Abstract
The presence of the South African Obscure Scale, Melanaspis corticosa (Brain) (Hemiptera, Diaspididae), was detected infesting olive trees, in Portugal. The identity of the scale insect was confirmed based on both morphological and molecular studies. Until now, this species was only known in a few African countries, including Guinea, Mozambique, South Africa and Zimbabwe. This is the first record of this species in Europe and in the Palearctic region. The scale was observed in 15 different locations, in the Algarve, since its first detection at the end of 2016. Samples were collected between 21 December 2016 and 10 March 2022, covering all seasonal periods. Most of the sampling sites resulted from private requests from farmers and proprietaries received by the Plant Protection Division of the Regional Directorate of Agriculture. Although it is considered a polyphagous species, it was not observed in other plant species, besides olive trees. The actual dispersion in the region suggests that M. corticosa became established and has been expanded its distribution since its arrival. This scale insect is a potential injurious pest of olive trees and needs to be studied to clarify its pest status and develop effective pest management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scale insects (Hemiptera, Coccomorpha) are small and cryptic, soft-body, piercing-sucking insects, that feed on plant sap. They include more than 8400 described species, distributed among 56 families. Armored scale insects (Diaspididae) are the largest family, with about 2700 species, including many economically important pests of agricultural crops and ornamentals (García Morales et al., 2016). Miller and Davidson (1990) published a list of 199 armored scale insect pests, of which about 20% were considered serious pests in different regions of the world. Economically important scales are often alien species (Miller & Miller, 2003; Miller et al., 2002; Pellizzari & Germain, 2010; Pellizzari & Porcelli, 2014).
Pellizzari and Germain (2010) estimated that the number of scale species present in Europe was about 400–450. According to the same authors, there were 129 alien-scale species reported in the region, up to 2007. More recently, other non-native scales invaded Europe, such as Delottococcus aberiae (De Lotto) (Beltrà et al., 2015), Paracoccus hakeae (Williams) (von Ellenrieder et al., 2016), Toumeyella parvicornis (Cockerell) (Garonna et al., 2018), and Phenacoccus solenopsis Tinsley (Ricupero et al., 2021). In Portugal, 48% of the reported 168 species of scale insects are alien (Franco et al., 2011). Armored scales are the largest group of alien scales in Europe, representing about 47% of the total number of species (Pellizzari & Germain, 2010).
Olive tree, Olea europaea L. is the most extensively cultivated fruit crop in the world (Migliorini, 2011). Mediterranean countries are responsible for about 93% of the world production of olive oil, and Spain, Italy, Greece and Portugal are the most important producers (IOC, 2022). In the Mediterranean region, 15–20 insect species are permanent or occasional pests of olive trees and about 50% of these species are scale insects (Pellizzari, 1997). The key-pest of olive trees worldwide is the olive fruit fly, Bactrocera oleae Gmelin (Daane & Johnson, 2010). The olive moth, Prays oleae Bern and, among scale insects, the black scale, Saissetia oleae Bern are examples of secondary pests (Haniotakis, 2005; Mansour et al., 2011).
Olea europaea has been reported as the host plant of about 100 scale insects worldwide, mostly belonging to Diaspididae (70 species), Coccidae (14), and Pseudococcidae (11) (García Morales et al., 2016), 43% of which in the Mediterranean basin (Panis, 1986). Argyriou (1990) presented a list of 18 armored scale insects that have originated damage or outbreaks in olive trees, including two species widely distributed, i.e., Parlatoria oleae (Colvée) and Aspidiotus nerii Bouché. Other armored scale insects may be of economic importance, at regional level, such as Pelionella cycliger (Leonardi) and A. nerii in Tunisia (Mansour et al., 2011), Pollinia pollini (Costa), Parlatoria oleae and Lepidosaphes ulmi (L.) in Italy (Longo & Suma, 2008), Leucaspis riccae Targioni Tozzetti and P. oleae in Egypt (Abd-Raboou & Ahmed, 2011), and Hemiberlesia rapax (Comstock), A. nerii, P. pollini, Lichtensia viburni Signoret in the Maltese Island (Haber & Mifsud, 2007).
