Abstract
The study described herein aimed to evaluate the impact of exercise on histone acetylation markers in striatum from Wistar rats at different stages of development. Male Wistar rats were submitted to two different exercise protocols: a single session of treadmill (running 20 min) or a moderate daily exercise protocol (running 20 min for 2 weeks). Striata of rats aged 39 days postnatal (adolescents), 3 months (young adults), and 20 months (aged) were used. The single exercise session induced persistent effects on global HDAC activity only in the adolescent group, given that exercised rats showed decreased HDAC activity 1 and 18 h after training, without effect on histone H4 acetylation levels. However, the moderate daily exercise did not alter any histone acetylation marker in adolescent and mature groups in any time point evaluated after training. In sum, our data suggest that exercise impacts striatal HDAC activity in an age- and protocol-dependent manner. Specifically, this response seems to be more evident during the adolescent period and might suffer a molecular adaptation in response to chronic training.
Similar content being viewed by others
Introduction
Recent evidence has pointed out that environmental stimulus, such as stress, nutrition, social interactions, maternal care, exercise, and environment enrichment may induce epigenetic modifications in brain, which can alter the transcriptional machinery of specific genes involved in neural function [25]. In this context, emerging findings demonstrated that exercise was able to modulate the enzymatic system involved in histone acetylation status in hippocampus from young adult and aged rats. This epigenetic process is controlled by histone acetyltransferases (HATs) and histone deacetylases (HDACs) enzymes, which respectively add and remove acetyl groups to amino-terminal tails of histones [14].
It is important to note that the studies reporting the exercise effects on acetylation parameters, such as HAT and HDAC activities and histone H3 and H4 acetylation levels have been focused in mature hippocampus [8, 10, 11, 18]. In addition, Spindler and colleagues (2014) recently reported that a single session of exercise enhanced HAT activity in frontal cortices from young adult Wistar rats, while the moderate daily exercise protocol reduced the HDAC activity [30]. Then, these previous findings support the idea that there are structure- and protocol-dependent effects of exercise on epigenetic parameters.
Interestingly, histone hypoacetylation and high levels of HDAC, which are usually associated with transcriptional repression, have been linked to the normal aging process, as well to neurodegenerative conditions [25]. It was reported that a daily exercise protocol was also able to reverse aging-related histone H4 hypoacetylation levels in hippocampi without any effect in young adult ones [19]. However, although the impact of early life experiences to several neurobiological process as well the potential role of epigenetic on these response are widely described [7, 27], studies about exercise effects on epigenetic markers are focused essentially on the mature brain and early periods have received little attention.
Besides, a moderate forced exercise protocol is able to increase a synaptic protein, synapsin I (SYN) and structural proteins (neurofilaments, NF68) in striatum, a brain area related to several brain functions, such as locomotor activity and learning [12]. Despite this finding can represent altered transcriptional activity induced by epigenetic mechanisms, to our knowledge, there are no works evaluating exercise effects on epigenetic parameters in striatum. In this context, some evidence has suggested the involvement of striatal HDAC in memory process. Gaglio and colleagues [13] recently suggested the requirement of epigenetic changes in ventral striatum to store and consolidate aversive memories. In this work, the authors performed immediate post-training focal administrations of the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) in the ventral striatum of mice trained in one-trial inhibitory avoidance task. SAHA administration was capable of improving memory retention, and increased acetylation of specific residues. The striatal SAHA administration indicates that this brain area can be vulnerable to epigenetic modulation [13]. Furthermore, it was recently reported that the expression of specific genes was modulated in response to a voluntary exercise paradigm (wheel running during 16 days) in striatum of adult rodents [9]. Although these findings can suggest altered transcriptional activity induced by epigenetic mechanisms, to our knowledge there are no works evaluating the impact of exercise on epigenetic parameters in this brain area.
Furthermore, a temporal profile of HDAC activities in brain areas was described, since hippocampal HDAC activity was significantly increased in the early morning when compared to the afternoon in Wistar rats [10, 26]. Indeed, a significant interaction between the aging process and temporal profile was observed in frontal cortices [26]. However, the temporal pattern of striatal HDAC activity in different developmental stages is poorly investigated.
