Abstract
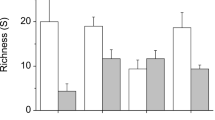
A depth gradient may influence both macrophytes and their associated epiphytic community through variations in hydrodynamics and light conditions, which in turn may affect the mobile organisms living on them. We investigated how a narrow depth gradient affects the associated peracarid assemblages in a Sargassum cymosum bed. This study was conducted on a rocky shore in Southeastern Brazil. A 20 × 3 m sampling area was defined parallel to the shore line, within which three perpendicular transects were sampled in October 1997 (spring), January 1998 (summer), April 1998 (fall), and July 1998 (winter). In each transect, three samples of Sargassum were randomly collected at three different depths—shallow (1 m), intermediate (2 m), and deep (3 m). Peracarid fauna was identified, quantified, and classified into feeding groups. Depth affected both species richness and total density only in January, with fewer species and individuals at the shallow depth, more species at the intermediate depth, and more individuals at the deep depth. The taxonomic and feeding group composition of peracarid assemblages were also affected by depth, with differences depending on the sampling period. Herbivores and omnivores were commonly more abundant at the shallow depth, while detritivores were more abundant at the deep depth. Carnivores seemed not to be affected by depth. Small-scale distribution patterns can be related to how peracarid species deal with the variability in turbulence and food availability imposed by a depth gradient, with morphological attributes and feeding habits guiding how species are distributed in the space and thus the peracarid assemblage as a whole.




Similar content being viewed by others
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austr Ecol 26:32–46
Baldinger AJ, Gable MF (1995) The occurrence of amphipods and other peracarid crustaceans in the rocky littoral zone of Bermuda. Pol Arch Hydrobiol 42:431–439
Bueno M, Tanaka MO, Flores AAV, Leite FPP (2016) Vertical differences in species turnover and diversity of amphipod assemblages associated with coralline mats. Estuar Coast Shelf Sci 181:153–159
Chu SH, Zhang QS, Liu SK, Tang YZ, Zhang SB, Lu ZC, Yu YQ (2012) Tolerance of Sargassum thunbergii germlings to thermal, osmotic and desiccation stress. Aquat Bot 96:1–6
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austr J Ecol 18:117–143
Connell JH (1978) Diversity in tropical rain-forest and coral reefs. Science 199:1302–1310
Cunha MR, Sorbe JC, Moreira MH (1999) Spatial and seasonal changes of brackish peracaridan assemblage and their relation to some environmental variables in two tidal channels of the Ria de Aveiro (NW Portugal). Mar Ecol Progr Ser 190:69–87
Curvelo RR, Corbisier TN (2000) The meiofauna associated withi Sargassum cymosum at Lázaro Beach, Ubatuba, São Paulo. Braz J Oceanogr 48:119–130
De Wreede RE (1976) The phenology of three species of Sargassum (Sargassaceae, Phaeophyta) in Hawaii. Phycologia 15:175–183
Dommasnes A (1968) Variation on the meiofauna of Corallina officianalis L. with wave exposure. Sarsia 34:117–124
Duffy JE (1990) Amphipods on seaweeds: partners or pests? Oecologia 8:267–276
Duffy JE, Hay ME (1994) Herbivore resistence to seaweed chemical defense: the roles of mobility and predation risk. Ecology 75:1304–1319
Edgar GJ (1983) The ecology of south-east Tasmanian phytal animal communities. I. Spatial organization on a local scale. J Exp Mar Bio Ecol 70:129–157
Edgar GJ, Aoki M (1993) Resource limitation and fish predation: their importance to mobile epifauna associated with japanese Sargassum. Oecologia 95:122–133