In 2016, new damage symptoms were observed on branches of ornamental olive trees in an urban area, in the Algarve, Portugal. The causal agent was recognized as an unknown armored scale insect. Since then, several detections of this scale have been registered. Here, we report the results of the morphological and molecular studies carried out on samples collected on olive trees from different types of habitats, in the Algarve, which allowed the identification of the causal agent as the South African Obscure Scale, Melanaspis corticosa (Brain). This scale insect is a potential injurious pest of olive trees and is reported for the first time in Europe, as well as in the Palearctic region. Until now, M. corticosa was only known in a few African countries, including Guinea, Mozambique, South Africa (where it was described), and Zimbabwe (García Morales et al., 2016).
Material and Methods
Sampling and field observations
Samples were collected in different locations and habitats, in the Algarve (Table 1). Most of the sampling sites resulted from private requests from farmers and proprietaries received by the Plant Protection Division of the Regional Directorate of Agriculture, in the Algarve. In each sampling site, 40–30 cm terminals of up to 5 damaged branches were collected from symptomatic olive trees and transported to the laboratory for study. Samples were kept in the fridge (ca. 5 °C), until observation. Adult-female specimens of the scale were collected and preserved in 80–90% alcohol within Eppendorf tubes for morphological and molecular studies.
Morphological studies
Infested shoots were studied in the laboratory under magnification (10-70x; EMZ13TR Meiji Techno) and photos of the nymphs and adult females were taken (software ProgRes CT5 USB Color, Meiji Techno).
A total of 25 adult females were slide-mounted in Canada balsam after being prepared following the method described in Watson (2002). The specimens were examined using a compound Nikon Labophot microscope at magnifications between 40 and 1000x, and compared with descriptions, illustrations, and keys to known species of the genus Melanaspis and other allied genera (Balachowsky, 1951, 1958; Brain, 1919; Ferris, 1938, 1941, 1942; Lupo, 1954; Normark, et al., 2019; Williams & Watson, 1988). Further information was also obtained from the ScaleNet database (García Morales et al., 2016). Pictures of slide-mounted specimens were taken with a Zeiss Axiophot microscope. Vouchers of the studied specimens were deposited at the Scale Insect Collection of the Department of Agriculture, Food and Environment, Section of Applied Entomology, University of Catania (Italy) (10 specimens) and Department of Agronomy, Food, Natural resources, Animals and Environment, University of Padua (Italy) (15 specimens).
Molecular studies
DNA extraction, amplification and sequencing
Two collected samples were used for molecular characterization. The first sample was composed of adults and the second sample, in addition to the adults, had also egg masses. Altogether six specimens were subsampled out of the samples for molecular analysis and all of them also undergone on morphological studies.. The insects were conserved in 100% ethanol until being used for DNA extraction.
The insects were briefly washed with sterile distilled water to remove residues of ethanol and to remove surface contaminants. The specimens from the different samples were individually extracted at the correspondent time of sampling which ensured the absence of cross-contamination among DNA extracts. Total genomic DNA was extracted from single adult specimens and from eggs using the DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. DNA extracts were stored at -20 °C until posterior use.
The mitochondrial cytochrome c oxidase subunit I locus (COI) gene was chosen as it has been designated as a DNA barcode for insect species suitable for population genetics and phylogenetic studies (Savolainen et al., 2005), but the commonly used 658 bp fragment produced with the primers HCO1490/HCO2198 (Folmer et al., 1994) was not efficiently amplified under our conditions. The PCR amplification of the 3’ region of the COI gene fragment was done by using the primers PCO-F1 (5-CCTTCAACTAATCATAAAAATATYAG-3’)/ Lep-R1 (5-TAAACTTCTGGATGTCCAAAAAATCA-3) (Amouroux et al., 2017).