Interestingly, previous data obtained in our laboratory showed that the single session and daily exercise affect transiently the acetylation parameters in hippocampus from young-adult and aged rats, specifically immediately and 1 h after, without any delayed effects [10, 19]. Accordingly, the daily exercise protocol improved aversive memory performance in the inhibitory avoidance [19]. In addition, there is evidence supporting the idea that exercise can impact methylation parameters in an protocol- and age-dependent manner. For instance, both single-session and daily exercise protocols diminished acutely, as well as at 18 h after, the H3-K9 methylation levels in young adult hippocampi, while the single session reversed the reductions on H3-K9 methylation levels induced by the aging process [11]. Even though these findings, the time course of the exercise effects on epigenetic modulation during the early stages are lack.
In view of these observations, our aim was to investigate the effect of two exercise protocols, a single session or daily treadmill protocol on HDAC activity in striatum of rats at different stages of development. Moreover, in order to verify the acute and delayed effects of exercise, we also evaluated 1 and 18 h after the last session of training.
Materials and methods
Animals
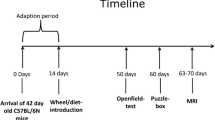
Male Wistar rats at different stages of development, periadolescents (25 days postnatal), young adults (3 months) and aged (20 months) were used. The animals were maintained under standard conditions (12 h light/dark cycle, 22 ± 2 °C) with food and water ad libitum. The NIH “Guide for the Care and Use of Laboratory Animals” (No. 80–23, revised 2011) was followed in all experiments. The Local Committee (CEUA/UFRGS) approved all handling and experimental conditions (nr. 21449).
Training
As recommended by previous studies, in order to minimize the non-specific effects associated with novelty and emotional stress, both exercise protocols, single exercise sessions and daily exercise training, started after 2 days of rest following the treadmill adaptation [10, 11, 30]. The peak oxygen uptake (VO2) was indirectly measured in all animals prior to training. Each rat ran on a treadmill at a low initial speed with the speed being increased by 5 m/min every 3 min until the point of exhaustion (i.e., failure of the rat to continue running). The time to fatigue (in min) and workload (in m/min) were taken as indexes of exercise capacity, which was in turn taken as VO2 max [4, 9]. Animals that initially refused to run were encouraged by a gentle tap to the backs. After the test, the animals that refused to run or who had the worst performance were assigned to the control/sedentary groups (SED). The animals that had the best performance were randomized and submitted to the single session or daily protocol (EXE groups). The single session of treadmill exercise consisted of running for 20 min, while in the daily treadmill protocol, rats run once daily for 20 min, for 2 weeks. SED was handled exactly as the experimental animals and left on the treadmill for 5 min without any stimulus to run. The exercise training consisted of running sessions on an adapted motorized rodent treadmill (INBRAMED TK 01, Porto Alegre, Brazil), with individual Plexiglas lanes, at 60 % of the animals’ maximal oxygen uptake [4].
Periadolescent rats (25 days old) were adapted to the treadmill by running, in the first two sessions, at 4.7 m/min for the first 2 min, 5.8 m/min for the next 4 min, 7 m/min for 8 min, 5.8 m/min for 4 min, and 4.7 m/min for the last 2 min. Thereafter, animals ran at 4.7 m/min for the first 4 min, 7 m/min for 12 min, and 4.7 m/min for the last 4 min.
Rats 3 months old were adapted to the treadmill by running, in the first two sessions, at 6.7 m/min for the first 2 min, 10 m/min for the next 4 min, 15 m/min for 8 min, 10 m/min for 4 min, and 6.7 m/min for the last 2 min. Thereafter, animals ran at 6.7 m/min for the first 4 min, 15 m/min for 12 min, and 6.7 m/min for the last 4 min.
Finally, the 20-month-old rats were adapted to the treadmill by running, in the first two sessions, at 4.5 m/min for the first 2 min, 5.6 m/min for the next 4 min, 6.8 m/min for 8 min, 5.6 m/min for 4 min, and 4.5 m/min for the last 2 min. Thereafter, animals ran at 4.5 m/min for the first 4 min, 6.8 m/min for 12 min, and 4.5 m/min for the last 4 min. Any animals that initially refused to run were encouraged by gently tapping their backs. Neither electric shock nor physical prodding was used in this study. All the procedures took place between 14:00 and 17:00 h.
Preparation of samples
To investigate both acute and delayed effects of the exercise protocols, rats were decapitated 1 and 18 h after a single session or after the last session of daily treadmill exercise. It is important to note that 1 h after exercise was at afternoon, while 18 h was early in the morning. Then it was possible to evaluate the impact of the circadian rhythm on the epigenetic markers.