Edgar GJ, Moore PG (1986) Macro-algae as habitats for motile macrofauna. Monogr Biol 4:255–277
Edgar GJ, Robertson AI (1992) The influence of seagrass structure on the distribution and abundance of motile epifauna: pattern and process in a western Australian Amphibolis bed. J Exp Mar Biol Ecol 160:13–31
Eston VR, Bussab WO (1990) An experimental analysis of ecological dominance in a rocky subtidal macroalgal community. J Exp Mar Biol Ecol 136:170–195
Fenwick GD (1976) The effect of wave exposure on the amphipod fauna of the alga Caulerpa brownii. J Exp Mar Biol Ecol 25:1–18
Fernandes MB (2015) Caracterização de microhabitats formados por algas calcáreas e sua utilização pelos anfípodes em costões rochosos do litoral norte de São Paulo. Thesis, Universidade Estadual de Campinas, São Paulo, Brazil
Garrabou J, Ballesteros E, Zabala M (2002) Structure and dynamics of North-Western Mediterranean rocky benthic communities along a depth gradient. Estuar Coast Shelf Sci 55:493–508
Gibbons M (1988) The impact of wave exposure on the meiofauna of Gelidium pristoides (Turner) Kuetzing (Gelidiales:Rodophyta). Estuar Coast Shelf Sci 27:581–593
Grande H, Reis M, Jacobucci GB (2012) Small-scale experimental contamination with diesel oil does not affect the recolonization of Sargassum (Fucales) fronds by vagile macrofauna. Zoologia 29:135–143
Guerra-García JM, García-Gómez JC (2001) The spatial distribution of Caprellidae (Crustacea: Amphipoda): a stress bioindicator in Ceuta (North Africa, Gibraltar Area). Mar Ecol 22:357–367
Guerra-García JM, Sánchez JA, Ros M (2009) Distribution and ecological patterns of caprellids (Crustacea: Amphipoda) associated with the seaweed Stypocaulon scoparium in the Iberian Peninsula. Mar Biod Rec 2:e151
Guerra-García JM, Rios M, Gordillo I, Cabezas MP, Baeza-Rojano E, Izquierdo D, Corzo J, Domínguez J, Varona S (2010) Distribution patterns of intertidal and shallow water caprellids associated with macroalgae along the Iberian Peninsula. Zool Baet 21:101–129
Guerra-García JM, Baeza-Rojano E, Cabezas MP, García-Gómez JC (2011) Vertical distribution and seasonality of peracarid crustaceans associated with intertidal macroalgae. J Sea Res 65:256–264
Guerra-García JM, Tierno de Figueroa JM, Navarro-Barranco C, Ros M, Sánchez-Moyano JE, Moreira J (2014) Dietary analysis of the marine Amphipoda (Crustacea: Peracarida) from the Iberian Peninsula. J Sea Res 85:508–517
Hagerman L (1966) The macro and microfauna associated with Fucus serratus L., with some ecological remarks. Ophelia 3:1–43
Hawkins SJ, Hartnoll RG, Kain JM, Norton TA (1992) Plant–animal interactions on hard substrata in the north-east Atlantic. In: John DM, Price JH (eds) Plant–animal interactions in the marine benthos. Systematics association special volume no. 46. Clarendon Press, Oxford, pp 1–32
Heiskanen A, Leppänen J (1995) Estimation of export production in the coastal Baltic Sea: effect of resuspension and microbial decomposition on sedimentation measurements. Hydrobiologia 316:211–224
Holmlund M, Peterson CH, Hay ME (1990) Does algal morphology affect amphipod susceptibility to fish predation? J Exp Mar Biol Ecol 139:65–83
Iribarne O (1996) Habitat structure, population abundance and the opportunity for selection on body weight in the amphipod Eogammarus oclairi. Mar Biol 127:143–150
Jacobi CM, Langevin R (1996) Habitat geometry of benthic substrata: effects on arrival and settlement of mobile epifauna. J Exp Mar Biol Ecol 206:39–54
Jacobucci GB, Güth AZ, Turra A, Leite FPP (2011) Influence of a narrow depth gradient and season on the morphology, phenology, and epibiosis of the brown alga Sargassum cymosum. J Mar Biol Assoc UK 91:761–770