The reaction master mix contained 1 × PCR buffer, 2.5 mM MgCl2, 0.08 mM of each dNTP, 0.5 µM of each primer, 4 Units of BIO-X-ACT short DNA polymerase (Bioline, London, UK), 5 µL of the extracted DNA and PCR grade water up to the final volume of 50 µL. The reactions were performed under the current conditions of the laboratory (initial denaturation of 2 min at 95 °C followed by 35 cycles of 20 s at 95 °C, 40 s at 48 °C and 30 s at 72 °C, with a final extension of 10 min at 72 °C, ended at 10 °C using a T-one Thermocycler instrument (Biometra, Göttingen, Germany). The amplified products were observed after electrophoresis of 8 µL in a 1.5% (w/v) agarose gel stained with GelRed®Nucleic Acid Gel Stain (Biotium, Fremont, USA). When a fragment of the expected size of 649 bp was observed, the remaining product was used for Sanger sequencing. Prior to sequencing, Exo-Sap enzymes (Applied Biosystems: Thermo Fisher Scientific, Waltham, USA) were used to remove non-used nucleotides and primers. The sequencing reactions were done using BigDye Terminator v3.1 Cycle sequencing kit and run on an 8-cappilary array for the ABI 3500xL Genetic Analyzer (Applied Biosystems: Thermo Fisher Scientific, Waltham, USA).
The obtained forward and reverse sequences were processed to remove the primers sequences prior to the assembling and alignment using the BioEdit Sequence Alignment Editor 7.2.5.3 (RRID:SCR_007361) (Hall, 1999). These sequences were compared with each other and also with the ones available in the National Centre of Biotechnology Information (NCBI) Genbank database (Geer et al., 2010) and in the Barcode of Life Database System (BoldSystems V4) (Ratnasingham & Hebert, 2007) with Clustal Omega (Geneious Prime®2022.2.1.). In the latter, the databases “Species Level Barcode Records” and “All barcode Records on Bold” were the consulted. As no similarity was found, the sequences generated for M. corticosa were deposited in the NCBI GenBank with the accession numbers OP442082 up to OP442087.
Phylogenetic analysis
A phylogenetic tree based on 1000 bootstrap replicates was built with Geneious Prime®2022.2.1. using the Neighbour Joining method (NJ) (Saitou & Nei, 1987) and the Tamura-Nei Genetic Distance Model which are followed as general protocols for barcoding study. One DNA sequence for Aspidiotus excisus (HM474079.1) as an outgroup taxon was obtained from GenBank.
Results and discussion
Observed symptoms and damage
Severe damages were observed in many olive trees in the studied locations, in which the scale originated dieback of branches, with leaf browning, followed by leaf abscission (Fig. 1). In most of the collected samples, the branches and shoots were completely covered by aggregated individuals of the scale, including adult females and nymphs (Fig. 2). This corresponds to the highest intensity level of infestation by scale insects, according to the classification proposed by Kosztarab (1990), with five categories (0–4), i.e., “4 = general or layered infestation (scales completely cover the infested parts of the plant)”.
The scale was present only on the bark. The observed pattern (Fig. 2) corresponded to that described by Brain (1919) for the attack of M. corticosa. According to Brain (1919), “Female scale varying greatly on different host-plants; on smooth-barked plants it is very large and flat, reaching 3.2 mm in diameter, brownish to black in colour with the blackish exuviae covered; as a rule, however, the scale is almost or entirely covered by the outer layers of bark of the host-plant; on Rhus this is usual, and it has been submitted on many occasions as a browning scale; on Robinia the scale takes the greyish appearance of the bark, but the black exuviae are very conspicuous with a greyish white concentric ring; on the wild olive, on the other hand, it forms a thick crust of blackish or greyish black scales, which easily flake off; the scale itself, without any admixture of tissues, is pitchy black, with concolorous exuviae; seen from below the scale is domed and very glossy; the ventral scale is delicate and usually remains on the host-plant”.