Periadolescent rats were 25 days old when they began the daily exercise protocol. Since the protocol consisted of 14 days, those rats were decapitated at adolescent period (39 days old). In order to evaluate the biochemical effects of single exercise session during early period, we used animals at the same age.
The striatum were quickly dissected out, immediately snap-frozen in liquid nitrogen, and stored at −80 °C. On the day of the assay, the striatum was homogenized in 1:3 volumes of ice-cold lysis buffer pH 7.4 containing (in mM): 250 sucrose; 20 Tris–HCl; 1 EDTA; 1 EGTA; 10 KCl; 1 DTT; 0.1 PMSF; 0.0001 okadaic acid). The lysates were subjected to centrifugation (16,000×g for 5 min) at 4 °C in a microcentrifuge tube, and the supernatant was removed for analysis.
Determination of global HDAC enzyme activity
The effect of exercise on global HDAC enzyme activity was determined using an HDAC Assay Kit (Fluorometric Detection catalog # 17–372, Upstate Biotechnology, Temecula, CA, USA) according to the manufacturer’s instructions. Briefly, the samples were incubated with the HDAC assay substrate at 30 °C for 45 min before addition of the activator solution. The mixtures were incubated for an additional 10 min at room temperature, and HDAC enzyme activity was measured on a microplate reader (excitation = 360 nm, emission = 450 nm). The protein concentration of each sample was measured by the Coomassie Blue method using bovine serum albumin as standard [2].
Determination of global histone H4 acetylation levels
Global histone H4 acetylation levels were determined using the Global Histone H4 Acetylation Assay Kit (Colorimetric Detection, catalog number P-4009, EpiQuik USA). Samples were homogenized with specific lysis buffer following histone extraction according to the manufacturer’s instructions. The pellet formed after incubations with TCA, HCl, acetone, and centrifugations were used for the H4 acetylation detection. The pellet were re-suspended and incubated with the capture antibody followed by detection antibody and developing solution. Stop Solution was added and the absorbance was measured on a microplate reader (450 nm). The Coomassie Blue method was used to determine the protein concentration of each sample using bovine serum albumin as standard [2].
Statistical analysis
The SED 1 h groups were taken as 100 %. Data were expressed by mean ± standard deviation. The results were analyzed by two-way analysis of variance (ANOVA) with exercise and time point after exercise as independent variables, followed by post hoc Tukey’s multiple range tests when appropriate. In all tests, p < 0.05 was considered to indicate statistical significance.
Results
We investigated the acute (1 h) and delayed (18 h after training) effects of global HDAC activity in striatum from Wistar rats at different stages of development submitted to a single session or a daily treadmill protocol.
The effects of the single session of treadmill exercise are illustrated in Fig. 1. Two-way ANOVA showed a significant effect of both factors, exercise and time point after exercise (respectively, F (1, 19) = 23.141, p < 0.001; F (1, 19) = 4.168; p = 0.047) in the adolescent group. It was observed that the single session induced short and delayed effects on global HDAC activity in the adolescent group, given that exercised rats showed a decrease on HDAC activity 1 h (about 30 %, p < 0.01) and 18 h (about 20 %, p < 0.01) compared to its sedentary groups (Fig. 1a). A clear temporal pattern on HDAC activity was also observed, since this parameter was reduced in both sedentary and exercised groups in the early morning (18 h groups) compared to the afternoon (1 h groups), as it was observed in Fig. 1a. Two-way ANOVA did not show a significant interaction between both factors of exercise and time point after exercise.
Effects of the single exercise session (20 min) on global HDAC activity in striatum from a adolescent, b young adult, and c aged Wistar rats. Results are expressed as percentage of the SED 1 h group and columns represent mean ± SD (n = 5–8). (Asterisk) values significantly different from its respective SED control group; (Hash) values significantly different from 1 h, as determined by ANOVA followed by Tukey’s test (p < 0.05)
The single session of treadmill exercise did not alter global HDAC activity in striatum of young adult and aged groups in evaluated time-points, 1 and 18 h (Fig. 1b, c).
Taken that the global HDAC activity was modified only in adolescent rats submitted to a single session protocol, we measured the striatal H4 acetylation levels in this group. However, this parameter was not significantly altered (p > 0.05; Fig. 2).
The daily exercise regimen did not change HDAC activity in any groups (p > 0.05; Fig. 3). Two-way ANOVA showed an effect of daytime only in the adolescent group (F (1,16) = 8.421, p = 0.012; Fig. 3a); since HDAC activity was significantly diminished in the afternoon, 18 h after training in both groups, SED and EXE.
