 26]. This study enrolled patients with type 2 diabetes mellitus who had inadequate glycemic control (HbA1c 7.5–10.0%) on metformin (≥1500 mg daily for ≥3 months) in Europe (including 9 centers in Spain) and North America. Patients were randomly allocated to 1.2 mg subcutaneous liraglutide once daily (n = 225), 1.8 mg subcutaneous liraglutide once daily (n = 221) or 100 mg oral sitagliptin once daily (n = 219). Mean age of the cohort was 55.3 years [standard deviation (SD) 9.2 years], with mean duration of diabetes of 6.0 years (SD 4.5 years), mean HbA1c of 8.4% (SD 0.80%), and mean body mass index (BMI) of 32.8 kg/m2 (SD 5.2 kg/m2).
Treatment Effects and Risk Factor Progression
In the previously published cost-effectiveness analysis of liraglutide 1.2 mg daily, treatment effect data were taken from the 26-week primary endpoint [20, 25]. In the present analysis, the treatment effects applied in the first year of the modeling analysis (Table 1) were taken from the 52-week time point as this longer follow-up data may represent a more robust source for long-term modeling, although it was not the primary endpoint of the trial (a sensitivity analysis using the primary endpoint data was conducted) [26]. Assumptions regarding progression of risk factors in the following years of the simulation were aligned with the cost-effectiveness analysis of liraglutide 1.2 mg versus sitagliptin [20]. HbA1c was assumed to remain unchanged for the duration of the analysis, as this allows the modeling analysis to capture the legacy effect, where benefits of reductions in HbA1c early in a patient’s life persist even after the HbA1c reduction has been abolished. Systolic blood pressure increased based on the UKPDS progression equation, whilst serum lipids followed the Framingham progression equations [21]. As in the previous analysis, patients were assumed to receive incretin therapy for 5 years, before intensifying treatment to basal insulin (with the previously received incretin therapy withdrawn). On treatment intensification, BMI was assumed to return to baseline and hypoglycemia event rates were assumed to be the same in both arms, but no other treatment effects were applied.
Costs and Utilities
Costs were accounted from the perspective of a healthcare payer in Spain in 2012 EUR. All costs were as per the previous cost-effectiveness evaluation of liraglutide 1.2 mg versus sitagliptin in Spain [20]. Utilities were in line with the previous cost-effectiveness analysis in Spain, and a cost-effectiveness analysis of liraglutide versus sitagliptin carried out in the UK setting [20, 27]. Full details of the costs and utilities are provided in the online supplementary information.
Sensitivity Analyses
A number of sensitivity analyses were conducted to evaluate the robustness of the modeled outcomes to changes in input parameters, and to identify key drivers of results. These were consistent with the previously published analysis of liraglutide 1.2 mg versus sitagliptin [20].
Scenarios with time horizons of 5, 10, 20 and 30 years, compared to 50 years in the base case, were run to evaluate the influence of the time horizon of the analysis on the projected outcomes. The effect of application of discount rates of 0% and 5% per annum on future cost and clinical outcomes was also investigated. The costs of diabetes-related complications were increased/decreased by 10% from those used in the base case analysis to examine the influence of over- or underestimating these costs. The importance of changes in physiological parameters were investigated in five sensitivity analyses, in which benefits in HbA1c, systolic blood pressure, blood lipids, BMI and hypoglycemia were individually abolished. Two scenarios with alternative assumptions around long-term progression of HbA1c were investigated. In the first, the HbA1c difference between the treatment arms was abolished when patients switched to insulin therapy. In the second, the United Kingdom Prospective Diabetes Study progression for HbA1c (as described by Palmer et al. [21]) was followed whilst patients received incretin therapy, and then HbA1c remained constant when patients switched to insulin. The effect of the timing of treatment switching was examined by varying the treatment switch to 7 and 3 years in both arms. A scenario was also investigated in which the 26-week primary endpoint data were used to inform the treatment effects used in the first year of the analysis.