Morphological studies
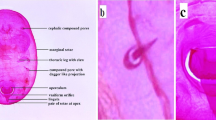
All adult females examined showed a remarkable matching with descriptions and illustrations of M. corticosa by Brain (1919) and Balachowsky (1958) and particularly they showed a perfect match with drawing of posterior dorsal area of pygidium of M. corticosa (= Chrysomphalus (Pseudischnaspis) corticosus) by Brain (1919), i.e.: the shape and proportion of the pairs of lobes (median, second and third lobes); the arrangement of orifices of dorsal ducts; the presence of plates; shape, proportion and arrangement of paraphysis (always 7 in number for each half of the pygidium, all of which are found to be arranged on segments VI, VII, and VIII) (Fig. 3).
However, the specimens examined showed some differences attributable to the intra-specific morphological variability of this species, concerning the position of the tubercles present on the mesothorax (1 on each side), which is generally closer to the prothorax than that indicated by Balachowsky (1958). In addition, differences emerged from what has been described by Balachowsky (1958), concerning the number of notches present on the lateral-external margin of lobes (in bracket the numbers indicated in the description): median lobes with 0–2 notches on external lateral margin (1); second lobes sometimes with 3 notches (2); third lobes sometimes with 1–2 notches (3–4). All specimens have small, rather short and sometimes not perfectly visible plates between the lobes and outside of third lobes; they always show the presence of a single plate between median and second lobes, detail that as Balachowsky (1958) points out distinguishes M. corticosa from other African Melanaspis. The arrangement of clusters of dorsal ducts remains constant and correspondent to description by Balachoswky (1958) with the total number of clusters sometimes lower, i.e.: 16–26 on segments VI-VII between median coupled paraphysis and third lobe (25–35); 8–19 with an anteriorly placed row of 6–12 elements in segments V-VI (18–20 with an upper row of 12–14 elements). Finally, the 5 distinct groups of perivulvar pores in the specimens examined respond to the formula 1–7 (11–17) 5–13, while Balachowsky (1958) indicates the formula 4–6 (12–16) 8–10 and Brain (1919) 6–9 (17–24) 9–16.
Molecular studies
For the first time COI sequences for M. corticosa were generated as during the Blast analysis that we performed, no sequences could be retrieved either from the GenBank or from the BoldSystems. The sequences obtained were aligned with full length COI fragments (> 649 bp) available for species of the Melanaspis genus. The genetic distances expressed in percent identity (Table 2) reveal significant interspecific variation. The identity variation between M. corticosa and other species varies from 34.7% to 89.4% and all specimens collected in Portugal could be grouped in one clade supported by a bootstrap value of 99.7%. The intraspecific distance in this population ranged from 0% to 0.3% for COI. The latter value was derived from the variation of the total length of the sequenced fragments rather than the variation within the nucleotide sequence. For the specimens 3–2002 and 4–2002, due to limitations of the sequencing process, the obtained sequences were shorter than expected, 612 bp and 610 bp, respectively. The COI sequences were not differentiated potentially reflecting the low extent of selection pressure that the specimens of this emerging species, currently representing a new entry in Europe, suffered to adapt to the new environment. Melanaspis corticosa can clearly be distinguished from other armored scales (Fig. 4).
Phylogenetic tree depicting genetic relationships derived from 27 COI sequences: 6 sequences from specimens collected in olive trees in Algarve, Portugal (M. corticosa); 20 sequences from all the Melanaspis species available in the GenBank; 1 sequence from an outgroup species (Aspidiotus excisus). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap is shown next to the branches
Geographical distribution and host plants
The scale was detected for the first time at Sagres, in 2016 (Table 1). Since then, it was observed in different locations, between Sagres and Tavira (Fig. 5), and different habitats, most often in urban trees and gardens (Table 1). Data suggest that M. corticosa established and has been expanding its distribution in the Algarve. It was not detected outside this region.
All samples were collected in olive trees. The presence of M. corticosa was not detected yet in other host plants in the Algarve. However, this armored scale insect is considered a polyphagous species, reported from host plants of different families, including Schinus molle, Sclerocarya birrea (Anacardiaceae), Celastrus, Ebenaceae, Diospyros pallens (Celastraceae), Erythrina caffra, Robinia, Virgilia oroboides (Fabaceae), Juglans spp. (Juglandaceae), Olea spp. (Oleaceae), Platanus (Platanaceae), Prunus, Prunus persica, Pyrus (Rosaceae), and Populus (Salicaceae) (García Morales et al., 2016). This is the first time this species is reported to originate economic damage in olive trees.
