Abstract
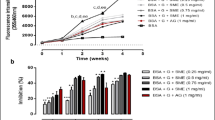
Siraitia grosvenorii (Swingle) is one kind of medical and edible plants with various health-promoting properties. Recently, its hypoglycemic and antidiabetic activities have been reported, but the underlying mechanism remains to be explored. The current study was aimed to investigate the antioxidant and antiglycation activities of mogroside extract (MGE) from Siraitia grosvenorii (Swingle). The results showed that compared to glycated BSA, MGE at middle (125 μg/mL) and high dose (500 μg/mL) significantly inhibited BSA glycation evidenced by decreased fluorescent AGEs formation, protein carbonyls and Nε-(carboxymethyl) lysine (CML) level at 500 μg/mL by 58.5, 26.7 and 71.2%, respectively. Additionally, the antiglycative activity of MGE (500 μg/mL) was comparable to aminoguanidine (AG) at the equal concentration. However, the inhibitory effect of MGE on glycation-induced increase of fructosamine level and decrease of thiol level was not remarkable. MGE was a potent peroxide radicals scavenger (851.8 μmol TE/g), moderate DPPH and ABTS radicals scavenger with IC50 1118.1 and 1473.2 μg/mL, respectively, corresponding to positive controls ascorbic acid of IC50 9.6 μg/mL, and trolox of IC50 47.9 μg/mL, respectively, and mild reducing power. These findings suggest that MGE may serve as a new promising antiglycative agent against diabetic complications by inhibiting protein glycation and glycoxidation.





Similar content being viewed by others
Abbreviations
- 1-DMF:
-
1-Deoxy-1-morpholino-fructose
- 3-DG:
-
3-Deoxyglucosone
- AAPH:
-
2,2′-Azobis (2-amidinopropane) dihydrochloride
- ABTS:
-
2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)
- AG:
-
Aminoguanidine
- AGEs:
-
Advanced glycation end products
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under curve
- BSA:
-
Bovine serum albumin
- CML:
-
Nε-(carboxymethyl)lysine
- DNPH:
-
2,4-Dinitrophenylhydrazine
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- DPTC:
-
4,5-Dimethyl-3-phenacylthiazolium chloride
- DTNB:
-
5,5′-Dithiobis-(2-nitrobenzoic acid)
- ELISA:
-
Enzyme-linked immunosorbent assay
- FI:
-
Fluorescence intensity
- GO:
-
Glyoxal
- GRAS:
-
Generally recognized as safe
- MGE:
-
Mogroside extract
- MGO:
-
Methylglyoxal
- NBT:
-
4-Nitro blue tetrazolium
- ORAC:
-
Oxygen radical absorbance capacity
- PBS:
-
Phosphate buffered saline
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- TCA:
-
Trichloroacetic acid
- Trolox:
-
6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid
References
Ahmed N (2005) Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res Clin Pract 67:3–21
Aljohi A, Matou-Nasri S, Ahmed N (2016) Antiglycation and antioxidant properties of Momordica charantia. PLoS ONE 11:e0159985
Allouche Y, Beltrán G, Gaforio JJ, Uceda M, Mesa MD (2010) Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem Toxicol 48:2885–2890
Bi L, Tian X, Dou F, Hong L, Tang H, Wang S (2012) New antioxidant and antiglycation active triterpenoid saponins from the root bark of Aralia taibaiensis. Fitoterapia 83:234–240
Chae S, Kang KA, Youn U, Park JS, Hyun JW (2010) A comparative study of the potential antioxidant activities of ginsenosides. J Food Biochem 34:31–43
Chen WJ, Wang J, Qi XY, Xie BJ (2007) The antioxidant activities of natural sweeteners, mogrosides, from fruits of Siraitia grosvenori. Int J Food Sci Nutr 58:548–556
Chompoo J, Upadhyay A, Kishimoto W, Makise T, Tawata S (2011) Advanced glycation end products inhibitors from Alpinia zerumbet rhizomes. Food Chem 129:709–715
Freedman BI et al (1999) Design and baseline characteristics for the aminoguanidine clinical trial in overt type 2 diabetic nephropathy (ACTION II). Control Clin Trials 20:493–510
Glomb MA, Monnier VM (1995) Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard Reaction. J Biol Chem 270:10017–10026
Hunt JV, Bottoms MA, Mitchinson MJ (1993) Oxidative alterations in the experimental glycation model of diabetes mellitus are due to protein-glucose adduct oxidation. Some fundamental differences in proposed mechanisms of glucose oxidation and oxidant production. Biochem J 291:529–535
Jariyapamornkoon N, Yibchok-anun S, Adisakwattana S (2013) Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement Altern Med 13:171–179