Secondary Analysis
As a further extension to the 1860-LIRA-DPP-4 trial, patients in the sitagliptin arm completing 52 weeks of treatment were randomly allocated to receive either liraglutide 1.2 mg or liraglutide 1.8 mg for a further 26 weeks [28]. Switching patients from sitagliptin to liraglutide 1.8 mg was associated with a further reduction in HbA1c and BMI, although these improvements were not as extensive as when liraglutide was initiated in the first year of the trial (Table 2). However, hypoglycemic event rates were lower than when liraglutide was initiated earlier. This may have been as a result of patients with a high susceptibility to hypoglycemia dropping out of the study (only 62% of the patients originally randomly allocated to sitagliptin entered the extension study at 52 weeks). A secondary cost-effectiveness analysis was conducted based on this extension to the 1860-LIRA-DPP-4 trial. In this comparison, 1 year of sitagliptin therapy followed by 4 years of liraglutide therapy was compared with 5 years of sitagliptin therapy (as in the base case analysis), with patients in both arms switched to insulin at the end of year five. All other assumptions were as per the base case analysis, and equivalent sensitivity analyses were performed.
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
Base Case Analysis
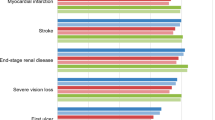
Treatment with liraglutide 1.8 mg was associated with a mean increase in discounted life expectancy of 0.37 years over treatment with sitagliptin (Table 3). Liraglutide was also associated with mean quality-adjusted life expectancy of 9.24 quality-adjusted life years (QALYs), compared to 8.84 QALYs with sitagliptin, a difference of 0.40 QALYs. The clinical benefits in the liraglutide arm were primarily driven by improved glycemic control with liraglutide over sitagliptin, resulting in a reduction in the projected incidence of all diabetes-related complications over patient lifetimes. Of particular note were the reductions in cumulative incidence of diabetic retinopathy, falling from 17.3% to 13.9% (relative risk reduction of 20.1%), and neuropathy, falling from 48.5% to 40.4% (relative risk reduction of 16.7%). The mean time to onset of diabetes-related complications was increased with liraglutide (Fig. 1). The mean time free from any complication was increased from 6.2 years with sitagliptin to 7.4 years with liraglutide, an increase of approximately 20%.
Liraglutide was associated with increased direct costs of EUR 4177 per patient versus sitagliptin (EUR 56,628 versus EUR 52,450) (Table 3; Fig. 2). The increased acquisition cost of liraglutide over sitagliptin (accrued during the first 5 years of the analysis) drove this difference. However, the reduced costs of treating diabetes-related complications partially offset this increased cost. The most notable savings were made as a result of avoided diabetic foot complications, where mean savings of EUR 2173 per patient were made (EUR 17,901 versus EUR 20,074).
Based on these estimates, liraglutide 1.8 mg was associated with an incremental cost-effectiveness ratio (ICER) of EUR 10,436 per QALY gained versus sitagliptin. Analysis of the incremental outcomes of the 1000 cohorts of 1000 patients run through the model found that in 97.7% of iterations, liraglutide was associated with increased quality-adjusted life expectancy and increased direct costs. In 95% of iterations, liraglutide was associated with an ICER of less than EUR 30,000 per QALY gained versus sitagliptin.
Sensitivity Analyses
Sensitivity analyses found that the cost-effectiveness outcomes were most sensitive to changes in the time horizon of the modeling analysis, with liraglutide less cost-effective over shorter time horizons (Table 4). As the time horizon was reduced, the ICER increased, with a 5-year time horizon producing an ICER of EUR 116,534 per QALY gained. This was primarily due to the improvements in physiological parameters associated with liraglutide resulting in reduced risk of long-term complications, with the benefits of this not fully realized over shorter time horizons. Changing the discount rate to 5% led to an increased ICER of EUR 14,955 per QALY gained, and applying a discount rate of 0% led to the ICER falling to EUR 5251 per QALY gained. This pattern also reflects the long-term benefits associated with liraglutide over sitagliptin. Abolishing the treatment effects in turn identified that the key driver of improved health outcomes with liraglutide was the improvement in HbA1c. When this difference was abolished (i.e., the change was assumed to be the same as in the sitagliptin arm) the incremental quality-adjusted life expectancy benefit fell from 0.40 QALYs to 0.10 QALYs. Using alternative assumptions around the long-term progression of HbA1c also resulted in changes in the cost-effectiveness outcomes, but in both cases the ICER remained below EUR 30,000 per QALY gained. Cost-effectiveness outcomes remained stable when the costs of complications were varied, when treatment switching was assumed to take place earlier or later, and when the 26-week trial data were used.