Conclusion
Both morphological and molecular studies indicate that the identity of the armored scale insect observed infesting olive trees in different locations, in the Southern region of Portugal (Algarve), corresponds to the South African Obscure Scale, M. corticosa. This is the first time this scale insect species is reported outside Africa and it is a first record in Europe and in the Palearctic region. Its presence in Portugal was reported to the National Plant Protection Organization. Due to the intraspecific variability observed in Melanaspis genus (Ramasubramanian et al., 2016), the DNA barcoding sequence generated in this study will constitute an important practical tool to help in the correct identification of M. corticosa. The observed level of damage indicates that the scale is a potential injurious pest of olive trees. Further studies are needed to clarify its pest status and develop effective pest management strategies.
References
Abd-Raboou, S., & Ahmed, N. (2011). Seasonal incidence of scale insects, whiteflies and psyllids (Hemiptera) of olive and their natural enemies in Egypt. Egyptian Academic Journal of Biological Sciences, 4(1), 59–74.
Argyriou, L. C. (1990). Olive. In D. Rosen (Ed.), Armored scale insects: Their biology, natural enemies and control (Vol. 4B, pp. 579–583). Elsevier.
Amouroux, P., Crochard, D., Germain, J.-F., Correa, M., Ampuero, J., Groussier, G., Kreiter, P., Malausa, T., & Zaviezo, T. (2017). Genetic diversity of armored scales (Hemiptera: Diaspididae) and soft scales (Hemiptera: Coccidae) in Chile. Scientific Reports, 7, 2014. https://doi.org/10.1038/s41598-017-01997-6
Balachowsky, A. S. (1951). Les cochenilles de France, d’Europe, du Nord de l’Afrique et du bassin Méditerranéen. VI. - Monographie des Coccoidea; Diaspidinae (Troisième partie) Aspidiotini (fin). Entomologie Appliquée Actualités Scientifiques Et Industrielles, 1127, 561–720.
Balachowsky, A.S. (1958). Les cochenilles du continent Africain Noir. Vol. 2 Aspidiotini (2me partie), Odonaspidini and Parlatorini. Annales du Musée Royal du Congo Belge, N.S., 4: 145–356.
Beltrà, A., Addison, P., Ávalos, J. A., Crochard, D., Garcia-Marí, F., Guerrieri, E., Giliomee, J. H., Malausa, T., Navarro-Campos, C., Palero, F., & Soto, A. S. (2015). Guiding classical biological control of an invasive mealybug using integrative taxonomy. PLoS One,10(6) e0128685. https://doi.org/10.1371/journal.pone.0128685
Brain, C. K. (1919). The Coccidae of South Africa - III. Bulletin of Entomological Research, 9, 197–239.
Daane, K. M., & Johnson, M. W. (2010). Olive Fruit Fly: Managing an Ancient Pest in Modern Times. Annual Review of Entomology, 2010(55), 151–169. https://doi.org/10.1146/annurev.ento.54.110807.090553
Ferris, G. F. (1938). Atlas of the scale insects of North America. Series 2. California: Stanford University Press Palo Alto.
Ferris, G. F. (1941). Atlas of the scale insects of North America. Series 3. California: Stanford University Press Palo Alto.
Ferris, G. F. (1942). Atlas of the scale insects of North America. Series 4. California: Stanford University Press Palo Alto.
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3(5), 294–299.
Franco, J. C., Russo, A., & Marotta, S. (2011). An annotated checklist of scale insects (Hemiptera: Coccoidea) of Portugal, including Madeira and Azores Archipelagos. Zootaxa, 3004, 1–32.
García Morales, M., Denno, B.D., Miller, D.R., Miller, G.L., Ben-Dov, Y., & Hardy, N.B. (2016). ScaleNet: A literature-based model of scale insect biology and systematics. Database. https://doi.org/10.1093/database/bav118. http://scalenet.info [Accessed on November 28, 2022].