Effects of the chronic treadmill protocol (2 weeks, 20 min daily) on global HDAC activity in striatum from a adolescent, b young adult, and c aged Wistar rats. Results are expressed as percentage of the SED 1 h group and columns represent mean ± SD (n = 5–8). (Hash) values significantly different from 1 h, as determined by ANOVA followed by Tukey’s test (p < 0.05)
Discussion
This study provides important insights about exercise-induced epigenetic modifications in the early stages of development, since it was observed that adolescent rats submitted to a single exercise session showed lower levels of global HDAC activity in striatum without any effect in young adult and aged groups. This is the first evidence describing that this brain area is vulnerable to epigenetic modulation induced by exercise.
Therefore, these data support the hypothesis that epigenetic modulation in striatum may be related, at least in part, on neuroprotective activity and memory-enhancing effects of exercise, since the striatum is involved in motor functions as well in memory process and executive functions [3, 20–23].
A relevant consideration concerning the striatum is that this brain area is crucially associated with reward processing. Interestingly, epidemiological and experimental studies reveal that exercise decreases the positive reinforcing effects of several classes of drugs [29]. Considering this, exercise seems to act especially in youths; for example, a tobacco cessation program induced elevated quit rates in high school [15]; we can propose that the impact of exercise on juvenile populations could be linked to epigenetic effects in adolescent striatum.
It is impossible to establish at this moment the exact mechanism of exercise, since it was determined the global HDAC activity; however we might suggest that exercise targets individual HDAC family members. Kennedy and colleagues [16] showed that the intra-nucleus accumbens administration of a HDAC1 inhibitor reversed the outcomes induced by chronic cocaine exposure. Indeed, mice with nucleus accumbens selective deletions of HDAC1, but not of two other Class I HDACs (HDAC2 or 3), demonstrated reduced behavioral responses to cocaine [16]. Taken the role of HDAC1 on addiction-related phenomena, our finding led us to hypothesize that exercise may change the activity and/or expression of HDAC1 in early stage of development.
In accordance with our findings, hippocampi of adolescent mice showed a down-regulation in the expression of several HDAC genes after 1 week of a voluntary exercise paradigm [1]. These authors also observed an increase on hippocampal H3 acetylation levels induced by training, which was associated with high levels of genes linked to synaptic plasticity and memory process, such as BDNF [1].
Although the lower HDAC activity in striatum of exercised adolescent rats could indicate a hyperacetylation status, the striatal acetylation levels of histone H4 were not changed in this group. It is important to note that enhances of acetylation levels could contribute to addiction-related phenomena. There is also evidence that drug exposure, such as cocaine and ethanol, increases global levels of acetylated H3 and H4 in different brain areas, such as nucleus accumbens and prefrontal cortex [17, 24, 28]. We are then allowed to speculate that an increase of acetylated H4 levels may not be fundamental to exercise effects in early periods. Besides, recently, it was demonstrated that the daily exercise protocol ameliorated aging-related memory decline and increased histone H4 acetylation levels in hippocampi 20-month-old rats, while it did not alter this parameter in the young adult group, showing age-dependent effects of exercise on this epigenetic mark [19].
The age-dependent exercise outcomes on HDAC modulation here observed are not surprising, since it was described in previous works that the environment stimulus effects on brain function, such as physical activity exposure may vary according to animal stage of development [6]. In agreement, recent findings showed that exercise can alter different epigenetic marks, specifically methylation parameters, in rat hippocampus in an age-dependent manner. It was observed that a single exercise session induced a decrease of DNA methyltransferase content, which was involved in DNA methylation process, in young adult rats, without any effect in the aged group. Besides, the single exercise session and chronic exercise protocols reduced significantly the methylation levels of histone H3 in young adult rats; on the other hand, only the single session diminished this parameter in the 20-months aged group [11]. All these data led us to hypothesize that epigenetic changes induced by exercise are linked to specific periods of development.
In the present study, as described above, exercise did not affect HDAC activity in the striatum from adult and aged rats. We can suggest a structure-dependent effect of exercise on epigenetic mechanism modulation, since it was previously observed that exercise acutely reduced HDAC activity in hippocampi and frontal cortices from young adult rats [10, 30]. Moreover, a daily exercise protocol increased H4 acetylation levels in hippocampi from 20-month-old rats [19]. This different profile might suggest brain regional differences in the epigenetic machinery.