Jacobucci GB, Leite FPP (2002) Distribuição vertical e flutuação sazonal da macrofauna vágil associada a Sargassum cymosum C. Agardh, na praia do Lázaro, Ubatuba, São Paulo, Brasil. Zoologia 19:87–100
Jacobucci GB, Leite FPP (2014) The role of epiphytic algae and different species of Sargassum in the distribution and feeding of herbivorous amphipods. Lat Am J Aquat Res 42:353–363
Jacobucci GB, Moretti D, Silva EM, Leite FPP (2002) Caprellid amphipods on Sargassum cymosum (Phaeophyta): depth distribution and population biology. Nauplius 10:27–36
Jacobucci GB, Tanaka MO, Leite FPP (2009) Temporal variation of amphipod assemblages associated with Sargassum filipendula (Phaeophyta) and its epiphytes in a subtropical shore. Aquat Ecol 43:1031–1040
Krapp-Schickel G (1993) Do algal dwelling amphipods react to the “critical zones” of a coastal slope? J Nat Hist 27:883–900
Lim STA, Alexander CG (1986) Reproductive behaviour of the caprellid amphipod Caprella scaura typica Mayer, 1890. Mar Behav Physiol 12:217–230
Lolas A, Vafidis D (2013) Population dynamics of two caprellid species (Crustacea: Amphipoda: Caprellidae) from shallow hard bottom assemblages. Mar Biodivers 43:227–236
Machado GBO, Dena SA, Neufeld AB, Siqueira SGL, Leite FPP (2015) Variation of amphipod assemblage along the Sargassum stenophyllum (Phaeophyta, Fucales) thallus. Nauplius 23:73–78
MacKenzie RA, Dionne M, Miller J, Haas M, Morgan PA (2015) Community structure and abundance of benthic infaunal invertebrates in Maine Fringing Marsh Ecosystems. Estuar Coast 38:1317–1334
Momota K, Nakaoka M (2017) Influence of different types of sessile epibionts on the community structure of mobile invertebrates in an eelgrass bed. PeerJ 5:e2952
Moore PG (1973a) The larger Crustacea associated with holdfasts of kelp (Laminaria hyperborea) in North-East Britain. Cah Biol Mar 14:493–518
Moore PG (1973b) The kelp fauna of north east Britain. II. Multivariate classification: turbidity as an ecological factor. J Exp Mar Biol Ecol 13:127–163
Negreiros-Fransozo M, Franzoso A, Pinheiro MAA, Mantelatto FLM, Santos S (1991) Caracterização física e química da Enseada da Fortaleza, Ubatuba, SP. Rev Bras Geoc 21:114–120
Palmer MA, Allan JD, Butman CA (1996) Dispersal as a regional process affecting the local dynamics of marine and stream benthic invertebrates. Trends Ecol Evol 11:322–326
Paula EJ, Oliveira Filho EC (1980) Phenology of two populations of Sargassum cymosum (Phaeophyta – Fucales) of São Paulo State coast, Brazil. Bolm Bot 8:21–39
Pedersen O, Colmer TD, Borum J, Zavala-Perez A, Kendrick GA (2016) Heat stress of two tropical seagrass species during low tides—impact on underwater net photosynthesis, dark respiration and diel in situ internal aeration. New Phytol 210:1207–1218
Plicanti A, Iaciofano D, Bertocci I, Brutto A (2016) The amphipod assemblages of Sabellaria alveolata reefs from the NW coast of Portugal: an account of the present knowledge, new records, and some biogeographic considerations. Mar Biodivers 47:521–534
Poznanska M, Kakareko T, Krzyzynski M, Kobak J (2013) Effect of substratum drying on the survival and migrations of Ponto-Caspian and native gammarids (Crustacea: Amphipoda). Hydrobiologia 700:47–59
Rohde S, Hiebenthal C, Wahl M, Karez R, Bischof K (2008) Decreased depth distribution of Fucus vesiculosus (Phaeophyceae) in the Western Baltic: effects of light deficiency and epibionts on growth and photosynthesis. Eur J Phycol 43:143–150
Rumbold C, Obenat S, Velazquez SN, Gancedo B, Spivak E (2017) Seasonal variation of peracarid assemblages in natural and artificial marine environments of the Southwestern Atlantic Ocean. Mar Biodivers. https://doi.org/10.1007/s12526-017-0663-x