Secondary Analysis
In the secondary analysis, receiving sitagliptin for 1 year followed by liraglutide 1.8 mg therapy for 4 years was associated with increased life expectancy (by 0.23 years) and quality-adjusted life expectancy (by 0.26 QALYs) compared with sitagliptin therapy for 5 years (with patients in both arms of the modeling analysis receiving glargine after year five for the remainder of their lifetime). As in the base case analysis, improvements were driven by a reduced incidence and increased time to onset of diabetes-related complications. Mean costs over patient lifetimes were found to be higher in the delayed liraglutide arm (EUR 56,008 versus EUR 52,450), driven by the acquisition cost of liraglutide in years 2–4 of the analysis. Based on these cost and clinical outcomes, delayed liraglutide therapy was associated with an ICER of EUR 13,628 per QALY gained versus sitagliptin.
Sensitivity analyses showed the same patterns as in the primary analysis. Analyses identified that the key driver of cost-effectiveness was the HbA1c improvement seen when patients switched from sitagliptin to liraglutide at the end of the first year of the analysis. Switching patients to liraglutide was found to be cost-effective as a result of a reduced incidence of diabetes-related complications over the long term, as shown by the analyses in which the time horizon and discount rates were changed.
Discussion
A previous cost-effectiveness analysis in the Spanish setting has suggested that liraglutide 1.2 mg is a cost-effective treatment option, versus sitagliptin, for patients with type 2 diabetes not achieving glycemic control on metformin monotherapy [20]. The present analysis has aimed to expand on the previously published work, by investigating the cost-effectiveness of liraglutide 1.8 mg in Spain based on the 52-week trial data. It was found that liraglutide 1.8 mg was associated with improved life expectancy and quality-adjusted life expectancy compared with sitagliptin in the Spanish setting. Clinical improvements resulted from a reduced incidence and increased time to onset of diabetes-related complications, driven predominantly by a greater reduction in HbA1c, but changes in systolic blood pressure, serum lipid levels and BMI were also found to be important. Liraglutide was associated with an increase in direct medical costs over patient lifetimes. This resulted from the increased acquisition cost of liraglutide over the short term, but was partially offset by avoidance of treatment of diabetes-related complications over the long term. Based on the projected outcomes, liraglutide 1.8 mg was associated with an ICER of EUR 10,436 versus sitagliptin in the Spanish setting for patients with type 2 diabetes not achieving glycemic targets on metformin monotherapy. This ICER falls below the commonly quoted willingness to pay threshold of EUR 30,000 per QALY gained [29–31], and therefore liraglutide 1.8 mg is likely to be considered a cost-effective treatment option in patients failing to meet glycemic targets on metformin monotherapy.
In the 1860-LIRA-DPP-4 study, the 1.8 mg dose was associated with greater reductions in HbA1c, blood pressure, and BMI at both 26- and 52-week time points than liraglutide 1.2 mg [25, 26]. Whilst care should be taken when results of the previous cost-effectiveness analysis and the present analysis are compared due to the different time points from which data were taken, these studies suggest that the greater improvements in surrogate outcomes with the 1.8 mg dose are likely to result in greater improvements in long-term clinical outcomes and both the 1.2 and 1.8 mg doses of liraglutide are likely to be cost-effective versus sitagliptin in the Spanish setting.
The secondary analysis represents a scenario in which liraglutide therapy is delayed by 1 year, with a year of sitagliptin treatment received previously. This analysis suggested that switching patients from sitagliptin to liraglutide was also a cost-effective treatment strategy for patients failing to meet glycemic targets on metformin monotherapy, compared to remaining on sitagliptin. As in the base case analysis, cost-effectiveness was driven by improvements in HbA1c and BMI when switching from DPP-4 to GLP-1 receptor agonist treatment. However, improvements in risk factors were not as large as when liraglutide 1.8 mg was initiated in the first year of the analysis. Therefore, the gains in life expectancy and quality-adjusted life expectancy were smaller in the secondary analysis of delayed liraglutide therapy than in the base case analysis in which liraglutide was initiated earlier. Furthermore, the ICER in the secondary was higher than in the base case analysis. This suggests that the best strategy for optimizing healthcare outcomes with a limited budget may be to initiate liraglutide earlier rather than later, as this resulted in improved health outcomes at a lower ICER.
As with all scientific studies, the limitations must be considered to put the results into context. A potential limitation of the present study (and also the previous analysis) is that all parts of the 1860-LIRA-DPP-4 study were open label. This included the initial treatment period used in the base case analysis and the extension used to inform the secondary analysis. Open label studies are less robust than double-blind trials, as patients may have expectations of the effects of the study medications and this may influence adherence to lifestyle recommendations. However, the impact of any potential effect is difficult to assess. The impact of this on the present study has been minimized by only using trial endpoints measured through objective tests (such as HbA1c and systolic blood pressure).