To our knowledge, we describe here, for the first time, the temporal profile of exercise outcomes on epigenetic parameters during the period of adolescence. It was observed that the diminished HDAC activity induced by exercise in striatum from adolescent rats persisted at least for 18 h, suggesting that this effect is more persistent than in late stages of development. Our data about exercise effects in adult hippocampus indicates a modulation in HAT and HDAC activities immediately and 1 h, without any alteration 18 h after training [10]. Similarly, it was demonstrated that forced swimming increased hippocampal phosphoacetylation histone levels up to 4 h, but not 24 h after training, in young adult rats [5]. These results suggest that epigenetic marks in response to a single exercise session are more persistent during the early stages of development. Although, remains unaltered after a chronic intervention, indicating a molecular adaptation.
Another remarkable point to discuss is that striatal HDAC activity was modulated by the circadian rhythm, given that this epigenetic mark was reduced in the early morning in striata of SED and EXE adolescent rats. The influence of the time of the day on HDAC activity in hippocampi from young adult Wistar rats was previously described, where this parameter is increased in the early morning [10]. Accordantly, higher HDAC activity in hippocampus and frontal cortex from aged rats in the early morning was also reported [26]. Therefore, we may postulate that the effects of anti-addiction approaches, such as HDAC inhibitors administration, can depend on test times/time of day of administration in adolescence period. Our results might suggest that a suitable time of day for administration of these drugs in rodent models is in the early morning.
Finally, it is widely described that exposure to a novel environment might trigger a stress response [31]. Then, to minimize these effects and avoid confounding responses, the rats used in the present study were submitted to a habituation 2 days before the single exercise session and the daily exercise protocol. Consequently, we can infer that the change on HDAC activity in striata of periadolescent rats was in response to the single exercise session. Future studies must be done in order to investigate and compare the impact of novelty and single bouts of exercise on epigenetic modulation in order to exactly elucidate this question.
Conclusions
Summarizing, the present study shows that exercise is an environmental stimulus able to induce epigenetic modification in striatum from Wistar adolescent rats. Further studies are necessary to establish the exact pathways through which region-specific modifications can be achieved and to better understand the route by which epigenetic mechanisms in response to exercise vary through lifespan.
Although this research was carefully prepared and the epigenetic analyses were chosen based on previous results obtained by the group, a limitation of the present study was that the levels of global histone H3 acetylation were not examined. Additional studies are required to elucidate whether this marker is influenced in striatum of adolescent rats following exercise.
References
Abel JL, Rissman EF (2013) Running-induced epigenetic and gene expression changes in the adolescent brain. Int J Dev Neurosci 31(6):382–390
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:218–254
Broadbent NJ, Squire LR, Clark RE (2007) Rats depend on habit memory for discrimination learning and retention. Learn Mem 14:145–151
Brooks GA, White TP (1978) Determination of metabolic and heart rate responses of rats to treadmill exercise. J App Physiol 45:1009–1015
Chandramohan Y, Droste SK, Arthur JS, Reul JM (2008) The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-d-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur J Neurosci 27(10):2701–2713
de Almeida AA, Gomes da Silva S, Fernandes J, Peixinho-Pena LF, Scorza FA, Cavalheiro EA, Arida RM (2013) Differential effects of exercise intensities in hippocampal BDNF, inflammatory cytokines and cell proliferation in rats during the postnatal brain development. Neurosci Lett 553:1–6
De Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475
de Meireles LC, Bertoldi K, Elsner VR, dos Moysés SF, Siqueira IR (2014) Treadmill exercise alters histone acetylation differently in rats exposed or not exposed to aversive learning context. Neurobiol Learn Mem 116:193–196
Darlington TM, McCarthy RD, Cox RJ, Miyamoto-Ditmon J, Gallego X, Ehringer MA (2016) Voluntary wheel running reduces voluntary consumption of ethanol in mice: identification of candidate genes through striatal gene expression profiling. Genes Brain Behav. 15(5):474–490