Garonna, A. P., Foscari, A., Russo, E., Jesu, G., Somma, S., Cascone, P., & Guerrieri, E. (2018). The spread of the non-native pine tortoise scale Toumeyella parvicornis (Hemiptera: Coccidae) in Europe: a major threat to Pinus pinea in Southern Italy. iForest, 11, 628–634. https://doi.org/10.3832/ifor2864-011
Geer, L. Y., Marchler-Bauer, A., Geer, R. C., Han, L., He, J., He, S., Liu, C., Shi, W., & Bryant, S. H. (2010). The NCBI BioSystems database. Nucleic Acids Research, 38, D492–D496.
Haber, G., & Mifsud, D. (2007). Pests and diseases associated with olive trees in the Maltese Islands (Central Mediterranean). The Central Mediterranean Naturalist, 4(3), 143–161.
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Window 95/98/NT. Nucleic Acids Symposium, 41, 95–98.
Haniotakis, G.E. (2005). Olive Pest Control: Present Status and Prospects. Proceedings of the IOBC/WPRS Conference on Integrated Protection of Olive Crops, Chania, 29–31 May 2003.
IOC – International Olive Council (2022). EU olive oil figures. Retrieved July 4, 2022, from: https://www.internationaloliveoil.org.
Kosztarab, M. (1990). Economic importance. In D. Rosen (Ed.), Armored scale insects: Their biology, natural enemies and control (Vol. 4B, pp. 307–311). Elsevier.
Longo, S., & Suma, P. (2008). The olive scales and their entomophagous in Italy. In Abstracts Book of the 1st International Symposium on Olive Tree Integrated Pest Management, November 25th-27th 2008 (p. 37). Sousse, Tunisia
Lupo, V. (1954). Revisione delle cocciniglie Italiane. X. (gen. Pelomphala, Aonidiella, Comstockaspis). Bollettino del R. Istituto Superiore di Agraria - Laboratorio di Entomologia Agraria, "Filippo Silvestri", 13, 34–63.
Mansour, R., Mkaouar, R., GrissaLebdi, K., Suma, P., & Russo, A. (2011). A survey of scale insects (Hemiptera: Coccoidea) occurring on olives in Tunisia. Journal of Entomological and Acarological Research, Ser. II, 43(3), 315–322.
Migliorini, P. (2011). Development of organic olive cultivation and its importance for the sustainability in the Mediterranean. In: Migliorini, Paola; Minotou, Charikleia; Lusic, Drazen; Hashem, Yousry and Martinis, Aristotelis (Eds.) Book of Abstract. International Conference on Organic Agriculture and Agro-Eco Tourism in the Mediterranean, DIO.
Miller, D. R., & Davidson, J. A. (1990). A list of the armored scale insect pests. In D. Rosen (Ed.), Armored scale insects: Their biology, natural enemies and control (Vol. 4B, pp. 299–306). Elsevier.
Miller, D. R., Miller, G. L., & Watson, G. W. (2002). Invasive species of mealybugs (Hemiptera: Pseudococcidae) and their threat to U.S. agriculture. Proceedings of the Entomological Society of Washington, 104, 825–836.
Miller, G. L., & Miller, D. R. (2003). Invasive soft scales (Hemiptera: Coccidae) and their threat to U. S. agriculture. Proceedings of the Entomological Society of Washington, 105, 832–846.
Normark, B. B., Okusu, A., Morse, G. E., Peterson, D. A., Itioka, T., & Schneider, S. A. (2019). Phylogeny and classification of armored scale insects (Hemiptera: Coccomorpha: Diaspididae). Zootaxa, 4616(1), 1–98.
Panis, A. (1986). Entomologie oleicole. In: FAO Food and Agricultural Organisation of the United Nations (Eds.), Madrid. Cours international d’entomologie oleicole (p. 230). FAO.
Pellizzari, G. (1997). Olive. In: Ben-Dov Y., Hodgson C.J. (eds.), Soft scale insects: their biology, natural enemies and control - World Crop Pests, Vol. 7, Part 2. Elsevier Science B.V., the Netherlands (pp. 217–229).