Jin JS, Lee JH (2012) Phytochemical and pharmacological aspects of Siraitia grosvenorii, luo han kuo. Orient Pharm Exp Med 12:233–239
Levine RL, Stadtman ER (2001) Oxidative modification of proteins during aging. Exp Gerontol 36:1495–1502
Liu F, Teodorowicz M, Wichers HJ, van Boekel MAJS, Hettinga KA (2016) Generation of soluble advanced glycation end products receptor (sRAGE)-binding ligands during extensive heat treatment of whey protein/lactose mixtures is dependent on glycation and aggregation. J Agric Food Chem 64:6477–6486
Matiacevich SB, Santagapita PR, Buera MP (2005) Fluorescence from the Maillard Reaction and its potential applications in food science. Crit Rev Food Sci Nutr 45:483–495
Meeprom A, Sompong W, Chan C, Adisakwattana S (2013) Isoferulic acid, a new anti-glycation agent, inhibits fructose- and glucose-mediated protein glycation in vitro. Molecules 18:6439–6454
Nagai R, Ikeda K, Higashi T, Sano H, Jinnouchi Y, Araki T, Horiuchi S (1997) Hydroxyl radical mediates Nϵ-(carboxymethyl)lysine formation from Amadori product. Biochem Biophys Res Commun 234:167–172
Obayashi H et al (1996) Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo. Biochem Biophys Res Commun 226:37–41
Odjakova M, Popova E, Sharif MA, Mironova R (2012) Plant-derived agents with anti-glycation activity. Glycosylation. Web. https://doi.org/10.5772/48186
Qi X, Chen W, Liu L, Yao P, Xie B (2006) Effect of a Siraitia grosvenori extract containing mogrosides on the cellular immune system of type 1 diabetes mellitus mice. Mol Nutr Food Res 50:732–738
Qi X, Chen W, Zhang L, Xie B (2008) Mogrosides extract from Siraitia grosvenori scavenges free radicals in vitro and lowers oxidative stress, serum glucose, and lipid levels in alloxan-induced diabetic mice. Nutr Res 28:278–284
Quiney C, Finnegan S, Groeger G, Cotter TG (2011) Protein oxidation. In: Vidal CJ (ed) Post-translational modifications in health and disease. Springer, New York, pp 57–78
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Singh R, Barden A, Mori T, Beilin L (2001) Advanced glycation end-products: a review. Diabetologia 44:129–146
Takeda A et al (1996) Immunohistochemical study of advanced glycation end products in aging and Alzheimer’s disease brain. Neurosci Lett 221:17–20
Thornalley PJ (2003) Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys 419:31–40
Ulrich P, Cerami A (2001) Protein glycation, diabetes, and aging. Recent Prog Horm Res 56:1–21
Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW (1995a) Identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry 34:3702–3709
Wells-Knecht MC, Thorpe SR, Baynes JW (1995b) Pathways of formation of glycoxidation products during glycation of collagen. Biochemistry 34:15134–15141
Wolff SP, Dean RT (1987) Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J 245:243–250
Wu CH, Huang SM, Lin JA, Yen GC (2011) Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct 2:224–234
Xi M, Hai C, Tang H, Chen M, Fang K, Liang X (2008) Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother Res 22:228–237
Xi M et al (2010) Antioxidant and antiglycation properties of triterpenoid saponins from Aralia taibaiensis traditionally used for treating diabetes mellitus. Redox Rep 15:20–28
Zheng L, Su G, Ren J, Gu L, You L, Zhao M (2012) Isolation and characterization of an oxygen radical absorbance activity peptide from defatted peanut meal hydrolysate and its antioxidant properties. J Agric Food Chem 60:5431–5437
Zheng H, Wu J, Jin Z, Yan LJ (2016) Protein modifications as manifestations of hyperglycemic glucotoxicity in diabetes and its complications. Biochem Insights 9:1–9
Acknowledgements
The authors thank Guilin Layn Natural Ingredients Corp. for generously providing MGE. This work was funded by National Natural Science Foundation of China (No. 31171780), Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University (No. 20171033) and general program from Department of Education of Zhejiang Province, China (Y201738544).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, H., Wang, C., Qi, X. et al. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J Food Sci Technol 55, 1880–1888 (2018). https://doi.org/10.1007/s13197-018-3105-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3105-2