Elsner VR, Lovatel GA, Bertoldi K, Vanzella C, Santos FM, Spindler C, de Almeida EF, Nardin P, Siqueira IR (2011) Effect of different exercise protocols on histone acetyltransferases and histone deacetylases activities in rat hippocampus. Neuroscience 192:580–587
Elsner VR, Lovatel GA, Moysés F, Bertoldi K, Spindler C, Cechinel LR, Muotri AR, Siqueira IR (2013) Exercise induces age-dependent changes on epigenetic parameters in rat hippocampus: a preliminary study. Exp Gerontol 48(2):136–139
Ferreira AF, Real CC, Rodrigues AC, Alves AS, Britto LR (2010) Moderate exercise changes synaptic and cytoskeletal proteins in motor regions of the rat brain. Brain Res 1361:31–42
Gaglio D, Capitano F, Mastrodonato A, Minicocci E, Deiana C, Fragapane P et al (2014) Learning induced epigenetic modifications in the ventral striatum are necessary for long-term memory. Behav Brain Res 265:61–68
Handel AE, Ebers GC, Ramagopalan SV (2010) Epigenetics: molecular mechanisms and implications for disease. Trends Mol Med 16:7–16
Horn K, Dino G, Branstetter SA, Zhang J, Noerachmanto N, Jarrett T, Taylor M (2011) Effects of physical activity on teen smoking cessation. J Dev Neurosci 31:382–390
Kennedy PJ, Robison AJ, Maze I, Feng J, Badimon A, Bassel-Duby R, Olson EN, Nestler EJ (2013) HDAC1 inhibition blocks cocaine-induced plasticity through targeted changes in histone methylation. Nat Neurosci 16:434–440
Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ (2008) Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48:303–314
Lovatel GA, Bertoldi K, Elsner VR, Piazza FV, Basso CG et al (2014) Long-term effects of pre and post-ischemic exercise following global cerebral ischemia on astrocyte and microglia functions in hippocampus from Wistar rats. Brain Res 1587:119–126
Lovatel GA, Elsner VR, Bertoldi K, Vanzella C, dos Moysés SF, Vizuete A, Spindler C, Cechinel LR, Netto CA, Muotri AR, Siqueira IR (2013) Treadmill exercise induces age-related changes in aversive memory, neuroinflammatory and epigenetic processes in the rat hippocampus. Neurobiol Learn Mem 101:94–102
Monchi O, Petrides M, Petre V, Worsley K, Dagher A (2001) Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci 21:7733–7741
Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J (2006) Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol 59:257–264
Owen AM (2004) Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist 10:525–537
Packard MG, McGaugh JL (1996) Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem 65:65–72
Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C (2012) Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rat are associated with ethanol-induced place conditioning. Neuropharmacol 62:2309–2319
Saha RN, Pahan K (2006) HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ 13(4):539–550
Sant’ Anna G, Elsner VR, Moysés F, Cechinel LR, Agustini GL, Siqueira IR (2013) Histone deacetylase activity is altered in brain areas from aged rats. Neurosci Lett 556:152–154
Schilling EA, Aseltine RH Jr, Gore S (2007) Adverse childhood experiences and mental health in young adults: a longitudinal survey. BMC Public Health 7:30
Shen S, Casaccia-Bonnefil P (2008) Post-translational modifications of nucleosomal histones in oligodendrocyte lineage cells in development and disease. J Mol Neurosci 35:13–22
Smith MA, Lynch WJ (2011) Exercise as a potential treatment for drug abuse: evidence from preclinical studies. Front Psychiatry 2:82
Spindler C, Cechinel LR, Basso C, Moysés F, Bertoldi K, Roesler R, Lovatel GA, Elsner VR, Siqueira IR (2014) Treadmill exercise alters histone acetyltransferases and histone deacetylases activities in frontal cortices from wistar rats. Cell Mol Neurobiol 34(8):1097–1101
Motoike T, Long JM, Tanaka H, Sinton CM, Skach A, Williams SC, Hammer RE, Sakurai T, Yanagisawa M (2016) Mesolimbic neuropeptide W coordinates stress responses under novel environments. Proc Natl Acad Sci USA 113(21):6023–6028
Acknowledgments
This work was supported, in part, by Grant 476634/2013-0 from Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq/Brazil. CNPq fellowships (Dr. I.R. Siqueira; V.R. Elsner; K. Bertoldi); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES fellowships (L.C. Meireles); Programa de Bolsas de Iniciação Científica—UFRGS (L.R. Cechinel; C.Basso).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Elsner, V.R., Basso, C., Bertoldi, K. et al. Differential effect of treadmill exercise on histone deacetylase activity in rat striatum at different stages of development. J Physiol Sci 67, 387–394 (2017). https://doi.org/10.1007/s12576-016-0471-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-016-0471-2