Scipione MB (2013) Do studies of functional groups give more insight to amphipod biodiversity? Crustaceana 86:955–1006
Stoner AW, Lewis IIIFG (1985) The influence of quantitative aspects of habitat complexity in tropical seagrass meadows. J Exp Mar Biol Ecol 94:19–40
Széchy MTM, Cordeiro-Marino M (1991) Feofíceas do litoral norte do Estado do Rio de Janeiro, Brasil. Hoehnea 18:205–241
Széchy MTM, Paula EJ (1997) Macroalgas epífitas em Sargassum (Phaeophyta - Fucales) do litoral dos Estados do Rio de Janeiro e São Paulo, Brasil. Leandra 12:1–10
Takeuchi I, Kuwabara R, Hirano R, Yamakawa H (1987) Species composition of the Caprellidea (Crustacea: Amphipoda) of the Sargassum zone on the Pacific coast of Japan. Bull Mar Sci 41:253–267
Tanaka MO, Leite FPP (2003) Spatial scaling in the distribution of macrofauna associated with Sargassum stenophyllum (Mertens) Martius: analyses of faunal groups, gammarid life habits, and assemblage structure. J Exp Mar Biol Ecol 293:1–22
Tanaka MO, Leite FPP (2004) Distance effects on short-term recolonization of Sargassum stenophyllum by mobile epifauna, with an analysis of gammarid life habits. J Mar Biol Assoc UK 84:901–910
Tararam AS, Wakabara Y, Leite FPP (1986) Vertical distribution of amphipods living on algae of a Brazilian intertidal rocky shore. Crustaceana 51:183–187
Taylor RB, Cole RG (1994) Mobile epifauna on subtidal brown seaweeds in northeastern New Zealand. Mar Ecol Prog Ser 115:271–282
Taylor RB (1998) Seasonal variation in assemblages of mobile epifauna inhabiting three subtidal brown seaweeds in northeastern New Zealand. Hydrobiologia 361:25–35
Thiel M, Vásquez JA (2000) Are kelp holdfasts islands on the ocean foor indication for temporarily closed aggregations of peracarid crustaceans? Hydrobiologia 440:45–54
Wakabara Y, Tararam AS, Takeda AM (1983) Comparative study of the amphipod fauna living on Sargassum of two Itanhaém shores, Brazil. J Crust Biol 3:602–607
Wallin M, Hakanson L (1992) Morphometry and sedimentation as regulating factors for nutrient recycling and trophic state in coastal waters. Hydrobiologia 235:33–45
Acknowledgements
A scholarship grant to GBJ was provided by CAPES – “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.” Financial support for FPPL was provided by CNPq – “Conselho Nacional de Desenvolvimento Técnico e Científico” (300337/82-5). We thank FAEPEX – “Fundo de Apoio ao Ensino, Pesquisa e Extensão,” which partially funded this research and CEBIMar-USP – “Centro de Biologia Marinha of Universidade de São Paulo,” for logistical support during sampling periods. We also thank A. Z. Güth and D. Silva for sampling help, L. Waters for English review, and the two anonymous reviewers for providing valuable comments and suggestions.
Funding
A scholarship grant to GBJ was provided by CAPES – “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.” Financial support for FPPL was provided by CNPq – “Conselho Nacional de Desenvolvimento Técnico e Científico” (300337/82–5). We also thank FAEPEX – “Fundo de Apoio ao Ensino, Pesquisa e Extensão,” which partially funded this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable.
Additional information
Communicated by S. Kaiser
Electronic supplementary material
ESM 1
(DOCX 41 kb)
Rights and permissions
About this article
Cite this article
Jacobucci, G.B., Vieira, E.A. & Leite, F.P.P. Influence of a narrow depth gradient on the spatial structure of Sargassum peracarid assemblages in Southeastern Brazil. Mar Biodiv 49, 1001–1011 (2019). https://doi.org/10.1007/s12526-018-0885-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-018-0885-6