Pellizzari, G, & Germain, J-F. (2010). Scales (Hemiptera, Superfamily Coccoidea). Chapter 9.3. In: Roques, A. et al. (Eds) Alien terrestrial arthropods of Europe. BioRisk, 4(1): 475–510. doi: https://doi.org/10.3897/biorisk.4.45
Pellizzari, G., & Porcelli, F. (2014). Alien scale insects (Hemiptera Coccoidea) in European and Mediterranean countries: The fate of new and old introductions. Phytoparasitica, 42, 713–721. https://doi.org/10.1007/s12600-014-0414-5
Ratnasingham, S., & Hebert, P. D. N. (2007). BOLD: The Barcode of Life Data System (www.barcodinglife.org). Molecular Ecology Notes, 7, 355–364. https://doi.org/10.1111/j.1471-8286.2006.01678.x
Ricupero, M., Biondi, A., Russo, A., Zappalà, L., & Mazzeo, G. (2021). The Cotton Mealybug Is Spreading along the Mediterranean: First Pest Detection in Italian Tomatoes. Insects, 12, 675. https://doi.org/10.3390/insects12080675
Saitou, N., & Nei, M. (1987). The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Molecular Biology and Evolution, 4, 406–425.
Savolainen, V., Cowan, R. S., Vogler, A. P., Roderick, G. K., & Lane, R. (2005). Towards writing the encyclopedia of life: an introduction to DNA barcoding. Philosophical Transactions of the Royal Society B - Biological Sciences, 360(1462), 1805–11. https://doi.org/10.1098/rstb.2005.1730
von Ellenrieder, N., Watson, G. W., Kimnee, S. A., Franco, J. C., & Mazzeo, G. (2016). Paracoccus leucadendri Mazzeo & Franco in Mazzeo, Franco & Russo, 2009, a junior synonym of Paracoccus hakeae (Williams, 1985) comb. nov. (Coccomorpha: Pseudococcidae). Zootaxa, 4093(4), 552–558. https://doi.org/10.11646/zootaxa.4093.4.6
Watson, G.W. (2002). Arthropods of economic importance: Diaspididae of the world. Series Title: World Biodiversity Database. Expert Center for Taxonomic Identification (ETI) Bioinformatics, Leiden. UNESCO Publishing, Paris. CD-Rom. Available from: https://diaspididae.linnaeus.naturalis.nl/linnaeus_ng/app/views/index/index.php?epi=155&&letter=l
Williams, D. J., & Watson, G. W. (1988). The Scale Insects of the Tropical South Pacific Region. Pt. 1. The Armoured Scales (Diaspididae) (p. 290). U.K.: CAB International Wallingford.
Acknowledgements
Thanks are due to the involved farmers and proprietaries for all the support during fieldwork.
Funding
Open access funding provided by Università degli Studi di Catania within the CRUI-CARE Agreement. Forest Research Centre (CEF) is a research unit funded by Fundação para a Ciência e a Tecnologia (FCT), Portugal, grant number UIDB/00239/2020 and Laboratory for Sustainable Land Use and Ecosystem Services—TERRA (LA/P/0092/2020).
Author information
Authors and Affiliations
Contributions
Conceptualization: JCF, CM; Methodology: GM, GP, JCF, EA; Field sampling and photos: CS, DT; Morphological studies: GM, GP, AR, SN; Molecular studies: EA; Laboratory photos and figures editing: EBS; Writing—original draft preparation: GM, EA, JCF; Writing—review and editing: all authors.
Corresponding author
Ethics declarations
Competing interests
One of the authors, José Carlos Franco is co-editor in chief of Phytoparasitica.
Conflicts of Interest
JCF is co-editor in chief of Phytoparasitica.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mazzeo, G., Pellizzari, G., Mateus, C. et al. Melanaspis corticosa: a new insect pest of olive trees in Europe. Phytoparasitica 51, 153–162 (2023). https://doi.org/10.1007/s12600-022-01041-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-022-01041-y