A further limitation of the present analysis may be the projection of long-term clinical events based on short-term trials measuring changes in surrogate outcomes. However, this limitation is applicable to the majority of health economic evaluations. Despite this, long-term modeling represents one of the best available options for making estimates of long-term clinical and economic outcomes in the absence of long-term clinical data, and this approach is recommended in guidelines [32]. The present study aims to minimize this limitation, through use of a recently validated model to conduct the analysis, and basing changes in physiological parameters on data collected in a randomized controlled trial [22, 23].
A key study in informing both the effectiveness and cost-effectiveness of diabetes medications in patients failing metformin therapy will be the Glycemia Reduction Approaches in Diabetes [(GRADE) NCT01794143] study, due to report in 2020 [33]. Patients will be randomly assigned to one of four diabetes medications (liraglutide, sitagliptin, glimepiride, and insulin glargine) and followed for 7 years. When this study is complete it will provide a large amount of clinical effectiveness data, and will form a key data source for future economic evaluation when the study reports in 2020.
A potential weakness of the secondary analysis is that treatment effects are taken from different time points, with the sitagliptin treatment arm informed by the 52-week data and the delayed liraglutide treatment arm informed by 52- and 78-week data. It was not possible to use equivalent time point data as all patients that received sitagliptin as part of the 1860-LIRA-DPP-4 trial were switched to liraglutide at 52 weeks. This is unlikely to have had a significant impact on the analysis, as in the 1860-LIRA-DPP-4 study the majority of change in measured outcomes when initiating sitagliptin (or liraglutide) occurred over the first 12 weeks, after which measured values remained stable up to 52 weeks [25, 26].
The impact of adherence to the two diabetes medications evaluated should also be considered. It has been suggested that injectable diabetes medications may be associated with lower adherence than oral medications as a result of the method of delivery [34]. However, it has also been proposed that the favorable clinical profile of incretin therapies, in terms of low hypoglycemic event rates and weight loss, may result in improved adherence compared to conventional diabetes treatments [35]. Whilst adherence to alternative GLP-1 receptor agonists has been assessed, currently there is no evidence to suggest that injectable GLP-1 receptor agonists are associated with lower adherence rates than oral DPP-4 inhibitors [36, 37]. Moreover, the impact of adherence on cost-effectiveness is difficult to assess, as both clinical outcomes and costs will be affected by adherence rates. The conclusions of the present analysis may only be valid for patients who are adherent to the diabetes medications received.
In conclusion, clinical trials have shown that both liraglutide and sitagliptin are effective treatments for patients not achieving glycemic targets on metformin monotherapy. In the recently published 1860-LIRA-DPP-4 trial, liraglutide 1.8 mg was associated with greater improvements in HbA1c and BMI than sitagliptin [25, 26]. Projecting these outcomes over patient lifetimes using a published and validated cost-effectiveness model suggested that liraglutide 1.8 mg is likely to be cost-effective versus sitagliptin in the Spanish setting for patients failing to meet glycemic targets on metformin monotherapy. The secondary analysis suggests that initiating liraglutide earlier, rather than after a year of sitagliptin therapy first, is the optimum method for maximizing health outcomes and cost-effectiveness. The results of the previous and present analyses suggest that both the 1.2 and 1.8 mg doses of liraglutide are likely to be cost-effective for patients with type 2 diabetes not achieving glycemic targets on metformin monotherapy from a healthcare payer perspective in Spain.
References
Garber AJ. Incretin-based therapies in the management of type 2 diabetes: rationale and reality in a managed care setting. Am J Manag Care. 2010;16(7 Suppl):S187–94.
Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93.
Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–91.
Griffin SJ, Borch-Johnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–67.
Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–43.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705.
Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–72.
Nauck MA, Hompesch M, Filipicazak R, Le TD, Zdravkovic M, Glumprecht J. Five weeks of treatment with the GLP-1 analogue liraglutide improves glycaemic control and lowers body weight in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2006;114:417–23.
Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin in type 2 diabetes mellitus (LEAD-2 Met). Diabetes Care. 2009;32:84–90.
Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–81.
DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–100.
Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–35.
Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–91.
Åhren B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2874–80.
Åhren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078–84.
Scott R, Herman G, Zhao P, Chen X, Wu M, Stein P. Twelve-week efficacy and tolerability of MK-0431, a dipeptidyl peptidase IV (DPPIV) inhibitor, in the treatment of type 2 diabetes (T2D). Diabetes Care. 2005;54(s1):10–1.
Hanefeld M, Herman G, Mickel C, et al. Effect of MK-0431, a dipeptidyl peptidase IV (DPP-IV) inhibitor, on glycemic control after 12 weeks in patients with type 2 diabetes. Diabetologia. 2005;48(s1):287–8.
Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care. 2013;29(2):117–22.
O’Reilly D, Gaebel K, Xie F, Tarride JE, Goeree R. Health economic evaluations help inform payers of the best use of scarce health care resources. Int J Circumpolar Health. 2011;70(4):417–27.
Mezquita Raya P, Pérez A, Ramírez de Arellano A, Briones T, Hunt B, Valentine WJ. Incretin therapy for type 2 diabetes in Spain: a cost-effectiveness analysis of liraglutide versus sitagliptin. Diabetes Ther. 2013;4(2):417–30.
Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):5–26.
Palmer AJ, Roze S, Valentine W, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):27–40.
McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE Diabetes Model. Value Health. 2014;17:14–24.
López-Bastida J, Oliva J, Antoñanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11:513–20.
Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, Thomsen AB, Søndergaard RE, Davies M, 1860-LIRA-DPP-4 Study Group. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375(9724):1447–56.
Pratley R, Nauck M, Bailey T, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65(4):397–407.
Davies MJ, Chubb BD, Valentine WJ. Cost-utility analysis of liraglutide compared with sulphonylurea or sitagliptin, all as add-on to metformin monotherapy in Type 2 diabetes mellitus. Diabet Med. 2012;29:313–20.
Pratley RE, Nauck MA, Bailey T, et al. Efficacy and safety of switching from the DPP-4 inhibitor sitagliptin to the human GLP-1 analog liraglutide after 52 weeks in metformin-treated patients with type 2 diabetes: a randomized, open-label trial. Diabetes Care. 2012;35(10):1986–93.
Davies A, Sculpher M, Barrett A, Huete T, Sacristán JA, Dilla T. Prasugrel compared to clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a Spanish model-based cost effectiveness analysis. Farm Hosp. 2013;37(4):307–16.
Lee D, Wilson K, Akehurst R, et al. Cost-effectiveness of eplerenone in patients with systolic heart failure and mild symptoms. Heart. 2014;100(21):1681–7.
Vallejo-Torres L, Castilla I, González N, Hunter R, Serrano-Pérez P, Perestelo-Pérez L. Cost-effectiveness of electroconvulsive therapy compared to repetitive transcranial magnetic stimulation for treatment-resistant severe depression: a decision model. Psychol Med. 2014;30:1–12.
American Diabetes Association Consensus Panel. Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27(9):2262–5.
Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254–61.
García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–94.
Zarowitz BJ, Conner C. The intersection of safety and adherence: new incretin-based therapies in patients with type 2 diabetes mellitus. Pharmacotherapy. 2009;29:55S–67S.
Malmenäs M, Bouchard JR, Langer J. Retrospective real-world adherence in patients with type 2 diabetes initiating once-daily liraglutide 1.8 mg or twice-daily exenatide 10 μg. Clin Ther. 2013;35:795–807.
Pelletier EM, Pawaskar M, Smith PJ, Best JH, Chapman RH. Economic outcomes of exenatide vs liraglutide in type 2 diabetes patients in the United States: results from a retrospective claims database analysis. J Med Econ. 2012;15:1039–50.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Novo Nordisk Health Care AG, Zürich, Switzerland. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Antonio Pérez is a scientific collaborator with Novo Nordisk and has participated in advisory boards and clinical trials. Pedro Mezquita Raya is a scientific collaborator with Novo Nordisk and has participated in advisory boards and clinical trials. Antonio Ramírez de Arellano is an employee of Novo Nordisk. Teresa Briones is an employee of Novo Nordisk. Barnaby Hunt is an employee of Ossian Health Economics and Communications, which received a consulting fee from Novo Nordisk to support the study. William Valentine is an employee of Ossian Health Economics and Communications, which received a consulting fee from Novo Nordisk to support the study.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pérez, A., Mezquita Raya, P., Ramírez de Arellano, A. et al. Cost-Effectiveness Analysis of Incretin Therapy for Type 2 Diabetes in Spain: 1.8 mg Liraglutide Versus Sitagliptin. Diabetes Ther 6, 61–74 (2015). https://doi.org/10.1007/s13300-015-0103-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-015-0103-5